原位聚合三维陶瓷骨架增强全固态锂电池电解质
2021-01-06颜一垣鞠江伟于美燕陈守刚崔光磊
颜一垣, 鞠江伟, 于美燕, 陈守刚, 崔光磊
原位聚合三维陶瓷骨架增强全固态锂电池电解质
颜一垣1, 鞠江伟2, 于美燕1, 陈守刚1, 崔光磊2
(1. 中国海洋大学 材料科学与工程学院, 青岛 266100; 2. 中国科学院 青岛生物能源与过程研究所, 青岛 266101)
有机/无机复合电解质被认为是全固态锂电池中最具潜力的固态电解质之一, 但由于无机填料易团聚, 通过提高无机填料含量来改善复合电解质的电导率难有成效。此外, 在全固态锂电池中, 电解质和电极之间松散的固–固接触造成过大的界面阻抗, 限制了全固态锂电池的性能。本研究采用固相法合成具有Li+连续传输通道的自支撑三维多孔Li6.4Al0.1La3Zr1.7Ta0.3O12骨架, 并利用原位聚合的方法构筑一体化电解质/电极固–固界面。此策略指导合成的复合电解质的室温电导率可达1.9×10−4S·cm−1。同时, 一体化的界面使得Li-Li对称电池的界面阻抗从1540 Ω·cm2降低至449 Ω·cm2, 因此4.3 V(. Li+/Li)的LiCoO2|Li全固态锂电池展现出良好的电化学性能。
固态复合电解质; 原位聚合; 多孔骨架; 全固态电池
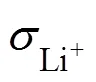
为弥补单一固态电解质的缺陷, 结合二者的优势, 将SIE与SPE进行复合是一种行之有效的方法。SPE中引入零维SIE颗粒或者一维SIE纤维能够降低SPE基体的结晶度或玻璃化转变温度[13-14], 一般可将SPE的电导率提升一个数量级[15]。但是零维或一维SIE填料的含量过高会产生团聚, 导致电导率降低[16-17]。并且, 高电导率的SIE填料或是被SPE相孤立或是被有机/无机界面相孤立, 阻碍了Li+在高电导率SIE相中的快速传导。不同于将SIE填料分散于SPE中, 相反, 将SPE浇注于多孔SIE骨架中, 即三维SIE填料中, 可得到具有连续SIE相的有机/无机复合电解质[17-18]。这种结构不仅能有效避免SIE填料的团聚, 还能为Li+的快速传输提供连续通道, 大幅提升电导率[19]。
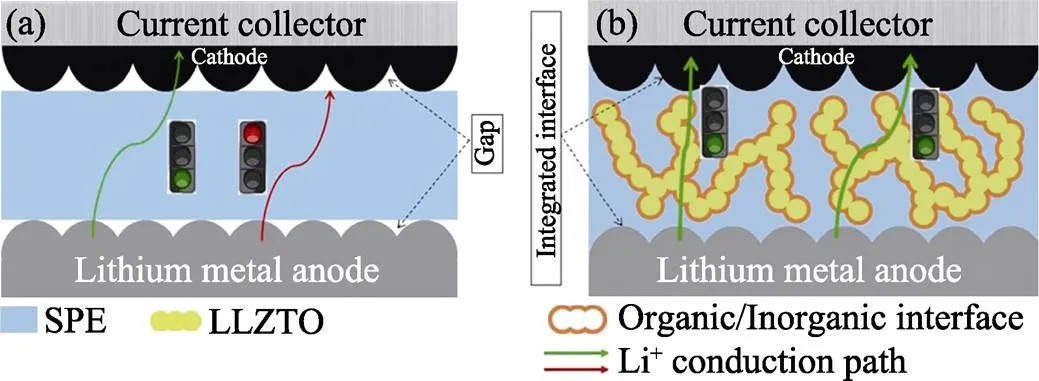
图1 (a)非原位聚合策略和(b)原位聚合策略制备的ASLB内部结构示意图
1 实验方法
固相反应法合成LLZTO粉末: 将Al2O3、ZrO2、LiOH、Ta2O5及La2O3按照Li6.4Al0.1La3Zr1.7Ta0.3O12的化学计量比称量后倒入球磨罐中。以异丙醇为球磨介质, 并于350 r/min的速率球磨10 h。之后, 将球磨所得浆料置于60 ℃烘箱中干燥, 并于1000 ℃的空气氛围中烧结5 h, 再将烧结所得块体进行研磨即可得到LLZTO粉末。
将上述所得LLZTO粉末与造孔剂石墨粉以质量比3.5 : 1.5均匀混合后, 在12 MPa的压力下压制成片。再将其置于氧化镁瓷舟中, 并用LLZTO粉末包覆, 在1150 ℃的空气氛围中烧结5 h, 得到p-LLZTO。直接将上述所得LLZTO粉末于12 MPa压力下压成片, 置于氧化镁瓷舟中, 并用LLZTO粉末包裹, 于1150 ℃的空气氛围中烧结5 h, 可得致密LLZTO样品。
为证明原位聚合对ASLB的积极作用, 本工作对原位聚合及非原位聚合的LiCoO2|Li ASLB性能进行对比。原位聚合LiCoO2|Li ASLB的制备: 将p-LLZTO或纤维素隔膜置于锂片上, 滴加100 μL的PEGMEA前驱体溶液, 放置正极片, 在手套箱中完成电池的组装后, 移入60 ℃烘箱中, 加热完成原位聚合。非原位聚合LiCoO2|Li ASLB的制备: 先将p-LLZTO置于聚四氟乙烯板上, 然后滴加100 μL PEGMEA前驱体溶液, 在60 ℃加热使PEGMEA完成聚合。将3D composite从聚四氟乙烯板上取下并置于锂片和正极片之间, 完成非原位电池组装。
2 结果与讨论
2.1 p-LLZTO的表征
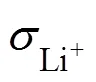
2.2 3D composite的表征
首先使用傅里叶红外光谱(Fourier transform infrared spectrometer, FT-IR)分析PEGMEA的聚合程度。在60 ℃加热24 h后, 如图3(a)所示, 位于1620 cm−1附近的C=C峰完全消失, 而其它官能团, 如C=O或者C−O−C的峰则仍保留, 证明在该条件下PEGMEA能够完全聚合, 转化为P(PEGMEA)。而3D composite中的P(PEGMEA)与纯P(PEGMEA)的红外光谱几乎相同, 说明LLZTO不影响PEGMEA的聚合且不与PEGMEA反应。为进一步分析P(PEGMEA)的分子结构, 又对PEGMEA及P(PEGMEA)进行核磁共振测试(Nuclear magnetic resonance, NMR)测试。在PEGMEA的氢谱(图3(b))中, 与C=C相连氢原子的峰分别位于6.4、6.2和6.0。在60 ℃加热24 h后, 这些峰完全消失, 同时在2.5~1.5的位置上出现了若干峰。这归结于C=C双键被打开, 同样证明PEGMEA完成了聚合。
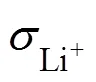
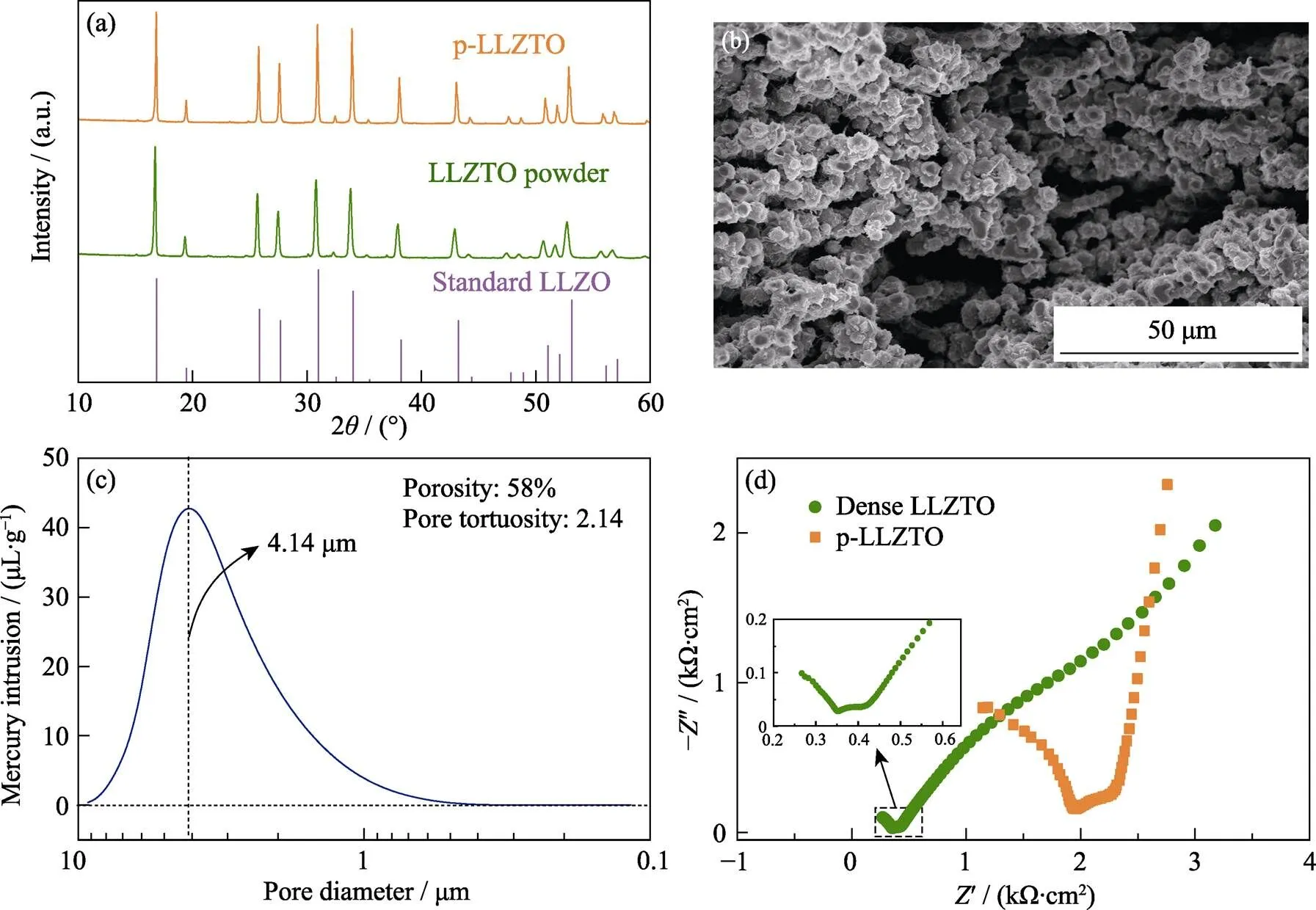
图2 (a)标准LLZO及本实验制备的LLZTO粉末和p-LLZTO的XRD图谱; (b) p-LLZTO的截面SEM照片; (c)p-LLZTO的孔径分布曲线; (d)致密LLZTO和p-LLZTO的室温阻抗图谱(插图: 局部放大的致密LLZTO阻抗谱)
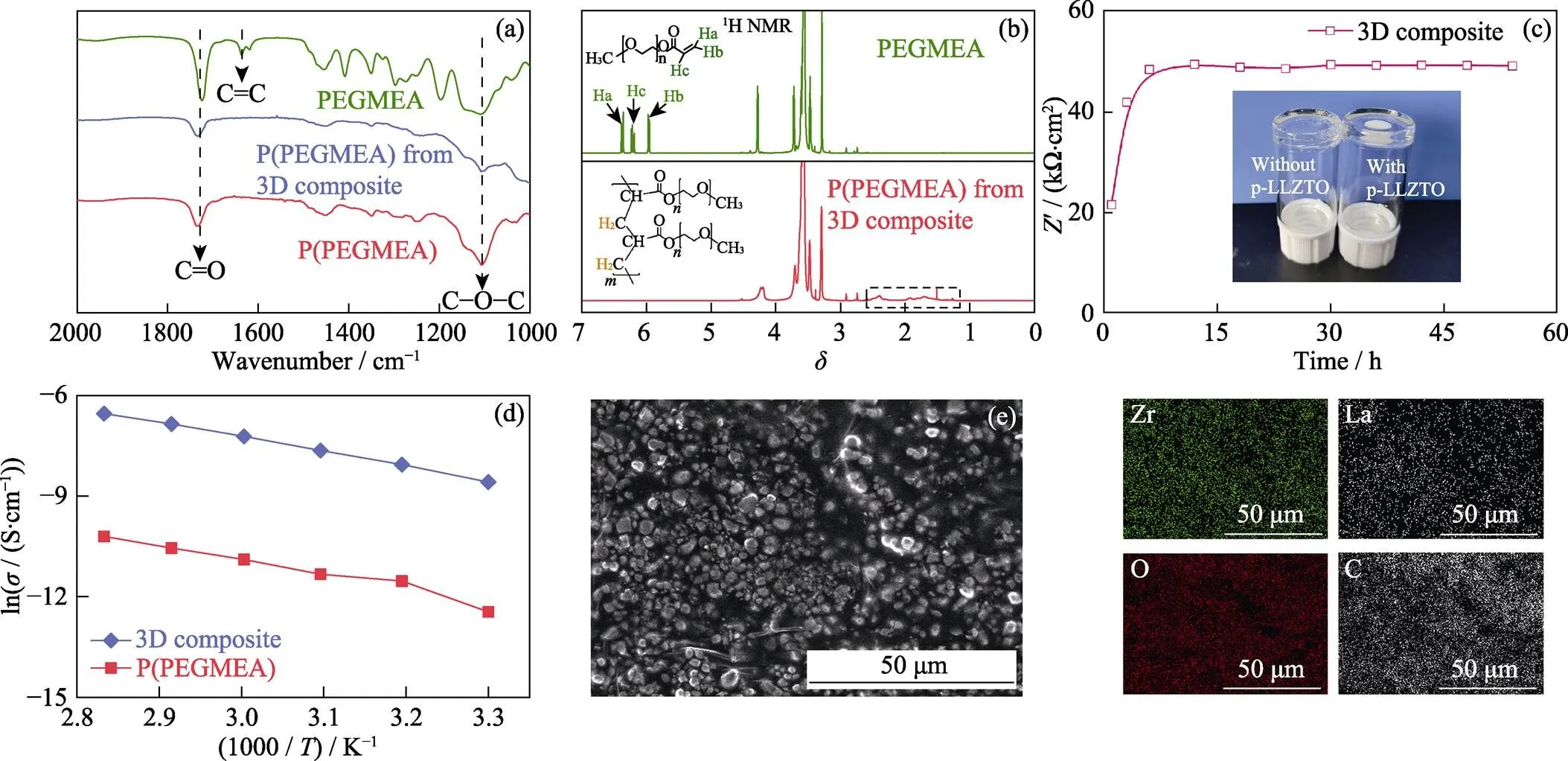
图3 (a)PEGMEA、P(PEGMEA)和3D composite中P(PEGMEA)的红外图谱; (b)PEGMEA和3D composite中P(PEGMEA)的核磁共振氢谱及相关结构式(溶剂为氘代N,N-二甲基甲酰胺); (c)60 ℃条件下steel|3D composite|steel电池欧姆阻抗与加热时间关系曲线, 插图为有/无p-LLZTOP的PEGMEA在小瓶中60 ℃加热24 h后的照片; (d)P(PEGMEA)和3D composite的电导率与温度的关系曲线; (e)3D composite的截面SEM照片及元素分布图
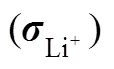
表1 不同固态电解质的室温电导率
a: ethylene oxide(–CH2–CH2–O–); b: lithium bis(trifluoromethanesulfonyl)imide); c: Li1.4Al0.4Ti1.6(PO4)3; d: Li0.35La0.55TiO3
2.3 3D composite与Li的相容性
对3D composite与Li的相容性进行测试以确定其能否应用于ASLB[12]。本实验对Li|3D composite|Li进行EIS和恒流极化测试, 并以Li|P(PEGMEA)|Li和Li|LLZTO|Li作为对照。图4(a~f)为基于不同电解质的电池在热处理前后的EIS图谱。加热前Li|PEGMEA|Li的EIS图谱由一个半圆和一条斜线组成, 半圆与实轴的交点为欧姆阻抗, 而半圆的跨度为界面阻抗[29-30]。Li|3D composite|Li的EIS图谱中存在两个半圆, 这可能是固态p-LLZTO所致。在60 ℃加热24 h后, Li|P(PEGMEA)|Li电池的EIS图谱同样出现两个半圆, 高频区半圆的跨度为欧姆阻抗, 而中频区半圆的跨度为界面阻抗[31]。
根据阻抗图谱的拟合结果, 上述电池热处理前后欧姆阻抗及界面阻抗的变化总结于图4(g, h)中。加热前, Li|3D composite|Li的欧姆阻抗为2736 Ω·cm, 仅为Li|PEGMEA|Li欧姆阻抗8550 Ω·cm的三分之一。加热后, Li|3D composite|Li的欧姆阻抗增至5996 Ω·cm,而Li|P(PEGMEA)|Li的欧姆阻抗陡增至216743 Ω·cm。同时, 加热后Li|3D composite|Li的界面阻抗从 115 Ω·cm2增加至449 Ω·cm2, 不到Li|P(PEGMEA)|Li界面阻抗956 Ω·cm2的二分之一。3D composite与P(PEGMEA)欧姆阻抗和界面阻抗的明显差异, 说明p-LLZTO在提升电导率上的巨大作用。图4(b, e)为Li|LLZTO|Li热处理前后的EIS图谱, 其欧姆阻抗和界面阻抗几乎没有变化, 分别约为8360 Ω·cm和1540 Ω·cm2, 表明LLZTO对锂金属稳定, 无化学反应发生, 与文献[3]报道相符。而3D composite的界面阻抗仅为LLZTO的三分之一说明原位聚合策略能够有效降低界面阻抗。
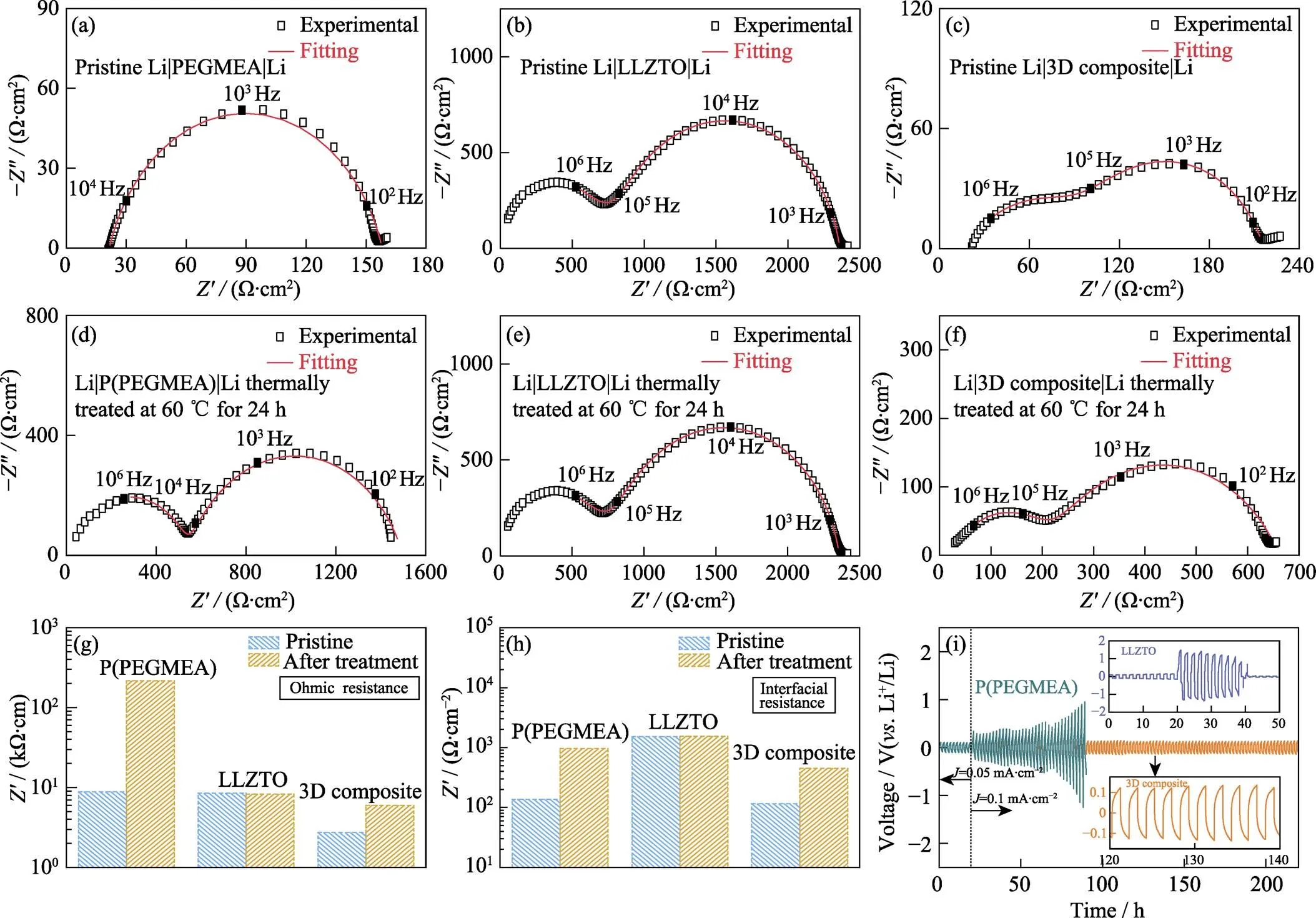
图4 热处理(a~c)前(d~f)后基于(a, d)PEGMEA、(b, e)LLZTO和(c, f)3D composite的Li-Li对称电池的EIS图谱; 基于不同电解质的Li-Li电池处理前后(g)欧姆阻抗和(h)界面阻抗对比; (i)P(PEGMEA)和3D composite的Li-Li电池室温下的直流恒流循环曲线(上插图为LLZTO的Li-Li电池室温下的直流恒流循环曲线, 下插图为3D composite的Li-Li电池的局部放大极化曲线, 电流密度为0.1 mA·cm−2)
P(PEGMEA)、LLZTO和3D composite与锂金属的相容性通过室温下的直流极化进行测试。图4(i)中, 电流密度为0.1 mA·cm−2时, Li|3D composite|Li的极化电压仅为0.12 V且能够稳定循环超过200 h, 说明3D composite与锂金属具有良好的相容性。作为对比的P(PEGMEA)在0.1 mA·cm−2的电流密度下, 不到90 h其极化电压从0.33 V增至0.95 V。这应当归因于P(PEGMEA)低的室温电导率(3.6×10−6S·cm−1)。而致密LLZTO在电流密度为0.1 mA·cm−2时, 其电池循环不到20 h就出现短路, 与文献[32]报道相符。这是由于LLZTO与金属锂接触不紧密, 局部产生较大的电流密度, 造成锂的不均匀沉积并使锂枝晶沿LLZTO中的晶界生长, 最终导致电池短路。相较之下, 利用原位聚合得到的3D composite与金属锂之间接触紧密, 电场分布均匀, 因此3D composite能够有效抑制锂枝晶的生长[33-34]。上述测试结果说明3D composite与锂金属有很好的相容性, 能够应用于ASLB。
2.4 3D composite在ASLB中的应用
以LiCoO2|Li ASLB对3D composite的性能进行测试。为探究原位聚合策略对ASLB的积极作用, 即图1中利用原位聚合形成一体化界面, 本工作对原位聚合及非原位聚合的LiCoO2|Li ASLBs性能进行对比。LiCoO2|Li ASLB工作电压范围为3.0~4.3 V (Li+/Li), 工作温度60 ℃。原位聚合LiCoO2|3D composite|Li ASLB在0.1(1=140 mAh·g−1)电流密度下, 首圈放电比容量为144 mAh·g−1, 首圈库仑效率为94%(图5(a, b))。电流密度为0.1、0.3、0.5时, 其放电比容量分别为144、138和129 mAh·g−1。在0.1循环90圈后, 其容量保持率为88%。对于原位聚合LiCoO2|P(PEGMEA)|Li ASLB, 即使在60 ℃下, 首圈放电比容量也仅为123 mAh·g−1, 且在40圈以内迅速衰减至10 mAh·g−1。而非原位聚合的LiCoO2|3D composite|Li ASLB的性能则更差, 首圈放电比容量仅为62 mAh·g−1, 在15圈左右便失效。

图5 (a)原位聚合LiCoO2|3D composite|Li、原位聚合LiCoO2|P(PEGMEA)|Li和非原位聚合LiCoO2|3D composite|Li ASLBs的循环性能; (b)原位聚合LiCoO2|3D composite|Li、原位聚合LiCoO2|P(PEGMEA)|Li和非原位聚合LiCoO2|3D composite|Li ASLBs的充放电曲线; (c)原位聚合和(d)非原位聚合LiCoO2|3D composite|Li ASLBs拆解后的LiCoO2/3D composite界面的截面SEM照片
分析上述ASLBs的失效机理, 首先, 低的离子电导率是造成原位聚合LiCoO2|P(PEGMEA)|Li ASLB性能不佳的主要原因, 即使在60 ℃下, P(PEGMEA)的电导率也仅为1.85×10−5S·cm−1, 这严重限制了Li+的快速输运。对于非原位聚合LiCoO2|3D composite |Li ASLB, 虽然3D composite具有较高的电导率, 但电极与电解质之间存在约30 μm的间隙, 不连续的界面接触严重阻碍了Li+在电解质–电极之间的传输, 造成电池性能的快速衰减。如图5(c, d)所示, 通过原位聚合制备的LiCoO2|3D composite|Li具有一体化的电解质/电极界面, 确保Li+在界面处顺利地传输。上述三种ASLBs的性能对比证明了高的电导率和一体化的电解质–电极界面是获得高性能ASLB的必要条件, 而本工作中通过原位聚合的策略成功实现了高电导率3D composite与电极之间的一体化界面的构建。
3 结论
综上, 本工作以石墨粉为造孔剂通过高温烧结成功制备自支撑三维多孔Li6.4Al0.1La3Zr1.7Ta0.3O12骨架。将聚乙二醇甲基醚丙烯酸酯浇注于多孔Li6.4Al0.1La3Zr1.7Ta0.3O12中, 聚合后得到三维有机无机复合电解质。连续的Li6.4Al0.1La3Zr1.7Ta0.3O12相能够为Li+的快速传输提供通道, 并将聚合后的聚乙二醇甲基醚丙烯酸酯的室温电导率提升53倍, 达到1.9×10−4S·cm−1。更重要的是, 原位聚合的聚乙二醇甲基醚丙烯酸酯能够在接触不良的三维有机无机复合电解质和电极之间形成一体化界面, 有效地将电池界面阻抗从1542 Ω·cm2降低至449 Ω·cm2。最后, 原位聚合三维有机无机复合电解质被成功应用于LiCoO2|Li全固态锂电池。本工作为制备与高电压正极、锂负极有良好化学机械相容性的高电导率有机/无机复合电解质提供了有价值的参考。
补充材料
本文相关补充材料可登陆https://doi.org/ 10.15541/ jim20200152查看。
[1] GAO Z, SUN H, FU L,. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries.,2018, 30(17): e1705702.
[2] BACHMAN J C, MUY S, GRIMAUD A,. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction.,2016, 116(1): 140–162.
[3] ZHENG F, KOTOBUKI M, SONG S,. Review on solid electrolytes for all-solid-state lithium-ion batteries.,2018, 389: 198–213.
[4] ZHANG B, TAN R, YANG L,. Mechanisms and properties of ion-transport in inorganic solid electrolytes.,2018, 10: 139–159.
[5] CHEN R, QU W, GUO X,. The pursuit of solid-state electrolytes for lithium batteries: from comprehensive insight to emerging horizons.,2016, 3(6): 487–516.
[6] FAN L, WEI S, LI S,. Recent progress of the solid-state electrolytes for high-energy metal-based batteries.,2018, 8 (11): 1702657.
[7] YUE L, MA J, ZHANG J,. All solid-state polymer electrolytes for high-performance lithium ion batteries.,2016, 5: 139–164.
[8] MANTHIRAM A, YU X, WANG S. Lithium battery chemistries enabled by solid-state electrolytes.,2017, 2(4): 16103
[9] GAO Y, WANG D, LI Y C,. Salt-based organic-inorganic nanocomposites: towards a stable lithium metal/Li10GeP2S12solid electrolyte interface.,2018, 57(41): 13608–13612.
[10] BUANNIC L, ORAYECH B. Dual substitution strategy to enhance Li+ionic conductivity in Li7La3Zr2O12solid electrolyte., 2017, 29(4): 1769–1778.
[11] ZHANG Z, SHAO Y, LOTSCH B,. New horizons for inorganic solid state ion conductors.,2018, 11(8): 1945–1976.
[12] CHENG X B, ZHAO C Z, YAO Y X,. Recent advances in energy chemistry between solid-state electrolyte and safe lithium- metal anodes.,2019, 5(1): 74–96.
[13] ZHA W, CHEN F, YANG D,. High-performance Li6.4La3Zr1.4Ta0.6O12/poly(ethylene oxide)/succinonitrile composite electrolyte for solid-state lithium batteries.,2018, 397: 87–94.
[14] ZHU P, YAN C, DIRICAN M,. Li0.33La0.557TiO3ceramic nanofiber- enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries.,2018, 6(10): 4279–4285.
[15] WAN Z, LEI D, YANG W,. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder.,2019, 29(1): 1805301.
[16] CHEN L, LI Y, LI S P,. PEO/garnet composite electrolytes for solid-state lithium batteries: from “ceramic-in-polymer” to “polymer- in-ceramic”.,2018, 46: 176–184.
[17] XIE H, YANG C, FU K K,. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose.,2018, 8(18): 1703474.
[18] BAE J, LI Y, ZHANG J,. A 3D nanostructured hydrogel- framework-derived high-performance composite polymer lithium-ion electrolyte.,2018, 57(8): 2096–2100.
[19] BAE J, LI Y, ZHAO F,. Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries.,2018, 15: 46–52.
[20] LIU Y, SUN Q, ZHAO Y,. Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition., 2018, 10(37): 31240–31248.
[21] JU J, WANG Y, CHEN B,. Integrated interface strategy toward room temperature solid-state lithium batteries., 2018, 10(16): 13588–13597.
[22] ZHAO Q, LIU X, STALIN S,. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries., 2019, 4(5): 365–373.
[23] DUAN H, YIN Y X, SHI Y,. Dendrite-free Li-metal battery enabled by a thin asymmetric solid electrolyte with engineered layers., 2018, 140(1): 82–85.
[24] PARANJAPE N, MANDADAPU P C, WU G,. Highly- branched cross-linked poly(ethylene oxide) with enhanced ionic conductivity., 2017, 111: 1–8.
[25] BAN X, ZHANG W, CHEN N,. A high-performance and durable poly(ethylene oxide)-based composite solid electrolyte for all solid-state lithium battery., 2018, 122(18): 9852–9858.
[26] GONG Y, FU K, XU S,. Lithium-ion conductive ceramic textile: a new architecture for flexible solid-state lithium metal batteries., 2018, 21(6): 594–601.
[27] LI D, CHEN L, WANG T,. 3D fiber-network-reinforced bicontinuous composite solid electrolyte for dendrite-free lithium metal batteries., 2018, 10(8): 7069–7078.
[28] LI Z, HUANG H M, ZHU J K,. Ionic conduction in composite polymer electrolytes: case of PEO:Ga-LLZO composites., 2019, 11(1): 784–791.
[29] WANG Q, WEN Z, JIN J,. A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries., 2016, 52(8): 1637–1640.
[30] HAN X, GONG Y, FU K K,. Negating interfacial impedance in garnet-based solid-state Li metal batteries., 2017, 16(5): 572–579.
[31] JU J, CHEN F, XIA C. Ionic conductivity of impregnated samaria doped ceria for solid oxide fuel cells., 2014, 136: 422–429.
[32] WU B, WANG S, LOCHALA J,. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries., 2018, 11(7): 1803–1810.
[33] HU J L, TIAN J, Li C L. Nanostructured carbon nitride polymer- reinforced electrolyte to enable dendrite-suppressed lithium metal batteries., 2017, 9: 11615–11625.
[34] HU J L, YAO Z G, CHEN K Y,High-conductivity open framework fluorinated electrolyte bonded by solidified ionic liquid wires for solid-state Li metal batteries., 2020, 28: 37–46.
Polymerization Integrating 3D Ceramic Framework in All Solid-state Lithium Battery
YAN Yiyuan1, JU Jiangwei2, YU Meiyan1, CHEN Shougang1, CUI Guanglei2
(1. School of Materials Science and Engineering, Ocean University of China, Qingdao 266100, China; 2. Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China)
Organic/inorganic composites have been considered as promising electrolyte candidates in all solid-state lithium batteries. Aiming at improving the conductivity significantly by increasing the frequently-used 0D or 1D ceramic nano-fillers to high content is unsuccessful due to the particle tendency to agglomeration. What's worse, the loose contact between the solid electrolyte and solid electrodes is much of a serious barrier to the performance and thus to the application of all solid-state lithium batteries. Herein, self-supported 3D porous Li6.4Al0.1La3Zr1.7Ta0.3O12frameworks are employed to provide percolated fast Li+conductive pathway whilepolymerization of poly(ethylene glycol) methyl ether acrylate can integrate the loose solid-solid interface and reduce the interfacial resistance efficiently. Inspiringly, the Li+conductivity of the composite exhibits 1.9×10−4S·cm−1at room temperature. The interfacial resistance in Li-Li batteries decreases significantly from 1540 to 449 Ω·cm2, rendering good capacity and cyclability of the 4.3 V (. Li+/Li) LiCoO2|Li all solid-state lithium battery.
solid composite electrolyte;polymerization; porous framework; all solid-state battery
TQ174
A
1000-324X(2020)12-1357-08
10.15541/jim20200152
2020-03-23;
2020-05-11
国家自然科学基金(51902325) National Natural Science Foundation of China(51902325)
颜一垣(1994–), 男, 硕士研究生. E-mail: yanyiyuan94@163.com
YAN Yiyuan(1994–), male, Master candidate. E-mail: yanyiyuan94@163.com
陈守刚, 教授. E-mail: sgchen@ouc.edu.cn; 崔光磊, 研究员. E-mail: cuigl@qibebt.ac.cn
CHEN Shougang, professor. E-mail: sgchen@ouc.edu.cn; CUI Guanglei, professor. E-mail: cuigl@qibebt.ac.cn
