Overexpression of Arabidopsis thaliana cysteine2/histidine2-type transcription factor 6 gene enhances plant resistance to a bacterial pathogen
2021-01-11WeiTangAnnaTang
Wei Tang · Anna Y. Tang
Abstract Transcription factors can be used to engineer plants for enhanced productivity. However, the mechanism(s) by which the C2H2-type zinc finger transcription factor enhances pathogen resistance in cells is not fully understood. Here, Agrobacterium tumefaciens carrying the gene for Arabidopsis thaliana cysteine2/histidine2-type transcription factor 6 (ZAT6) was used to engineer rice (Oryza sativa L.), cotton (Gossypium hirsutum L.), and slash pine(Pinus elliottii Engelm.) to generate transgenic cell lines.Transgenic cells were then inoculated with the pathogenic bacterium Pseudomonas syringae. Compared to the control, cell viability of transgenic cells increased 39-47% and growth rate increased 9-15% by 7 days after inoculation in rice, cotton and slash pine. Acid phosphatase activity and alkaline phosphatase activity and transcript levels of Ca 2+ -dependent protein kinase genes OsCPK1, OsCPK2,OsCPK6, and OsCPK8 and mitogen-activated protein kinase genes OsMAPK1, OsMAPK2, OsMAPK3, and OsMAPK8 increased significantly in transgenic rice cells by 3 day after inoculation, and extracellular pH had decreased by 10-14%by 96 min after inoculation in transgenic rice, cotton and slash pine cells. These results suggest that ZAT6 enhances P.syringae resistance in plant cells by modulating transcription of CPK and MAPK and oxidase activity.
Keywords Ca2 + -dependent protein kinase · Mitogenactivated protein kinase · Oxidase · Pathogen · Pinus
Introduction
Because biotic and abiotic stresses can reduce plant production, agricultural production should be improved by decreasing the damage from such stress. Transcription factors play an important role in plant responses and tolerance to biotic and abiotic stresses and thus can be used to engineer plants for enhanced productivity. For example, Arabidopsis thaliana cysteine2/histidine2-type transcription factor 6 (ZAT6)positively regulates biotic and abiotic stress by modulating the transcription activity of GSH1, GSH2, PCS1, and PCS2(Chen et al. 2016). Transcription factor ZAT6 carries the classic C2H2 zinc finger motif that can bind to DNA, RNA and proteins to regulate cell function, and overexpression of ZAT6 leads to altered root architecture and phosphate (Pi)acquisition and enhanced root development (Devaiah et al.2007). From genomic analyses, 176 zinc finger proteins have been identified in A. thaliana and 33 A. thaliana zinc finger proteins are known to be conserved in other eukaryotes(Englbrecht et al. 2004). However, the function of ZAT6 in pathogen resistance has not been reported.
Mitogen-activated protein kinases (MAPKs) also have diverse roles in plant response to stresses such as drought,salt, cold, and pathogens. Transgenic Arabidopsis plants overexpressing the tomato SpMPK3 showed improved antioxidant capacity under osmotic stress via enhancing transcript levels of RD29A, RAB18, RD22, ZAT6, ZAT10,and MYB44 under salt stress (Li et al. 2014). In transgenic plants overexpressing ZAT6 and in vitro, ZAT6 interacts with MPK6 to enhance abiotic stress tolerance, indicating that phosphorylation by MPK6 is required for the function of ZAT6 under stress (Liu et al. 2013). Transgenic plants of A. thaliana that expressed repressors constructed using transcription factors AtMYB102, ANAC047, HRS1, ZAT6 or AtERF5 were tolerant to NaCl, demonstrating that increased expression of DREB- and ZAT-related genes are involved in abiotic stress tolerance and that expression of ZAT6 provides an effective strategy for enhancing tolerance to abiotic stress (Mito et al. 2011). Transcription factor ZAT6 is also involved in plant response to freezing stress; its expression is upregulated by cold stress, and ZAT6-activated CBF pathway seems to be essential for freezing stress response in A.thaliana (Shi and Chan 2014). It is not known how ZAT6 interacts with transcription factors to enhance pathogen resistance.
Among the transcription factors involved in stress tolerance, MYB transcription factors have been widely studied for their role in response to both biotic and abiotic stresses.In Betula platyphylla , MYB46 improves abiotic stress tolerance by affecting the expression of genes encoding superoxide dismutase (SOD), peroxidase (POD), and Δ-pyrroline-5-carboxylate synthetase (P5CS) (Guo et al. 2017). Transgenic A. thaliana cells that express GmMYB12B2 are more tolerant than the wild type to salt (Li et al. 2016). In Jatropha curcas, MYB2-overexpressing plants are more tolerant than wild-type plants to salt and cold stresses (Peng et al. 2016).In Solanum lycopersicum, R1-MYB-type transcription factor ARS1 improves salt tolerance without affecting yield(Campos et al. 2016). MYB transcription factor MYB20 also enhances plant tolerance to biotic and abiotic stresses in numerous plant species including A. thaliana (Cui et al.2013), Solanum tuberosum (Cheng et al. 2013b), Leymus chinensis (Cheng et al. 2013a), Oryza sativa (Yang et al.2012), Chrysanthemum morifolium (Shan et al. 2012), and Triticum aestivum (Qin et al. 2012). In S. lycopersicum,expression of the R2R3MYB transcription factor SlAIM1 is induced by pathogens. Ectopic expression of SlAIM1 increases tolerance to high salinity and oxidative stress and decreases susceptibility to the necrotrophic fungus Botrytis cinerea, suggesting SlAIM1 is required to integrate tomato responses to biotic and abiotic stresses (Nagaoka and Takano 2003; Villalobos et al. 2004; Jung et al. 2008; Abuqamar et al. 2009).
Ca 2+ -dependent protein kinases (CPKs) play important roles in plant Ca 2+ -mediated signal transduction and MAPKs function in signaling pathways related to modulating and regulating abiotic and biotic stress responses to external stimuli in plant cells. In A. thaliana, there are 34 CPK genes, and expression of CPK13 regulates stressrelated stomatal opening (Ronzier et al. 2014). Boron (B)in the cell wall transmits an environmental signal to the nucleus to elicit a response to nutrition def iciency by modulating the expression of Ca 2+ -related genes (Gonzalez-Fontes et al. 2013). There is also crosstalk between CPK3 and the major salt-activated MAPKs, MPK4 and MPK6,during salt stress responses. CPK3 kinase activity is also induced by salt stress in protoplasts (Mehlmer et al. 2010).CPK genes are also important for cell viability (Leroch et al. 2008). Functional integration of CPK proteins in the cytoplasmic membrane reveals high specificity for an ATP/ADP antiport (Leroch et al. 2008). CPKs have also been immunodetected in ER membranes (Leroch et al. 2008).
CPKs and MAPKs are also involved in essential signaltransduction pathways. MAP kinase cascades that respond to different extracellular signals during stress and are regulated at the posttranslational and transcriptional levels in cells (Mizoguchi et al. 1996). MAPKs also play an important role in the development of fungal pathogens and their virulence on their hosts. In maize, the MAPK Chk1 functions in virulence of Cochliobolus heterostrophus via regulating expression of cellulase-encoding genes (Lev and Horwitz 2003). MAPKs in nuclei phosphorylate transcription factors and activate gene expression. In tobacco,MAPK kinase MEK2 induces expression of 3-hydroxy-3-methlyglutaryl CoA reductase (HMGR), basic pathogenesis-related (PR) genes, systemic acquired resistance gene 8.2 (Sar 8.2), and harpin-induced gene1 (Hin1) (Kim and Zhang 2004). In Lotus japonicus , Medicago truncatula ,and Phaseolus vulgaris , comparative genomics of MAPK genes demonstrated that MAPKs modulate signaling of abiotic and biotic stresses in cells (Neupane et al. 2013a,b). In Brassica napus, 66 MAPKKK genes were identified to be involved in plant immunity and hormone responses and in biotic and abiotic stress responses (Sun et al. 2014).In Hevea brasiliensis, MAPKs expressed in roots, stems,leaves, and latex regulate responses to abiotic stresses and latex yields (Jin et al. 2017).
In this investigation, we examined the function of ZAT6 in rice (O. sativa L.), cotton (Gossypium hirsutum L.), and slash pine (Pinus elliottii Engelm.) in elevating resistance to a bacterial pathogen, Pseudomonas syringae pv. aceris and analyzed the relationship of between CPKs and ZAT6 overexpression in enhancing resistance to P. syringae pv.aceris in rice. Our experimental results showed that, compared to the control, ZAT6-transgenic cells increased cell viability, growth rate, acid phosphatase activity, and alkaline phosphatase activity in rice, cotton, and slash pine,after treatment with P. syringae. Transcription levels of Ca 2+ -dependent protein kinase genes OsCPK1, OsCPK2,OsCPK6, and OsCPK8 and transcription levels of mitogen-activated protein kinase genes OsMAPK1, OsMAPK2,OsMAPK3, and OsMAPK8 were examined using qRT-PCR.Significant increase of CPKs and MAPKs had observed in ZAT6-transgenic rice cells after inoculation. These results suggest that ZAT6 enhances pathogen resistance in plant cells through enhancing transcription of CPKs and MAPKs.
Materials and methods
Expression vector
For constructing an expression vector for overexpression of ZAT6, cDNA of ZAT6 and the pBI121 binary vector was digested using restriction enzymes KpnI and XbaI (Promega,Madison, WI, USA) at 37 °C. The digested pBI121 was ligated with a 717-bp fragment of ZAT6 (Tang et al. 2007a,b; Tang and Page 2013) to produce the expression vector pBI-ZAT6. Vector pBI-ZAT6 was introduced into Agrobacterium tumefaciens strain GV3101 by electroporation.
Agrobacterium-mediated transformation
ZAT6-transgenic cell lines of rice (O. sativa L.), cotton (G.hirsutum L.), and slash pine (P. elliottii Engelm.) were generated as described before (Tang et al. 2007a, b; Tang and Page 2013) using A. tumefaciens strain GV3101 carrying the pBI-ZAT6 expression vector. Cells in 50-mL flasks were incubated with the Agrobacterium suspension in a shaker at 60 rpm and at room temperature for 20 min. Infected cells were washed 5 times, then placed onto culture medium and incubated for 3 days. Cells were then transferred onto selection medium supplemented with 150 mg/L kanamycin, 250 mg/L cefotaxime, and 500 mg/L carbenicillin. Six weeks after transformation, the cell cultures were used for PCR and southern and northern blotting.
Polymerase chain reaction analyses of transgenic cells
Polymerase chain reaction (PCR) analysis of transgenic cells was conducted as previously described (Tang et al.2005; Tang and Page 2013). Five grams of control cells and transgenic cells were used to isolate genomic DNA, using a Genomic DNA Isolation Kit (Sigma, St. Louis, MO, USA).The primers used for neomycin phosphotransferase II gene(NPTII) were forward primer nfp (5′-GAA CTC GTC AAG AAG GCG ATA-3′) and reverse primer nrp (5′-TGA TTG AAC AAG ATG GAT TGC-3′), for transcription factor ZAT6 DNA fragments were forward primer zf (5′-GTC GAC ATG GCG GAG GAA TTT GGA AGC ATA G-3′) and reverse primer zr (5′-CCA TGG TAG ACT CCT GCT TCG ACA TCA TGG-3′). The reaction mixture, conditions, and gel electrophoresis were described previously (Tang et al. 2007a, b; Tang and Page 2013). PCR was done in a PTC-100TM thermal cycler (MJ Research, San Francisco, CA, USA). Genomic DNA (300 ng) was added to a 50 μL PCR reaction volume as a template with 200 μM each of dATP, dCTP, dGTP,dTTP, primers (35 pmoL), Taq DNA polymerase (2.5 U,Promega), and 10 × reaction buffer (5 μL, 500 mM KCl,100 mM Tris-HCl pH 9.0 at 25 °C, 1% Triton X-100, and 15 mM MgCl 2 ). The thermal cycling conditions were 95 °C for 5 min; 30 cycles of 95 °C for 60 s, 57 °C for 40 s, and 72 °C for 90 s; 72 °C for 8 min.
Southern blot analysis of transgenic cells
The southern blotting protocol for O. sativa, G. hirsutum,and P. elliottii transgenic cells were previously described(Tang et al. 2005; Tang and Page 2013). Five grams of control cells and transgenic cells were used to isolate genomic DNA using a Genomic DNA Isolation Kit (Sigma). Twentyf ive micrograms of DNA were digested 16 h with restriction enzyme Xba I (Roche Diagnostics GmbH) at 37 °C. The 717-bp fragment of ZAT6 was labeled with digoxigenin (DIG)(Roche Diagnostics, Indianapolis, IN, USA).
RNA isolation and northern blot analysis
Five grams of suspension cultures of transgenic and control cells of O. sativa, G. hirsutum, and P. elliottii were used to isolate total RNA, using an RNeasy Mini Plant Kit (Qiagen,Germantown, MD, USA) according to the instructions. Six micrograms of total RNA was used for northern blotting as described before (Tang et al. 2005; Tang and Page 2013)with the DIG-labelled ZAT6 DNA hybridization probe.Tobacco 25S rRNA was used as the loading control for RNA samples. After the southern and northern blotting, three transgenic cell lines of O. sativa (Os8, Os9, and Os13); three G. hirsutum transgenic cell lines (Gh7, Gh12, and Gh29);and three P. elliottii transgenic cell lines (Pe11, Pe17, and Pe21) were selected for further experiments.
Treatment of transgenic cell lines with pathogen
Pseudomonas syringae was grown in LB broth at 37 °C overnight and cell density estimated spectrophotometrically at OD 600 and adjusted to OD600= 0.5 by adding LB broth.Transgenic suspension cells were then inoculated by adding 10 μL of the P. syringae suspension (OD 600 = 0.5) per 1 million transgenic suspension cells of rice, cotton, and slash pine as previously described (Felix et al. 1999). Mixture of transgenic suspension cells and P. syringae cells was cultured in a tissue culture incubator. Samples were taken at different times (0, 1, 3, 5, and 7 days) for analyses.
Measurement of cell viability
The stain 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide (MTT) was used to test cell viability (Koiwai et al. 2007; Nagai et al. 2011; Nath et al. 2016). Cell suspension samples (1 mL) were aseptically washed three times with 50 mM phosphate buffer, pH 7.5. Cells were resuspended in 1 mL of the same buffer. MTT was added to samples at a final concentration of 1.25 mM, then samples incubated for 8 h in the dark at 25 °C and solubilized with formazan salts in 1.5 mL 50% methanol containing 1% SDS at 60 °C for 30 min. Samples were centrifuged three times at 1875 × g for 5 min, and the supernatant was removed each time. The supernatants were combined and absorbance measured at 570 nm. Because fluorescein accumulates in cells with intact membranes, its green fluorescence can be used as a marker of cell viability. Cell viability was measured 0, 1, 3, 5, and 7 days after the addition of 10 μL P.syringae (OD 600 = 0.5) per 1 million transgenic cells. Cell viability was expressed as percentage oflive cells in total cells assayed (Tang et al. 2006, 2007a, b).
Measurement of cell growth rate
Samples (10 mL) transgenic and control cell cultures were withdrawn from the culture flasks using sterile de-tipped pipettes (Lassig et al. 2014; Lalau et al. 2015; Mei et al.2015). For determination of fresh cell mass, the cells were centrifuged (at 1000 × g for 5 min) in 10 mL of distilled water, filtered on a pre-washed and pre-weighed 0.45-μm Millipore filter (Millipore, Bedford, MA, USA) and the mass was measured. Cell numbers were quantified 0, 1, 3,5, and 7 days after the addition of 10 μL bacterial suspension (OD 600 = 0.5) per 1 million transgenic cells and average growth rate was expressed as milligrams per gram fresh mass per day (Tang et al. 2006, 2007a, b).
Measurement of pH of medium with transgenic cell lines
Medium was taken from flasks of cell cultures to measure pH using a method that requires a potassium hydroxide extraction procedure and high-pH anion-exchange chromatography (Riondet et al. 2005). Plant cells were stained with 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt,that acts as a suitable fluorescent pH indicator (Bogoslavsky and Neumann 1998; Flythe and Russell 2004; Barbez et al.2017). The extracellular pH was determined at 0, 6, 12, 24,48, and 96 min after transgenic cells were inoculated with 10 μL bacterial suspension OD 600 = 0.5) per 1 million cells.
Acid phosphatase and alkaline phosphatase activity
At 0, 1, 3, 5, and 7 days after the addition of 10 μL bacterial suspension (OD 600 = 0.5) per 1 million transgenic cells, activity for the two enzymes was quantified at using the method previously described (Kaida et al. 2009;Johnson et al. 2015) and calculation of Sakthivel and Guruvayoorappan (2013). Methanolic extract of cells was then tested for acid phosphatase and alkaline phosphatase activities as previously described (Ma et al. 2013; Selin-Rani et al. 2016).
Determination of relative transcript level of Ca2 + -dependent protein kinase genes
Expression of Ca2+-dependent protein kinase genes in transgenic cells of O. sativa, G. hirsutum, and P. elliottii was examined using qPCR as previously described (Wan et al. 2007). Total RNA was extracted from frozen sample cells using TRIzol reagent and the manufacturer’s protocol(Invitrogen). cDNA for the samples was synthesized using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). Samples were analyzed in triplicate using the Applied Biosystems 7900HT System and the manufacturer’s manual. The primers for qPCR are listed in Table 1. The U6 gene was used as an internal control. Gene expression was measured at 0, 1, 3, 5, and 7 days after the addition of 10 μL P. syringae (OD 600 = 0.5) per 1 million transgenic cells.
Determination of relative transcript level of MAPK genes
Expression of MAPK genes in transgenic cells of O. sativa,G. hirsutum, and P. elliottii was quantified using qPCR as previously described (Wan et al. 2007). Total RNA was extracted from frozen sample cells using TRIzol reagent and the manufacturer’s protocol (Invitrogen), and cDNA was synthesized using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). Samples were analyzed in triplicate using the Applied Biosystems 7900HT System and the manufacturer’s manual. The primers for qPCR are listed in Table 1. U6 was used as an internal control. Gene expression was measured after treatment with 10 μL bacterial suspension (OD 600 = 0.5) per 1 million transgenic cells at different time (0, 1, 3, 5, and 7 days).
Statistical analyses
Means for inoculated cells a specific sampling time were compared to those of the control using the general linear model procedure of SAS 9.4 (SAS Institute, Cary, NC, USA)and one-way ANOVA.
Results
Molecular analysis of transgenic cells
After cells of O. sativa, G. hirsutum, and P. elliottii were exposed to A. tumefaciens strain GV3101 transformed withZAT6, 26 transgenic cell lines of O. sativa , 38 transgenic cell lines of G. hirsutum, and 39 transgenic cell lines of P. elliottii were obtained on selection medium. Oryza sativa transgenic cell lines Os8, Os9, and Os13; G. hirsutum transgenic cell lines Gh7, Gh12, and Gh29; and P. elliottii transgenic cell lines Pe11, Pe17, and Pe21 were selected for PCR analysis to conf irm the presence of NPTII (Fig. 1 b) and ZAT6(Fig. 1 c). PCR-positive cell lines were further conf irmed by Southern (Fig. 1 d) and Northern blotting for ZAT6 (Fig. 1 e).The cell lines were then inoculated with pathogen for functional analysis.
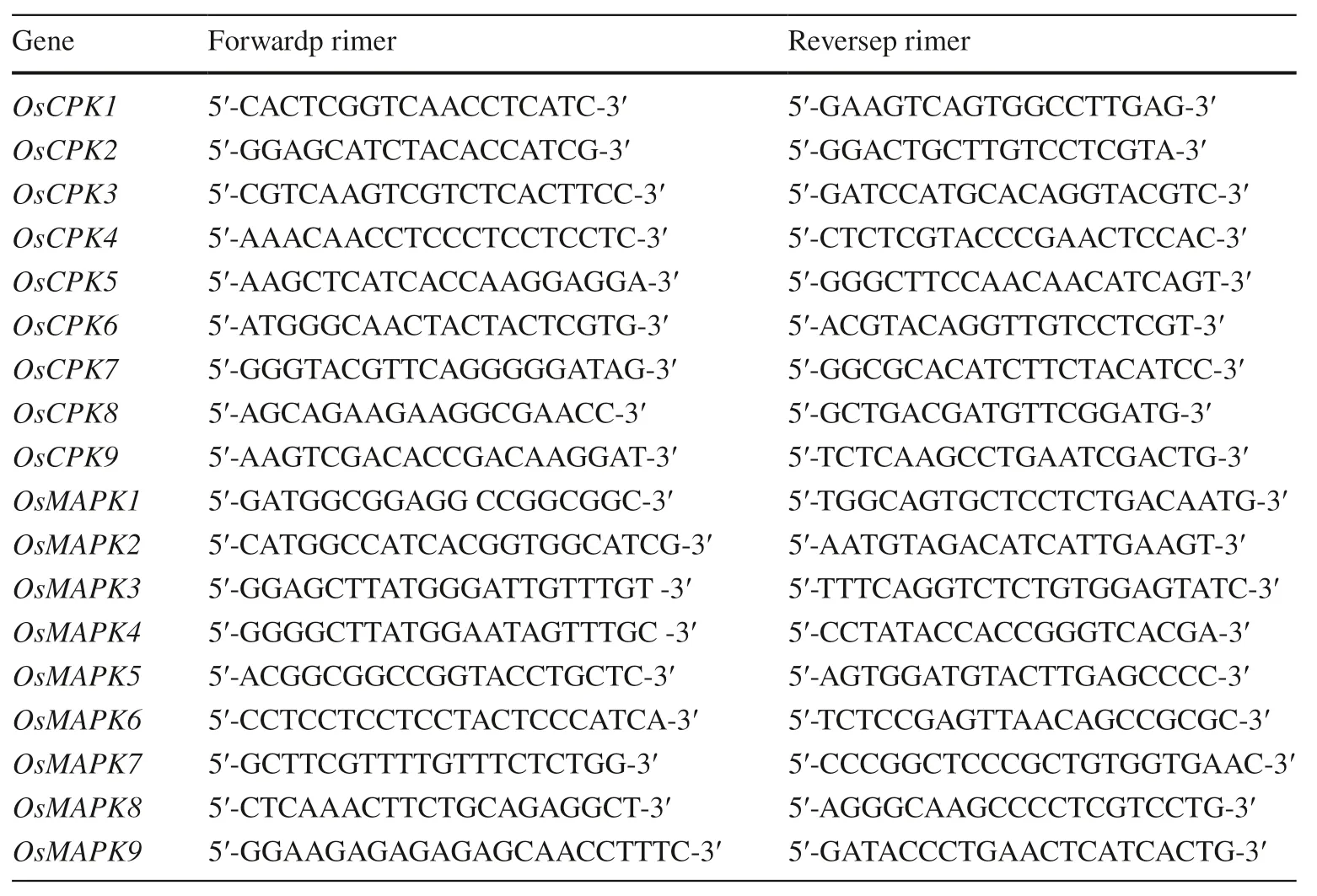
Table 1 Primers used in this study
Cell viability and growth rate of transgenic cell lines
Compared to the viability of the control cells at 7 days after inoculation (dai), cell viability was 39-45% higher in O.sativa transgenic cell lines Os8, Os9, and Os13 (Fig. 2 a);40-46% higher in G. hirsutum transgenic cell lines Gh7,Gh12, and Gh29 (Fig. 2 b); and 41-47% higher in P. elliottii transgenic cell lines Pe11, Pe17, and Pe21 (Fig. 2 c). In addition, cell growth rate was 9-15% higher in the three transgenic lines of O. sativa (Fig. 2 d), 10-16% higher in the three lines of G. hirsutum t (Fig. 2 e), and 21-27% higher in the three lines of P. elliottii by 7 dai.
Acid phosphatase and alkaline phosphatase of ZAT6-transgenic cell lines
Compared to the control levels at 3, 5 and 7 dai, acid phosphatase activity and alkaline phosphatase activity were significantly higher in all transgenic cell lines of rice, cotton and slash pine (Fig. 3 a-c). Acid phosphatase activity also was significantly higher in all the transgenic cell lines tested(Fig. 3 d-f); by 3 dai.
Expression of CPK genes in ZAT6-transgenic rice lines
Compared to expression of the CPK genes in the control,the expression of OsCPK1 (Fig. 4 a), OsCPK2 (Fig. 4 b),OsCPK6 (Fig. 4 f), and OsCPK8 (Fig. 4 h) was significantly higher in transgenic rice cells at 3-7 dai, but that of OsCPK3(Fig. 4 c), OsCPK4 (Fig. 4 d), OsCPK5 (Fig. 4 e), OsCPK7(Fig. 4 g), and OsCPK9 (Fig. 4 i) did not differ significantly.
Expression of MAPK genes in ZAT6-transgenic rice lines
Compared to expression in the control at 3-7 dai, the expression of OsMAPK1 (Fig. 5 a), OsMAPK2 (Fig. 5 b), OsMAPK3(Fig. 5 c), and OsMAPK8 (Fig. 5 h) was significantly higher in transgenic rice cells, but that of OsMAPK4 (Fig. 5 d),OsMAPK5 (Fig. 5 e), OsMAPK6 (Fig. 5 f), OsMAPK7(Fig. 5 g), and OsMAPK9 (Fig. 5 i) did not differ significantly .
pH of media of transgenic cell lines
Compared to the pH for the respective controls, the extracellular pH for the three transgenic lines of O. sativa cell lines was 10-11% lower (Fig. 6 a), 10-14% lower for the G.hirsutum transgenic cell lines (Fig. 6 b) and 10-12% lower for the P. elliottii transgenic cell lines (Fig. 6 c). Different cell shapes were observed for all the inoculated transgenic cells of the three species (Fig. 6 e-k) compared with the noninoculated cells (control, Fig. 6 d) at 0-7 dai.
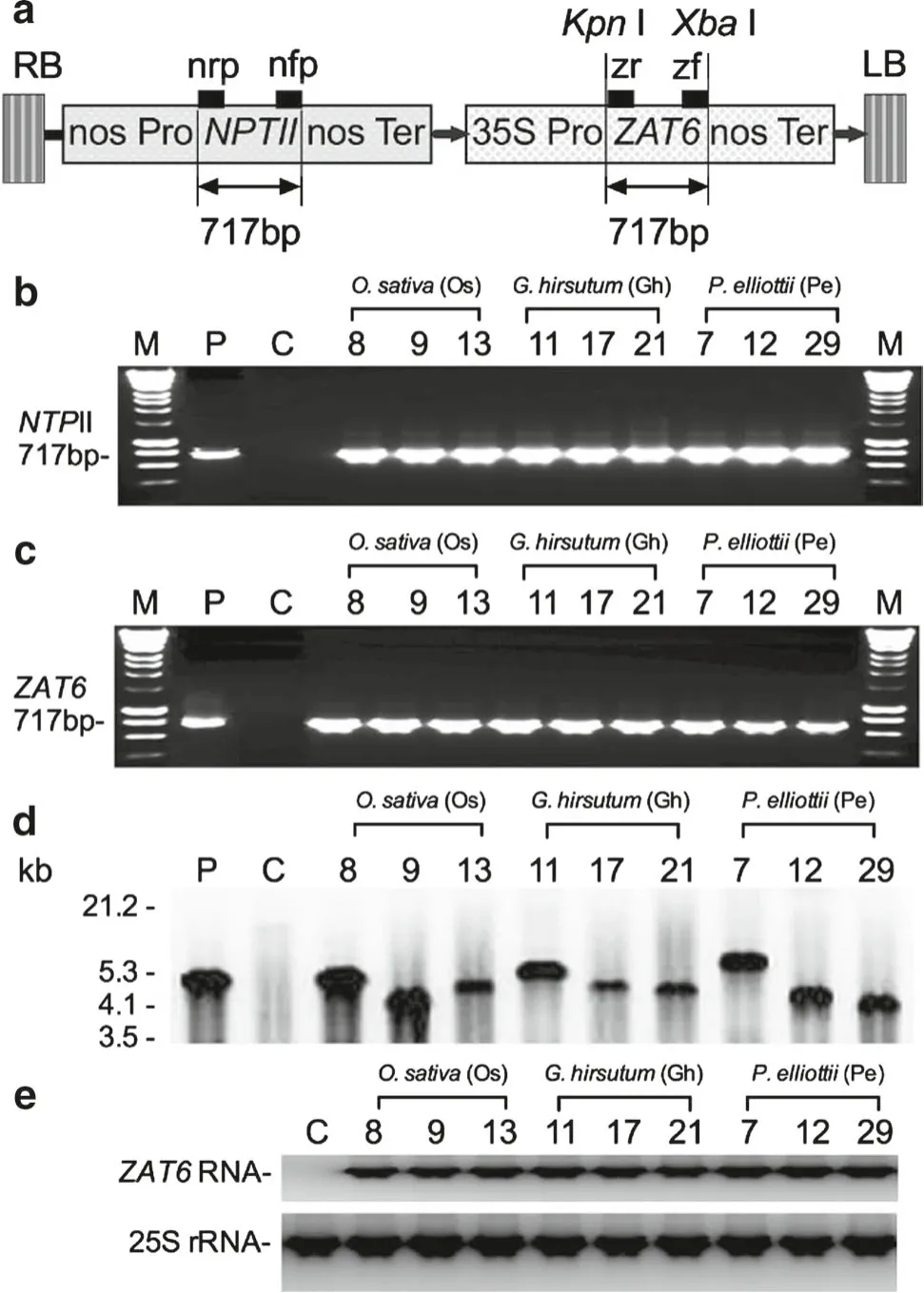
Fig. 1 Expression vector and molecular analysis of ZAT6-transgenic cells. a Linear plasmid map indicating NPTII (neomycin phosphotransferase II) gene, ZAT6 (C2H2-type zinc finger transcription factor of Arabidopsis thaliana 6), nos Pro (promoter of nopaline synthase gene), 35S Pro (), nos Ter (terminator from nopaline synthase gene), LB (left border), and RB (right border). Binding sites of PCR primers zr, zf, nrp, and nfp are shown as black squares. b PCR results for NPTII from O. sativa, G. hirsutum, and P. elliottii transgenic cell lines. c PCR results for ZAT6 from O. sativa, G. hirsutum, and P. elliottii transgenic cells. d Southern blot analysis of O. sativa, G.hirsutum, and P. elliottii transgenic cell lines using a DNA fragment of ZAT6 as probe. e Northern blot analysis of O. sativa, G. hirsutum,and P. elliottii transgenic cell lines for ZAT6. Lanes: M: HyperLadder I DNA molecular markers (Bioline), P: pBI-At ZAT6 plasmid control, C: wild-type control cells; 8, 9, 13: transgenic cell lines from O.sativa: 11, 17, 21: transgenic cell lines from G. hirsutum; 7, 12, 29:transgenic cell lines from P. elliottii
Discussion
Stress responses are accompanied by altered transcription of specific genes in plants. Ca 2+ -dependent protein kinases genes activate a stress-inducible promoter when induced by a stress signal. In Triticum aestivum, CDPK1 transduces this signal through its catalytic domain, the autoinhibitory function domain, and the C-terminal calmodulin-domain (Bulgakov et al. 2003; Preuss et al. 2006; Martinez-Noel et al. 2007;Mehlmer et al. 2010). In rice, CDPK7 is induced by cold and salt stresses. Overexpression of OsCDPK7 enhances induction of some stress-responsive genes in response to salinity,drought, but not to cold (Saijo et al. 2000). PCaPK-alpha and PCaPK-beta from a Paramecium genomic DNA library are activated by Ca 2+ and responsive to abiotic stress (Kim et al.1998). On the basis of mutational analyses, CDPK1 may be a positive regulator controlling stress signal transduction in plants (Sheen 1996; Kim et al. 1998; Liu et al. 1998; Wang and Poovaiah 1999). Twenty-nine CDPK genes have been identified in the rice genome. Five (OsCPK6, OsCDPK7,OsCPK13, OsCPK17 and OsCPK25) are induced by chilling, dehydration and salt stresses, indicating that they may be important components in the signal transduction pathways for stress responses (Wan et al. 2007). Transcription factors may suppress virulence factor for bacteria pathogenic to plants. In cells of tomato, peptides acted as specific inhibitors to suppress the plant’s response to crude bacterial extracts and living bacterial cells (Felix et al. 1999). In the present study, the function of ZAT6 was analyzed in O.sativa, G. hirsutum, and P. elliottii. Our experimental results demonstrated that ZAT6-transgenic cells had higher growth rate and cell viability, as well as higher acid phosphatase and alkaline phosphatase activity in O. sativa, G. hirsutum, and P. elliottii after inoculation with P. syringae, compared to those of the control. Increased acid phosphatase activity and alkaline phosphatase activity may contribute to P. syringae resistance in ZAT6-transgenic cells. Compared to those of the control, transcription levels of Ca 2+ -dependent protein kinase genes OsCPK1, OsCPK2, OsCPK6, and OsCPK8 in ZAT6-transgenic rice cells increased significantly after inoculation with P. syringae. These results showed that ZAT6 could increase resistance to a pathogen, P. syringae, through increasing expression of CPK genes in rice.
Mitogen-activated protein kinases (MAPKs) are response to drought, cold, and salinity stress. MAPKs respond to a variety of extracellular signals in plants and function in transducing signals in the presence of environmental stress(Mizoguchi et al. 1996). Mitogen-activated protein kinase Chk1 plays an important role in the development of fungal pathogens on their hosts (Lev and Horwitz 2003). Comparative genomics analysis showed MAPK genes are associated with biotic and abiotic stresses (Neupane et al. 2013a, b).In soybean, 38 MAPKs have been identified and some of them are involved in stress responses (Kim and Zhang 2004;Neupane et al. 2013a, b). In Capsicum annuum, 19 pepper MAPK genes and five MAPKK genes were identified, some of which function as pathogen resistance factors (Sun et al.2014; Liu et al. 2015; Zhou et al. 2016; Jin et al. 2017).Here, our experimental results showed that transcription levels of mitogen-activated protein kinase genes OsMAPK1,OsMAPK2, OsMAPK3, and OsMAPK8 were significantly higher in ZAT6-transgenic rice cells, compared to the non-ZAT6-transgenic control, that ZAT6-enhanced expression of MAPK genes may contribute to resistance to P. syringae.

Fig. 2 Cell viability and growth rate of ZAT6-transgenic cell lines.Cell viability of a O. sativa transgenic cell lines Os8, Os9, and Os13;b G. hirsutum transgenic cell lines Gh7, Gh12, and Gh29: c P. elliottii transgenic cell lines Pe11, Pe17, and Pe21. Growth rate of d O. sativa transgenic cell lines Os8, Os9, and Os13; e G. hirsutum transgenic cell lines Gh7, Gh12, and Gh29; P. elliottii transgenic cell lines Pe11,Pe17, and Pe21. Viability and growth rates were measured daily after inoculation with 10 μL Pseudomonas syringae (OD 600 = 0.5)per 1 million plant cells. The experiment was repeated three times,with 6-16 250-mL flasks per repeat for each cell line. Values are means ± SD. The asterisk indicates significant differences compared to the control in a t test at * P < 0.05
In the ZAT6-transgenic lines of O. sativa, G. hirsutum,and P. elliottii, the viability and growth rate of the transgenic cells inoculated with P. syringae were higher than those of the wild-type control. The increased transcript levels at 3 dai for OsCPK1, OsCPK2, OsCPK6, and OsCPK8 and OsMAPK1, OsMAPK2, OsMAPK3, and OsMAPK8 and decrease in extracellular pH of the medium with the transgenic cells at 96 min after inoculation compared with those of the wild-type controls suggest that ZAT6 enhances pathogen resistance in plant cells by modulating transcription of CPK and MAPK.
On the basis of the data we obtained, we propose a ZAT6-enhanced pathogen resistance mechanism. After the ZAT6-transgenic lines are inoculated with the bacterial pathogen,expression of Ca 2+ -dependent protein kinase genes, mitogen-activated protein kinase genes, and oxidase genes are enhanced. Increased expression of these genes activates transcription of pathogen-resistance genes that lead to resistance(Fig. 7). Understanding this mechanism of ZAT6-enhanced pathogen resistance in more detail should increase our understanding of the biology of biotic stress tolerance.

Fig. 3 Acid phosphatase and alkaline phosphatase activities for ZAT6-transgenic cell lines after inoculation with Pseudomonas syringae. Acid phosphatase activity of a three O. sativa transgenic cell lines Os8, Os9, and Os13; b G. hirsutum transgenic cell lines Gh7,Gh12, and Gh29; c P. elliottii transgenic cell lines Pe11, Pe17, and Pe21. Alkaline phosphatase activity of d O. sativa transgenic cell lines Os8, Os9, and Os13; e G. hirsutum transgenic cell lines Gh7,Gh12, and Gh29; f P. elliottii transgenic cell lines Pe11, Pe17, and Pe21. Cells were inoculated with 10 μL Pseudomonas syringae(OD 600 = 0.5) per 1 million plant cells. The experiment was repeated 3 times, with 6-16 250-mL flasks per repeat for each cell line. Values are means ± SD. The asterisk indicates significant differences compared to the control in a t test at * P < 0.05

Fig. 4 Expression of Ca2 + -dependent protein kinase genes in transgenic cell lines. Expression of CPK genes in three O. sativa transgenic cell lines Os8, Os9, and Os13, including OsCPK1 (a), OsCPK2(b), OsCPK3 (c), OsCPK4 (d), OsCPK5 (e), OsCPK6 (f), OsCPK7(g), OsCPK8 (h), and OsCPK9 (i). Gene expression was quantified at 0, 1, 3, 5, and 7 days after treatment by 10 μL Pseudomonas syringae (OD600 = 0.5) per 1 million cells. The experiment was repeated 3 times, with 6-16 250-mL flasks per repeat for each cell line. Values are means ± SD. The asterisk indicates significant differences compared to the control in a t-test at * P < 0.05

Fig. 5 Expression of mitogen-activated protein kinase genes in ZAT6-transgenic cell lines after inoculation with Pseudomonas syringae. Expression of OsMAPK1 (a), OsMAPK2 (b), OsMAPK3(c), OsMAPK4 (d), OsMAPK5 (e), OsMAPK6 (f), OsMAPK7 (g),OsMAPK8 (h), and OsMAPK9 (i) in O. sativa transgenic cell lines Os8, Os9, and Os13. Gene expression was quantified at 0, 1, 3,5, and 7 days after treatment with 10 μL Pseudomonas syringae(OD 600 = 0.5) per 1 million cells. The experiment was repeated 3 times, with 6-16 250-mL flasks per repeat for each cell line. Values are means ± SD. The asterisk indicates significant differences compared to the control in a t test at * P < 0.05

Fig. 7 Proposed mechanism of ZAT6-enhanced resistance to Pseudomonas syringae. After stimulation by the bacteria, transcription factor ZAT6 enhances expression of Ca 2+ -dependent protein kinase genes, mitogen-activated protein kinase genes, and oxidase genes.This increased gene expression activates transcription of pathogenresistance genes that confer resistance
AcknowledgementsWe acknowledge the University Council of Scientific Research and thank Dr. Page, Dr. Bradshaw, Dr. Lischewski,and Dr. Thompson for their critical reading and suggestions during the preparation of this manuscript.
杂志排行
Journal of Forestry Research的其它文章
- A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing
- Reconciliation of research on forest carbon sequestration and water conservation
- A theory to link relationships of stand volume, density, mean diameter and height in forestry data
- A new model for predicting the total tree height for stems cut-to-length by harvesters in Pinus radiata plantations
- Comparative performances of new and existing indices of crown asymmetry: an evaluation using tall trees of Eucalyptus pilularis(Smith)
- Tree mortality and biomass loss in drought-affected forests of East Texas, USA
