Decrement of Sugar Consumption in Rice Young Panicle Under High Temperature Aggravates Spikelet Number Reduction
2020-12-28WangYaliangZhangYikaiShiQinghuaChenHuizheXiangJingHuGuohuiChenYanhuaWangXiaodanWangJunkeYiZihaoZhuDefengZhangYuping
Wang Yaliang, Zhang Yikai, Shi Qinghua, Chen Huizhe, Xiang Jing, Hu Guohui, Chen Yanhua, Wang Xiaodan, Wang Junke, Yi Zihao, Zhu Defeng, , Zhang Yuping
Research Paper
Decrement of Sugar Consumption in Rice Young Panicle Under High Temperature Aggravates Spikelet Number Reduction
Wang Yaliang1, 2, #, Zhang Yikai1, #, Shi Qinghua2, Chen Huizhe1, Xiang Jing1, Hu Guohui1, Chen Yanhua1, Wang Xiaodan1, Wang Junke1, Yi Zihao1, Zhu Defeng1, 2, Zhang Yuping1
(China National Rice Research Institute / State Key Laboratory of Rice Biology, Hangzhou 310006, China; College of Agronomy, Jiangxi Agricultural University / Key Laboratory of Crop Physiology, Ecology and Genetics Breeding, Ministry of Education, Nanchang 330045, China; These authors contributed equally to this work)
Two rice genotypes Huanghuazhan (HHZ, heat-resistant) and IR36 (heat-susceptible) were subjected to high-temperature (HT, 40 ºC) and normal-temperature (NT, 32 ºC) treatments at the spikelet differentiation stage. HT treatment inhibited spikelet differentiation, aggravated spikelet degeneration, reduced spikelet size, and disordered carbohydrate allocation. Meanwhile, HT treatment increased nonstructural carbohydrate content in leaves, but decreased that in stems and young panicles, and the same tendencies of sucrose and starch contents were observed in leaves and stem. However, HT treatment significantly increased the sucrose content and sharply decreased the glucose and fructose contents in young panicles. Lower activity levels of soluble acid invertase (EC3.2.1.26) and sucrose synthase (EC2.4.1.13) were observed under HT treatment. Moreover, HT treatment reduced the activities of key enzymes associated with glycolysis and the tricarboxylic acid cycle, which indicated sucrose consumption was inhibited in young panicles under HT treatment. Exogenous glucose and fructose applied under HT treatment increased the spikelet number more than exogenous sucrose. In conclusion, the results demonstrated that the reduction of spikelet number under high temperature was more affected by the decrease in sugar consumption than the blocking of sucrose transport. The impairment of sucrose hydrolysis was the main reason for the inhibition of sugar utilization.
rice; high temperature; panicle development; spikelet number; carbohydrate allocation; sugar consumption
Spikelet number of rice is important for the yield. The optimum temperature for spikelet development ofrice is 33.1 ºC, while that ofrice is 26.7 ºC (Sanchez et al, 2014). Previous studies have shown that temperature stress do harm to spikelet development (Wang et al, 2015; Zeng et al, 2015; Wu et al, 2016). Because of intensifying climate change, high temperature weather seems to be occurring much more frequently in the rice growth season. In recent years, extreme high temperature events (> 40 ºC) have coincided with the rice panicle initiation stage and reduced the number of spikelets per panicle, resulting in an increase of rice yield losses.
Spikelet development relies on the endogenous supply assimilated carbon from leaf photosynthesis, which supplies energy for plant development and cell differentiation (van den Ende, 2014). Previous studies showed that spikelet number is correlated to the dry weight of rice plants (Liu et al, 2005). Spikelet number per panicle is determined by spikelet differentiation and degeneration during the panicle initiation stage. In field conditions, more dry matter accumulation during panicle development stage contributes to spikelet differentiation, while no significant correlation is observed between spikelet degeneration and dry weight (Wang et al, 2017). Leaf net photosynthesis has been reported to be slightly increased under high temperature due to strong transpiration (Wang et al, 2016), however, the reason for the dry weight reduction in young panicles induced by high temperatures and its relationship with the decrease in spikelet number has not been determined.
The reduction in carbohydrate accumulation in growing tissue under high temperatures has been reported to be associated with sucrose transport, which has been reported to be heat susceptible (Kaushal et al, 2013). Sucrose is the main carrier for carbohydrate utilization. During the rice filling stage, a higher expression level of, which encodes a sucrose transporter protein, contributes to increased heat tolerance under high temperatures (Miyazaki et al, 2013; Phan et al, 2013), and therefore, the carbohydrate distribution seems to be more important than carbohydrate assimilation for spikelet development.
It has been suggested that the sucrose content in sink organs should present a decreasing tendency due to the blocking of sucrose transport under high temperatures (Zhang et al, 2018; Cheng et al, 2019). However, some studies have reported that the sucrose content is increased due to sucrose hydrolysis inhibition at the panicle initiation stage, which inhibits starch accumulation (Li et al, 2006; Chaturvedi et al, 2017). Sucrose metabolism is an important index of sink activity in growing tissues, and stronger sink activity presents as higher heat tolerance (Scharte et al, 2009; Ishibashiet al, 2014). Young developing panicles are the main sink for carbohydrate accumulation during rice panicle initiation, and sucrose metabolism plays an important role in cellulose synthesis and energy supply for cell development rather than starch accumulation. Thus, the effects of high temperature on sucrose utilization may differ between the rice panicle initiation and grain filling stages. Additionally, whether sucrose transport or consumption has a greater effect on the spikelet number remains unclear.
After sucrose is transported into sink organs, it is hydrolyzed into fructose and glucose, and consumed by glycolysis and the tricarboxylic (TCA) cycle to supply energy for plant growth (Bao et al, 2015). Glaubitz et al (2015) reported that high night temperatures strongly affect the TCA cycle. Yu et al (2017) reported that elevated CO2could upregulate glycolysis and the TCA cycle to improve heat tolerance in Bermudagrass. However, there have few studies on glycolysis and TCA cycle under extreme high temperatures in young panicles, while glycolysis and the TCA cycle provide the intermediate products for spikelet development. Heng et al (2018) reported that a lack ofmalate in young panicles promotes optimal spikelet degeneration.
In this study, the carbohydrate and sucrose contents, the activity levels of sucrose metabolism enzymes, the expression levels of sucrose transporter genes (SUTs), invertase genes (INVs) and sucrose synthase genes (SUSs), and the activities and expression of key enzymes in glycolysis and the TCA cycle were determined at high temperatures compared with normal temperatures to explore the carbohydrate distribution and sugar utilization changes in young panicles. Furthermore, exogenous sucrose, glucose, and fructose were applied to verify the results.
Materials and methods
Plant materials and growth conditions
The heat-resistant inbredrice cultivar Huanghuazhan (HHZ) (Cao et al, 2008) and the heat-susceptible inbredrice cultivar IR36 (Fang et al, 2006) were used.
Pot experiments were conducted at the China National Rice Research Institute (119º55′48′′ E, 30º2′24′′ N), Hangzhou, China. Pre-germinated seeds were sown in seed trays and grown for 20 d, and then the seedlings were transplanted into plastic pots (four seedlings per pot). Each pot (24.0 cm length × 22.5 cm width × 21.5 cm height) contained 10 kg air-dried paddy soil. The pots were kept under natural environmental conditions. Before transplanting, about 3.5 g compound fertilizer (the ratio of N, P and K as 15%:15%:15%) was applied to each pot. At the tillering stage, 0.6 g urea was applied to each pot, and 0.3 g urea and 0.5 g potassium chloride were applied to each pot at the panicle initiation stage before the high temperature treatment. During rice growth, a 1–2 cm layer of water was maintained. Pests, diseases and weeds were intensively controlled in accordance with local recommendations for high yield production.
Temperature treatments
The high temperature (HT, 40 ºC) and normal temperature (NT, 32 ºC) treatments were carried out in automatic temperature- and humidity-controlled climate chambers. The climate chambers were made of transparent glass, which enabled the internal illumination to be kept consistent with natural conditions, the light intensity was about 50 000 lux. The HT and NT treatments were set according to a model of air temperature in recent years. Different temperatures were set in the chambers at different times of the day (0:00–24:00 h). A difference of 8 ºC was kept between HT and NT for 8 h from 9:30 to 17:30 (Supplemental Table 1). The humidity in the chamber was maintained at 75%–80%.
Rice plants were grown under natural conditions before and after the HT and NT treatments. The plants were treated with HT and NT on the approximate date of spikelet differentiation, when the panicle length was 0.2–0.5 cm. Each treatment had three replicates (15 pots per chamber/replicate), and the plants were treated with HT and NT for 15 d and then returned to ambient conditions.
Exogenous application of sucrose, glucose and fructose
According to the results of preliminary experiments, totally 200 mL of 2.5% exogenous sucrose, 2.5%-(+)-glucose, and 2.5%-fructose (Sigma Co., St. Louis, MO, USA) were sprayed on the whole plant per pot at 1 d before HT treatment and 7 d under the HT treatment to verify the effects of high temperature on sucrose metabolism. Upon rice panicle heading, the panicle of the primary tiller was sampled to determine the spikelet number per panicle.
Sampling and data collection
The net photosynthetic rate of top expanded leaves was determined using a Li-6400 portable photosynthesis system (Li-Cor Inc., Lincoln, NE, USA). The measurement conditions were as follows: leaf area, 6 cm2; ambient CO2, 450 µmol/mol; photosynthetic photon flux density, 1000 µmol/(m2·s); and flow speed, 500 µmol/s.The temperature of leaf chamber was maintained according to the treatment.
Panicle lengths and spikelet differentiation or degeneration of the primary tiller panicles were determined at the heading stage. Ten primary tillers were sampled from five plants per replicate. The leaves, sheaths and stems, and panicles were separated, oven-dried at 80 ºC for 72 h, and then weighed. To quantify spikelet differentiation and degeneration, the number of degenerated spikelets was calculated by counting the vestiges present on the panicles. The number of differentiated spikelets was calculated as the sum of the surviving and degenerated spikelets. Proportion of degenerated spikelets (%) = (Number of degenerated spikelets / Number of total differentiated spikelets) × 100.
Spikelet morphology was observed under a stereo- microscope (Olympus SZX7, Olympus Corporation, Tokyo, Japan), and length (mm) and width (mm) were measured using a digital camera (DP70) at 0.63× and 2.50×. Altogether, 30 spikelets were collected from the top, middle and lower positions of panicles.
Upon rice maturity, the spikelet numbers, seed-setting rates, and grain weights of the primary tillers were determined. Ten panicles of primary tillers were collected per replicate.
The top expanded leaves, stems and young panicles of the primary tillers were collected at 7 and 15 d of the treatments. The samples were quickly frozen in liquid nitrogen for 30 min, and then stored at -80 ºC until use. The nonstructural carbohydrate content was the sum of the soluble sugar content and starch content.
To determine soluble sugar content, 0.1 g sample (frozen leaves, stems and young panicles) was ground to a fine powder and extracted in 2 mL distilled water at 100 ºC for 30min according to Dubois et al (1956) with some small modifications. Then, the mixture was centrifuged at 10 000 ×for 10 min. The pellet was dried for starch determination as described below. About 0.1 mL aliquot of the supernatant was mixed with 0.5 mL of anthracnose/ethyl acetate solution (1 g anthracnose dissolved in 50 mL ethyl acetate), and reaction with 5 mL H2SO4. After boiling for 1 min, the absorbance of the reaction mixture was monitored at 630 nm. Soluble sugar content was quantified from a standard curve prepared using serial dilutions of standard sucrose solution.
The starch content was measured by the anthrone method (McCready et al, 1950). The pellet was treated with 80% alcohol to remove sugars. Then, the starch was extracted with 9.2 mol/L perchloric acid, and hydrolyzed into glucose with 5 mL sulfuric acid-anthrone reagent (150 mg anthrone dissolved in 100 mL dilute sulfuric acid). The absorbance of the reaction mixture was measured at 620 nm. The starch content was quantified from a standard curve prepared using serial dilutions of a starch standard solution.
The method for extracting sucrose, glucose and fructose was the same as for soluble sugar extraction. The sucrose, glucose and fructose contents were determined according to Zhang (1977) with some modifications. For sucrose content determining, 0.1 mL of supernatant was mixed with 50 µL of 2 mol/L NaOH, boiled for 5 min, and then incubated for 10 min at 80 ºC with 0.7 mL of 30% HCl and 0.2 mL of 0.1% resorcinol. After cooling, the change in the absorbance of the reaction mixture was monitored at 480 nm. The sucrose content was quantified from a standard curve prepared using a serially diluted standard sucrose solution.
For glucose content determination, 4 mL enzyme mixture (10 mg-dianisidine, 10 mg horseradish peroxidase and 0.1 mL glucose oxidase dissolved in 100 mL of 0.1 mol/L acetic acid buffer) was added to 2 mL supernatant, then the mixture was bathed at 30 ºC for 5 min, and 8 mL of 10 mol/L H2SO4solution was added to terminate the reaction, and then monitored at 505 nm.
To determine the fructose content, 0.7 mL H2O2and 0.2 mL of 0.1% resorcinol were added into 0.1 mL supernatant. After incubating for 10 min at 80 ºC, the absorbance of the mixture was measured at 480 nm.
Frozen young panicles (0.1 g) were ground to a fine powder in liquid nitrogen and homogenized in an extraction buffer (20%) containing 50 mmol/L HEPES (pH 7.5), 5 mmol/L MgCl2, 1 mmol/L ethylene diamine tetraacetic acid disodium salt (EDTA-Na2), 0.5 mmol/L dithiothreitol (DTT), 1% Triton X-100, 2% polyvinyl pyrrolidone and 10% glycerol. The supernatant was desalted immediately on a Sephadex G-25 column equilibrated with extraction buffer at 4 ºC. The filtrate was used immediately to determine enzyme activities. The enzyme activities of S-AI (EC3.2.1.26) and SuSy (EC2.4.1.13) were determined using test kits from the Cominbio Company Ltd. (Suzhou, China).
Hexokinase and Pyruvate Kinase Determination Kit (Cominbio Co., Suzhou, China) were used to determine the activities of hexokinase and pyruvate kinase activity according to the manufacturer’s instructions. Hexokinase (HK, EC2.7.1.1) activities were determined by the change of the NADPH concentration during the dehydrogenation of 6-phosphoric acid by glucose 6-phosphate dehydrogenase. The reduction of NADH that induced by pyruvate kinase catalyzation the production of lactic and NAD+from NADH and pyruvate was used to monitor the activities of pyruvate kinase (PK, EC2.7.1.40).
Pyruvate dehydrogenase (PDH, EC1.2.1.51) and α-ketoglutarate dehydrogenase activities (α-KGDH, EC1.2.4.2) were analyzed using a Pyruvate Dehydrogenase and α-Ketoglutarate Dehydrogenase Determination Kit (Cominbio Co., Suzhou, China). Before determining PDH and α-KGDH activities, mitochondria were extracted according to Reidet al(1977). Young panicles (0.1 g) were ground into a homogenate with 1 mL of 50 mmol/L Tris-HCl buffer (pH 7.8) [containing 57 mmol/L 2-mercaptoethanol, 2 mmol/L EDTA, 0.7 mol/L sucrose and 0.5% albumin from bovine serum (BSA) (Fraction V; Sigma, St Louis, MO, USA)]. Then, the homogenate was filtered through four layers of cheesecloth and centrifuged at 2 000 ×for 10 min, and the supernatants were centrifuged at 30 000 ×for 45 min under 4 ºC. The precipitate was resuspended in 180 mL grinding buffer solution and centrifuged twice at 30 000 ×for 45 min under 4 ºC. Finally, the precipitate containing mitochondria was resuspended in cold acetone, centrifuged, and stored at -20 ºC for desiccation.
Total RNA was extracted from leaf, stem and young panicle tissues by using TRIzol according to the manufacturer’s instructions (Invitrogen, USA). First strand cDNA was synthesis from 1 μg RNA by using ReverTra Ace quantitative PCR RT Master Mix with gDNA Remover Kit (TOYOBO). The quantitative real-time PCR (qRT-PCR) was performed as Czechowski et al (2004). The qRT-PCR experiment was performed three biological replicates andgene in rice was used as the control. All primers used were shown in Supplemental Table 2.
Statistical analysis
Statistical differences between the HT and NT treatments at 7 and 15 d were detected by the Student’s-test (0.05) using Excel 2016 (mean of three replicates). One-way analysis of variance (ANOVA) was conducted to compare the differences among the exogenous sucrose, glucose and fructose treatments under HT with a least significant difference (LSD) test using SAS9.1 (SAS Corp., Cary, NC, USA) at the 0.05 level.
Results
Panicle initiation, spikelet formation, and dry matter accumulation
Panicle growth was inhibited by HT treatment (Fig. 1-A). After 7 and 15 d of HT treatment, the dry weight per panicle of HHZ was reduced by 9.6% and 28.4%, and that of IR36 was reduced by 12.0% and 55.1%, respectively (Fig. 1-B). The reduction in young panicle length was more pronounced in IR36 than that in HHZ after 15 d under high temperature (Fig. 1-C). The fully formed panicles were much smaller in the HT treatment than those in the NT treatment (Fig. 1-D). Panicle length showed a slight decrease under HT treatment (Fig. 1-E). Compared with the NT treatment, the HT treatment showed decreases in the number of differentiated spikelets by 15.9% and 29.3%, and the number of surviving spikelets by 40.7% and 69.9%, and increases in the proportion of degenerated spikelets of 51.0% and 64.4% in HHZ and IR36, respectively (Fig. 1-F). In addition, spikelet development was inhibited by the HT treatment. In the HT treatment, the spikelet lengths of HHZ and IR36 were reduced by 12.3% and 20.9% (Fig. 1-G) and the widths were reduced by 13.7% and 9.2%, respectively, compared with the NT treatment (Fig. 1-H). Significant decreases in grain weight of 13.0% and 21.4% were observed in HHZ and IR36, respectively (Fig. 1-I), while the seed-setting rate per panicle decreased by 25.0% and 48.3%, respectively, as a result of HT treatment (Fig. 1-J). However, net photosynthesis rate in the top expanded leaf increased slightly (Fig. 1-K) and dry matter accumulation per tiller remained unchanged under HT (Fig. 1-L). These results indicated that HT at the spikelet differentiation stage caused major retardation of spikelet development by inhibiting spikelet differentiation and aggravating spikelet degeneration, and that heat damage was less severe in HHZ than IR36. In addition, the inhibition of panicle growth was associated with a decrease in dry matter accumulation in young panicles rather than the whole plant, indicating that the photosynthate distribution was disordered under high temperature.
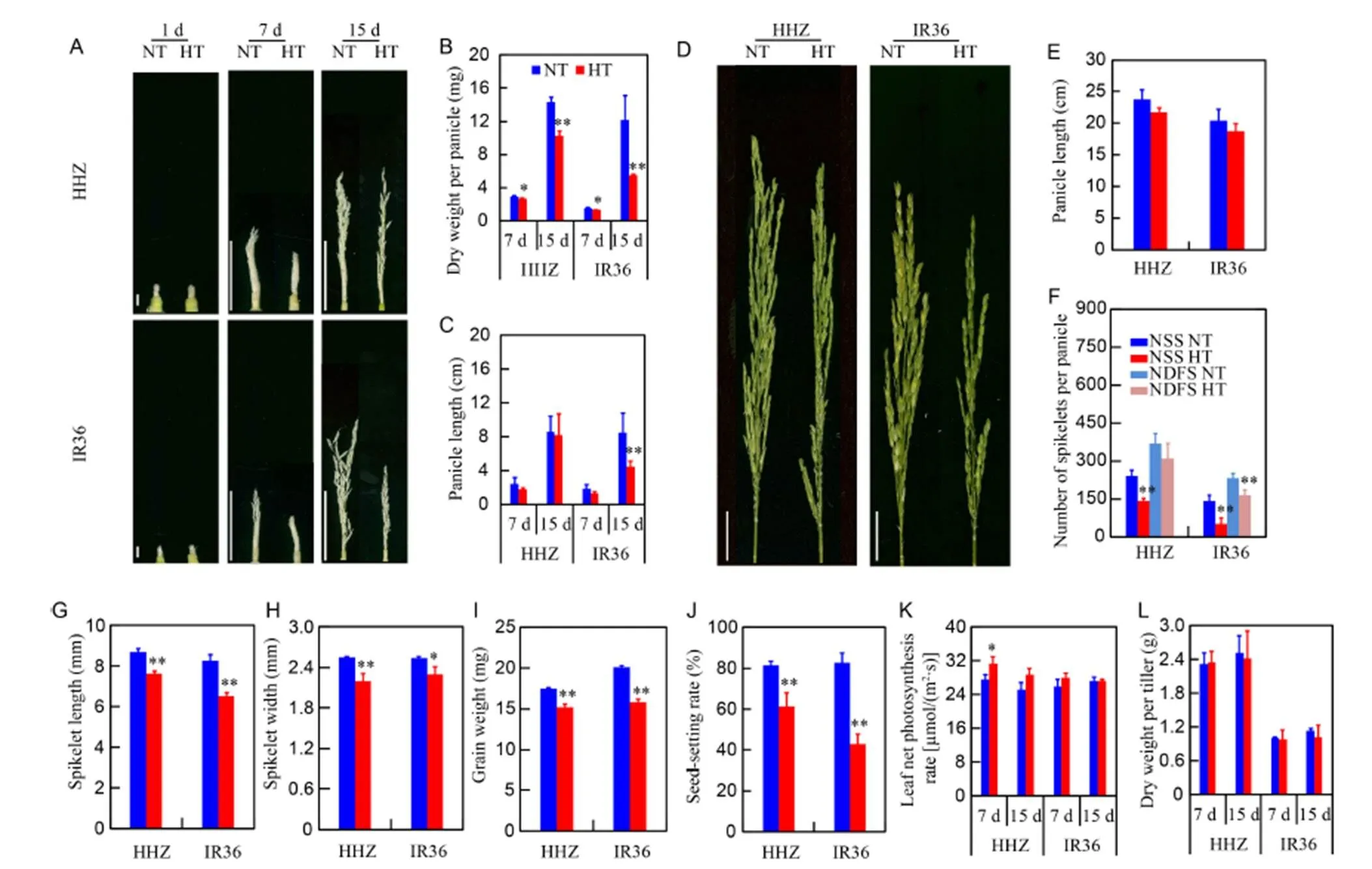
Fig. 1. Effects of high temperature (HT) on panicle development, numbers of differentiated and degenerated spikelets, spikelet size, spikelet fertility, and leaf photosynthesis of Huanghuazhan (HHZ) and IR36 compared with normal temperature (NT)after 7 and 15 d.
Nonstructural carbohydrate, soluble sugar and starch contents
In leaves, the nonstructural carbohydrate content after 7 and 15 d of HT treatment was increased by 9.7% and 17.5% in HHZ, and by 8.9% and 48.7% in IR36, respectively (Fig. 2-A). However, the nonstructural carbohydrate content in young stem was decreased by 28.4% and 28.7% (Fig. 2-B), while that in young panicles was decreased by 13.1% and 17.7% (Fig. 2-C) in HHZ and IR36 under HT compared with NT treatment at 15 d. Moreover, the trends of soluble sugar and starch contents were the same as those of the nonstructural carbohydrate in leaves, stem and panicles (Fig. 2-D to -I). HT treatment disrupted the allocation of carbohydrates, to be a greater degree in IR36 than in HHZ.
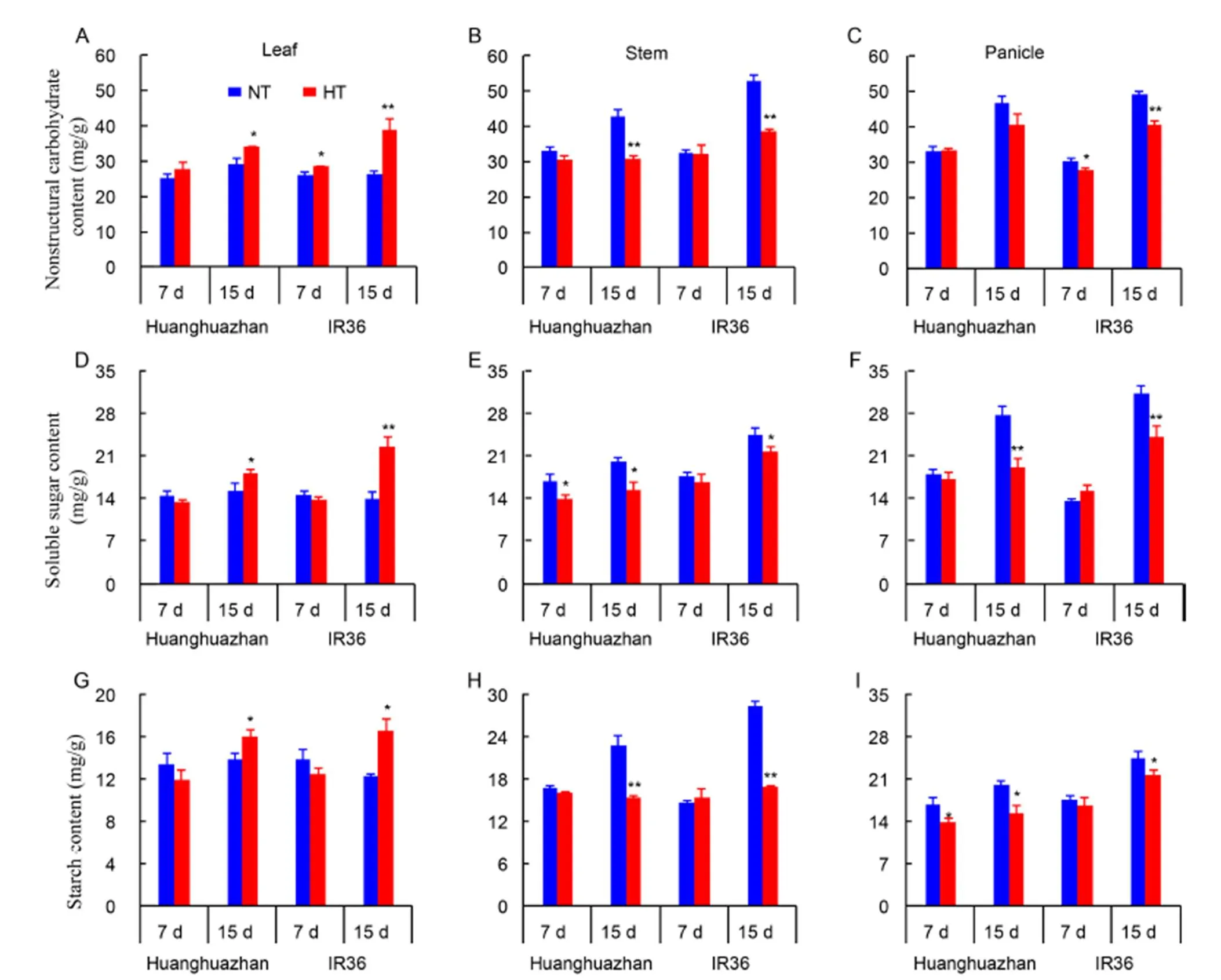
Fig. 2. Nonstructural carbohydrate, soluble sugar, and starch contents in the leaf, young stem, and young panicle of Huanghuazhan and IR36 under normal temperature (NT) and high temperature (HT) treatments after 7 and 15 d.
Transcript levels of sucrose transporter genes
Among all carbohydrates, sucrose is the most commonly transported sugar. On 15 d of the HT treatment, the transcript levels of,andin the leaf were decreased by 64.2%, 85.1% and 51.3% in HHZ, respectively, and by 88.2%, 91.0% and 77.1%, in IR36 respectively (Fig. 3-A, -D and -G). In the stem,andshowed higher expression levels under HT treatment than under NT treatment at 7 d. However, the transcript levels ofin the stem were decreased by 5.9% and 48.8% in HHZ and IR36, respectively. On 15 d of the HT treatment, the transcript levels of,andin the stem were decreased to a greater extent in IR36 than in HHZ (Fig. 3-B, -E and -H). In panicles, the expression patterns of,andwere similar to those in the leaf, and greater decreases were observed in IR36 than in HHZ under the HT treatment (Fig. 3-C, -F and -I). These results demonstrated that prolonging the HT treatment inhibited carbohydrate transport from leaves to young panicles.
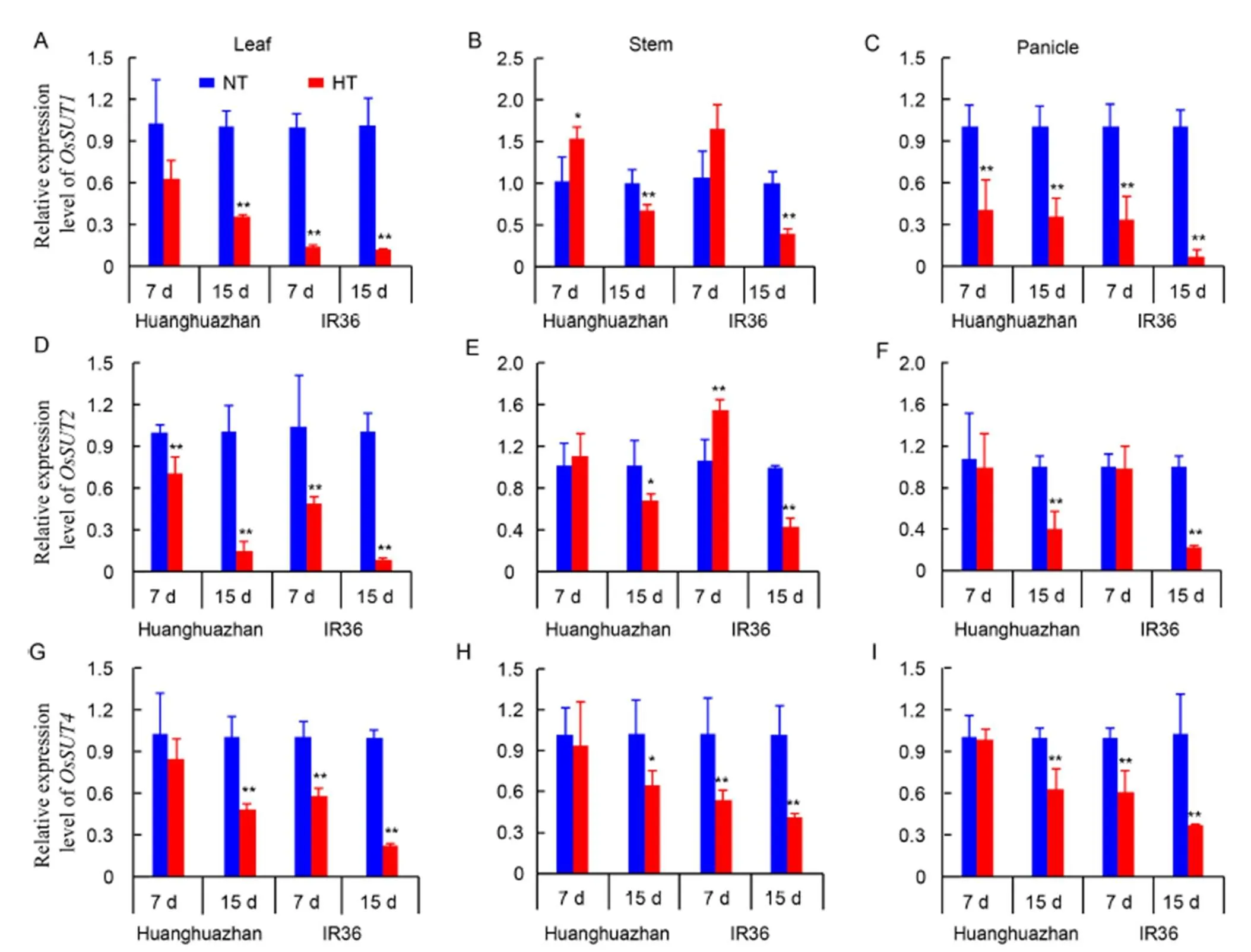
Fig. 3. Transcript levels of sucrose transporter genes in the leaf, stem, and young panicleof Huanghuazhan and IR36 under normal temperature (NT) and high temperature (HT) treatments after 7 and 15 d.
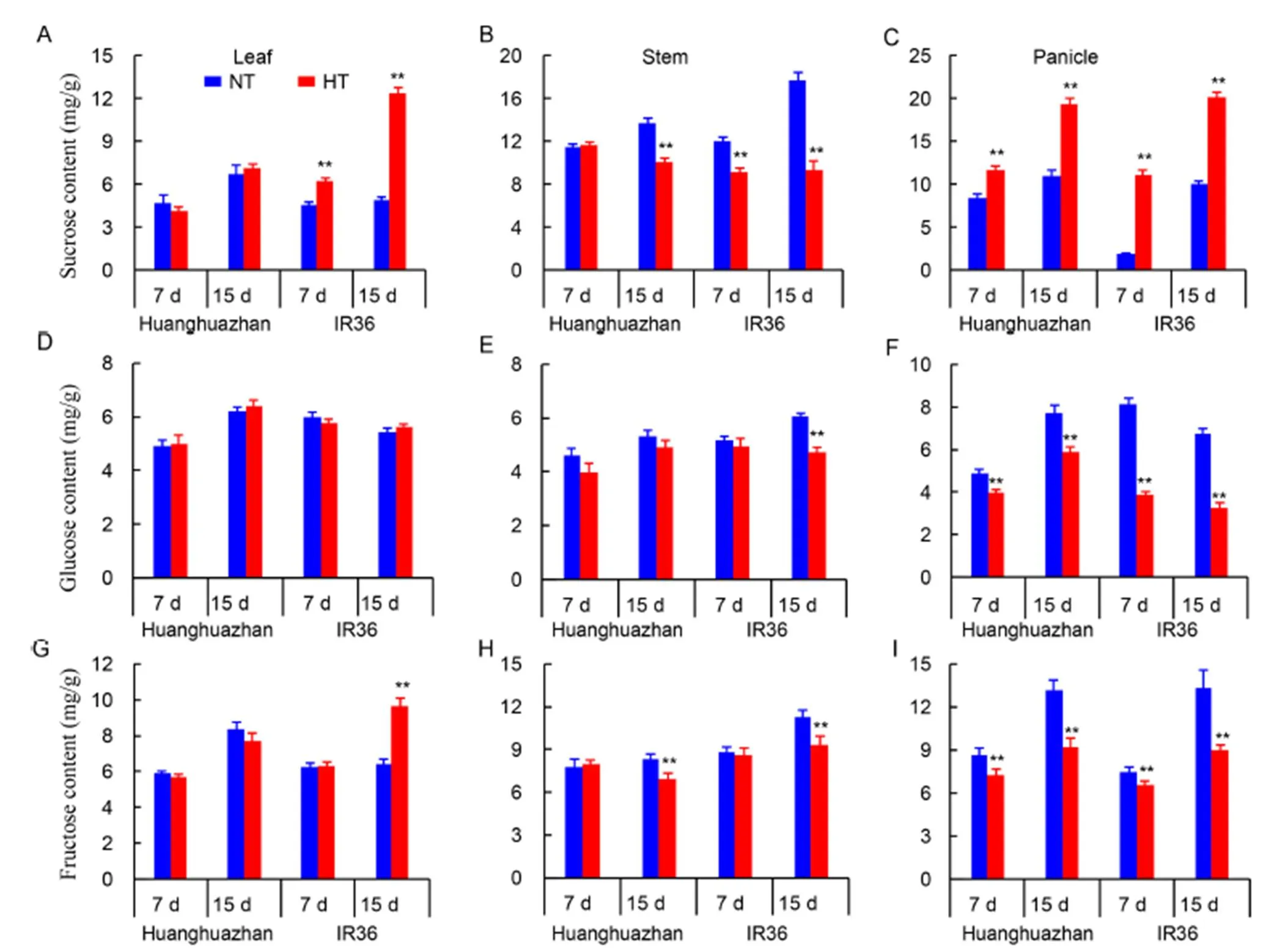
Fig. 4. Sucrose, glucose and fructose contents in the leaf, stem and young panicle of Huanghuazhan and IR36 under normal temperature (NT) and high temperature (HT) treatments after 7 and 15 d.
Sucrose, glucose and fructose contents
The sucrose content was increased significantly in the leaf, especially in IR36 (Fig. 4-A). The glucose content did not differ between the HT and NT treatments in the leaf (Fig. 4-D). However, the fructose content was increased significantly under the HT treatment when compared with NT treatment at 15 d (Fig. 4-G). In the stem, the sucrose content was decreased with prolonged HT treatment. The sucrose content in young stems of HHZ and IR36 was reduced by 26.0% and 47.1% under HT treatment at 15 d respectively (Fig. 4-B), and the glucose and fructose contents in IR36 also decreased under HT (Fig. 4-E and -H). The sucrose content in young panicles was increased by 39.6% and 75.4% in HHZ and by 495.4% and 99.8% in IR36 after 7 and 15 d under HT treatment, respectively (Fig. 4-C). The glucose and fructose contents were sharply decreased under HT in young panicles at both 7 and 15 d, and the decrease was more pronounced in IR36 (Fig. 4-F and -I). Based on these results, we inferred that sucrose hydrolysis in young panicles was significantly inhibited by the HT treatment.
Activities of sucrose hydrolysis enzymes and expression levels of related genes
The activities of soluble acid invertase (S-AI) and sucrose synthase (SuSy) tended to be lower in HT-treated panicles than in NT-treated panicles (Fig. 5-A and -D). In both cultivars, the activities of S-AI were lower at 7 d than 15 d under the HT treatment, and the decrease in S-AI activity was greater in IR36 (41.2%) than in HHZ (37.4%) (Fig. 5-A). Transcript levels of the genesand, which encode acid invertases, were increased significantly at 7 d of the HT treatment, but were decreased sharply at 15 d of the HT treatment when compared with the NT treatment. The reductions were greater in IR36 than in HHZ (Fig. 5-B and -C). The activity of SuSy was decreased greatly at 15 d of the HT treatment in HHZ, and significant decreases were observed at both 7 and 15 d under HT treatment compared with the NT treatment (Fig. 5-D). The transcript levels of the genes encoding SuSy did not differ significantly between the HT and NT treatments at 7 d. However, the transcript levels ofandwere decreased by 34.5% and 44.8% in HHZ, and by 74.1% and 82.8% in IR36 under HT compared with NT at 15 d (Fig. 5-E and -F). These results showed that the HT treatment retarded sucrose hydrolysis in young panicles of IR36 more than HHZ.
Activities of key enzymes associated with glycolysis and tricarboxylic acid cycle
Glycolysis and TCA cycle play an important role in glucose and fructose consumption. Hexokinase and pyruvate kinase are the two key enzymes in glycolysis. HT reduced the activities of hexokinase and pyruvate kinase, and the reductions were more pronounced in IR36 than in HHZ after prolonged treatment (Fig. 6-A and -B). Pyruvate dehydrogenase and ɑ-ketoglutaric dehydrogenase are the two rate-limiting enzymes in the TCA cycle. Prolonged exposure to the HT treatment reduced the enzyme activities in IR36 more than in HHZ (Fig. 6-C and -D). These results suggested that HT inhibited glycolysis and the TCA cycle in young panicles.
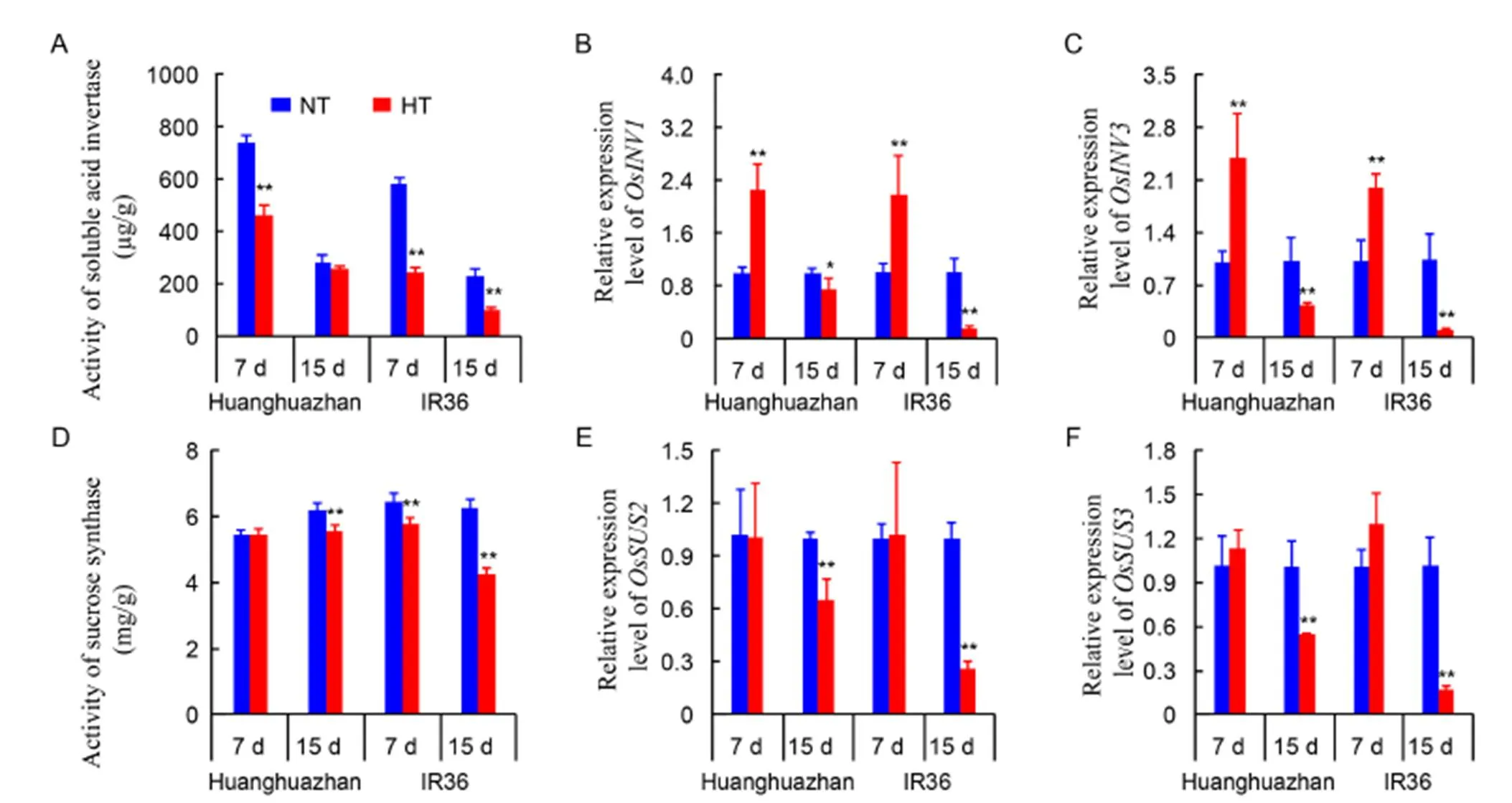
Fig. 5. Activities of soluble acid invertase and sucrose synthase, and transcript levels of related genes in the panicles of Huanghuazhan and IR36 undernormal temperature (NT) andhigh temperature (HT) treatments after 7 and 15 d.
Effects of exogenous sucrose, glucose and fructose on rice spikelet numbers under high temperature
In young panicles, sucrose decomposition and metabolism were inhibited by high temperature. Exogenous sucrose, glucose and fructose were applied to verify these results. Exogenous sucrose, glucose and fructose increased the number of surviving spikelets per panicle by 11.1%, 23.5% and 33.4% in HHZ, and by 7.6%, 44.5% and 53.1% in IR36, respectively, compared with the absence of exogenous regulators (Fig. 7-A). Exogenous glucose and fructose significantly increased the number of differentiated spikelets (Fig. 7-B), and decreased the proportion of degenerated spikelets (Fig. 7-C). These results confirmed that the retardation of spikelet formation under high temperature is associated with sucrose hydrolysis and sugar consumption impairment.
Discussion
In the present study, HT treatment at spikelet differentiation stage significantly reduced the number of spikelets per panicle to a larger extent than the seed-setting rate and grain weight (Fig. 1). Because the HT treatment ended at the beginning of the pollen meiosis stage, seed-setting rate was reduced under HT due to the disruption of pollen development (Sakata et al, 2010; Storme and Geelen, 2014), and this was more pronounced in IR36 (Fig. 1-D). Additionally, grain weight losses were induced by the decrease in spikelet size. The number of spikelets per panicle is determined by the numbers of differentiated and degenerated spikelets. Carbohydrate supply is important for spikelet differentiation and panicle development. In this study, photosynthesis of the top expanded leaf was slightly increased under HT treatment compared with NT treatment (Fig. 1-J), and as a result, total dry matter accumulation was not affected by HT (Fig. 1-K), but HT reduced the young panicle weight (Fig. 1-B). The results showed a disordered assimilate distribution between leaves and young panicles under HT stress, and the developing young panicles were more sensitive to HT than fully formed panicles, which is similar to a study in maize that high temperature inhibited ear expansion induced by photosynthate transport disorders (Suwa et al, 2010).
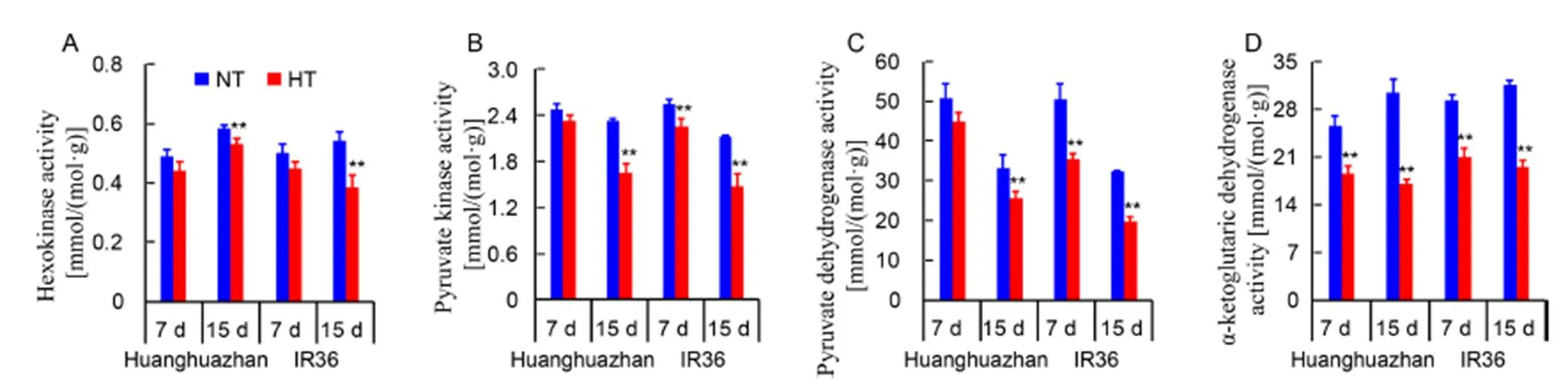
Fig. 6.Activities of hexokinase, pyruvate kinase, pyruvate dehydrogenase, and ɑ-ketoglutaric dehydrogenase in panicles of Huanghuazhan and IR36 under normal temperature (NT) andhigh temperature (HT) treatments after 7 and 15 d.
Values are presented as Mean ± SD (= 3). **, Significant differences between NT and HT at the 0.01 level by the one-tailed Student’s-test.
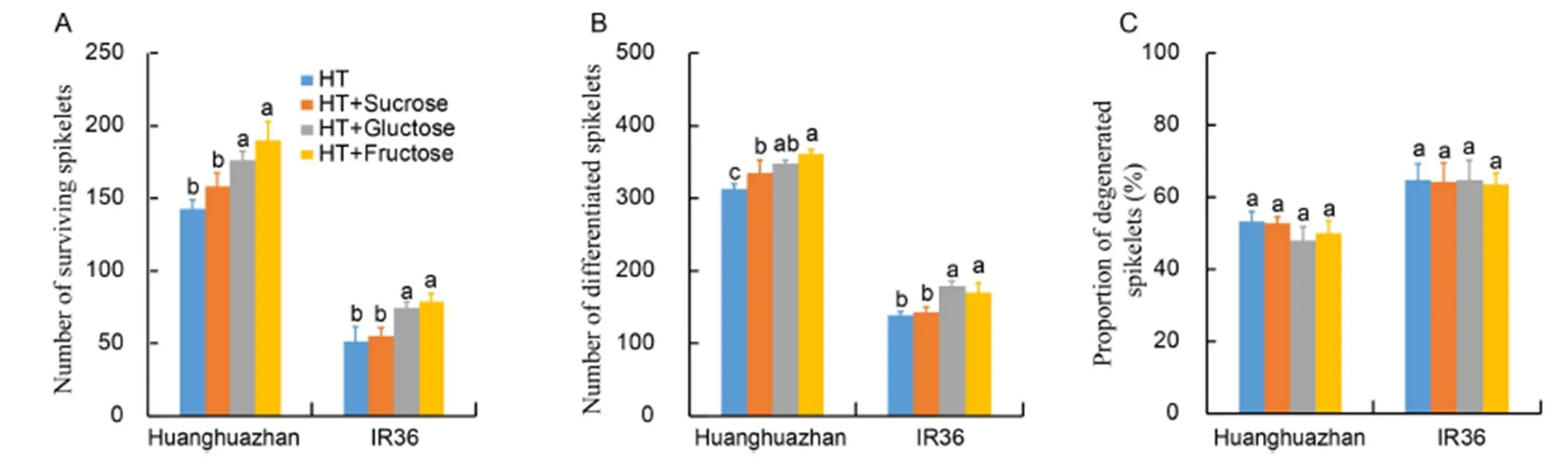
Fig. 7. Effects of exogenous sucrose, glucose, and fructose on spikelet formation of Huanghuazhan and IR36 after 15 d of high temperature (HT).
Values are presented as Mean ± SD (= 3). *, Significant differences at the 0.05 and 0.01 levels by the one-tailed Student’s-test.
With prolonged HT treatment, the expression levels of,and, which encode SUT family proteins involved in long-distance sucrose transport (Scofield et al, 2007), were significantly down-regulated in the leaf, stem and panicle (Fig. 3). As a result, low nonstructural carbohydrate, soluble sugar and starch contents were observed in the stem and young panicles (Fig. 2-B and -C), which decreased dry matter accumulation in young panicles (Fig. 1-B). The transcript levels ofandin the young stem were increased slightly on 7 d of the HT treatment. This may be related to feedback on the sucrose reduction at the beginning of the HT treatment, which is detected in the stem under short-term heat stress in a previous study (Matsukur et al, 2000). Zhang et al(2018) reported that high temperature could destroy plasmodesmata to retard sucrose transportation, and a greater effect is observed in a high-temperature susceptible mutant. In the present study, larger decrease in the expression levels ofgenes were observed in the high temperature-susceptible cultivar IR36 than in the heat-tolerant cultivar HHZ, which is consistent with a previous study showing that OsSUTs contribute to heat tolerance improvement in rice cultivars (Miyazaki et al, 2013).
However, the blocking of carbohydrate transport seems not to be the main reason for the spikelet number reduction. As a result of sucrose transport inhibition, the total nonstructural carbohydrate content (Fig. 2-C) and soluble sugar content (Fig. 2-F) were decreased in young panicles. However, the sucrose content in the panicles was significantly increased (Fig. 4-C) when the soluble sugar content was decreased under HT (Fig. 2-F). The sucrose content in young panicles compared with that in young stem indicated that sucrose metabolism was disrupted under high temperature. Prolonged exposure to HT treatment reduced the activities of S-AI and SuSy, and down- regulated the expression of related genes (Fig. 5), which together contributed to sucrose accumulation. S-AI has been reported to promote adaptation to high temperature in rice plants by providing hexoses, and SuSy decomposes sucrose into UDP-glucose and fructose to provide energy for rice growth (Ruan, 2012; Sharma and Sharma, 2018). In IR36, HT treatment increased the accumulation of sucrose, and decreased the glucose and fructose contents more than in HHZ, when compared with the corresponding NT-treated cultivars, with a heavier reduction in S-AI and SuSy activities (Fig. 5). This suggested that high temperature had greater negative effects on spikelet development in the heat-susceptible rice cultivars. Exogenous applications of glucose and fructose increased the spikelet number by increasing spikelet differentiation and decreasing spikelet degeneration, and a greater increase was observed in IR36 (Fig. 7), while the increase in spikelet number under exogenous sucrose was lower than under exogenous applications of glucose and fructose. However, Zhang et al(2018) reported that exogenous application of sucrose in the rice grain filling stage could alleviate the grain weight reduction caused by the blocking of sucrose transport under high temperature, which suggests that sucrose transport blocking is not the major reason for spikelet formation inhibition at the spikelet development stage. However, glucose and fructose could be used in practice to alleviate heat damage at the spikelet formation stage.
Decreases in the activities of key glycolysis and TCA cycle enzymes were observed in the present study (Fig. 6), as reported in heat-stressed Bermuda grass (Yu et al, 2017), demonstrating that glycolysis and the TCA cycle were inhibited by high temperature, retarding glucose and fructose consumption. Generally, the inhibition of glycolysis and the TCA cycle lead to glucose and fructose accumulation. However, the glucose and fructose contents under HT were significantly reduced in young panicles both in HHZ and IR36 (Fig. 4-F and -I), indicating that the inhibition of sucrose hydrolysis might be the main process impairing sugar consumption. In rice production, allele mining of sucrose utilization genes and applied in rice cultivar modifying, which would promote carbohydrate utilization and enhance the heat-tolerance of rice cultivars.
Conclusions
The reduction of spikelet numbers under high temperaturewas associated with carbohydrate distribution disorders. High temperature inhibited sucrose transport, and increased the accumulation of carbohydrates including sucrose in leaves, but decreased the accumulation in young stems. Sucrose transport inhibition decreased the dry matter accumulation of young panicles. However, high temperature increased the sucrose content in young panicles while the total carbohydrates were reduced due to sucrose hydrolysis inhibition, and a greater degree of damage was observed in the heat- susceptible cultivar. Additionally, high temperature inhibited glycolysis and the tricarboxylic acid cycle. In summary, sugar consumption inhibition aggravated the spikelet development injury under high temperature. Increased sugar utilization contributed to alleviating the spikelet number reduction induced by heat stress at the spikelet development stage.
Acknowledgements
This study was funded by the National Key Research and Development Program of China (Grant No. 2017YFD0300409), the Special Fund for China Agricultural Research System (Grant No. CARS-01-07B), Agricultural Sciences and Technologies Innovation Program of Chinese Academy of Agricultural Sciences, National Natural Science Foundation (Grant No. 31701374), Zhejiang Provincial Natural Science Foundation (Grant No. LY16C130006) and Basic Research Foundation of National Commonweal Research Institute (Grant No. 2017RG004-4) in China.
supplemental data
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. Temperatures setting in climate chambers.
Supplemental Table 2. Primers used for quantitative real-time PCR.
Bao A L, Zhao Z Q, Ding G D, Shi L, Xu F S, Cai H M. 2015. The stable level ofplays an important role in rice growth and in carbon-nitrogen metabolic balance., 16(6): 12713–12736.
Cao Y Y, Duan H, Yang L N, Wang Z Q, Zhou S C, Yang J C. 2008. Effect of heat-stress during meiosis on grain yield of rice cultivars differing in heat-tolerance and its physiological mechanism., 34(12): 2134–2142. (in Chinese with English abstract)
Chaturvedi A K, Bahuguna R N, Shah D, Pal M, Jagadish S V K. 2017. High temperature stress during flowering and grain filling offsets beneficial impact of elevated CO2on assimilate partitioning and sink-strength in rice., 7: 8227.
Cheng C, Zeng Y J, Cheng H H, Tan X M, Shang Q Y, Zeng Y H, Shi Q H. 2019. Effects of different temperature from full heading to milking on grain filling stage on grain hormones concentrations, activities of enzymes involved in starch synthesis and accumulation in rice Nanjing 9108., 33(1): 57–67. (in Chinese with English abstract)
Czechowski T, Bari R P, Stitt M, Scheible W R, Udvardi M K. 2004. Real-time RT-PCR profiling of over 1400transcription factors: Unprecedented sensitivity reveals novel root- and shoot-specific genes., 38(2): 366–379.
Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. 1956. Colorimetric method for determination of sugars and related substances., 28(3): 350–356.
Fang X W, Tang L H, Wang Y P. 2006. Selection on rice germplasm tolerant to high temperature., 7(3): 342–344. (in Chinese with English abstract)
Glaubitz U, Erban A, Kopka J, Hincha D K, Zuther E. 2015. High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity- dependent manner., 66(20): 6385–6397.
Heng Y Q, Wu C Y, Long Y, Luo S, Ma J, Chen J, Liu J F, Zhang H, Ren Y L, Wang M, Tan J J, Zhu S S, Wang J L, Lei C L, Zhang X, Guo X P, Wang H Y, Cheng Z J, Wan J M. 2018.maintains panicle size and grain yield in rice by mediating malate transport., 30(4): 889–906.
Ishibashi Y, Okamura K, Miyazaki M, Phan T, Yuasa T, Iwaya- Inoue M. 2014. Expression of rice sucrose transporter genein sink and source organs shaded during grain filling may affect grain yield and quality., 97: 49–54.
Kaushal N, Awasthi R, Gupta K, Gaur P, Siddique K H M, Nayyar H. 2013. Heat-stress-induced reproductive failures in chickpea ()are associated with impaired sucrose metabolism in leaves and anthers., 40(12): 1334–1349.
Li T, Liu Q H, Ohsugi R, Yamagishi T, Sasaki H. 2006. Effect of high temperature on sucrose content and sucrose cleaving enzyme activity in rice grain during the filling stage., 13(3): 205–210.
Liu X W, Meng Y L, Zhou Z G, Cao W X. 2005. Dynamic characteristics of floret differentiation and degeneration in rice., 31(4): 451–455. (in Chinese with English abstract)
Matsukura C, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J. 2000. Sugar uptake and transport in rice embryo: Expression of companion cell-specific sucrose transporter () induced by sugar and light., 124(1): 85–93.
McCready R M, Guggolz J, Silviera V, Owens H S. 1950. Determinationof starch and amylose in vegetables., 22(9): 1956–1958.
Miyazaki M, Araki M, Okamura K, Ishibashi Y, Yuasa T, Iwaya-Inoue M. 2013. Assimilate translocation and expression of sucrose transporter,, contribute to high-performance ripening under heat stress in the heat-tolerant rice cultivar Genkitsukushi., 170(18): 1579–1584.
Phan T T T, Ishibashi Y, Miyazaki M, Tran H T, Okamura K, Tanaka S, Nakamura J, Yuasa T, Iwaya-Inoue M. 2013. High temperature-induced repression of the rice sucrose transporter () and starch synthesis-related genes in sink and source organs at milky ripening stage causes chalky grains., 199(3): 178–188.
Reid E E, Thompson P, Lyttle C R, Dennis D T. 1977. Pyruvate dehydrogenase complex from higher plant mitochondria and proplastids., 59(5): 854–858.
Ruan Y L. 2012. Signaling role of sucrose metabolism in development., 5(4): 763–765.
Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A. 2010. Auxins reverse plant male sterility caused by high temperatures., 107(19): 8569–8574.
Sanchez B, Rasmussen A, Porter J R. 2014. Temperatures and the growth and development of maize and rice: A review., 20(2): 408–417.
Scharte J, Schön H, Tjaden Z, Weis E, Schaewen A V. 2009. Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants., 106(19): 8061–8066.
Scofield G N, Hirose T, Aoki N, Furbank R T. 2007. Involvement of the sucrose transporter,, in the long-distance pathway for assimilate transport in rice., 58(12): 3155–3169.
Sharma K P, Sharma N. 2018. Influence of high temperature on sucrose metabolism in chalky and translucent rice genotypes., 88(3): 1275–1284.
Storme D N, Geelen D. 2014. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms., 37(1): 1–18.
Suwa R, Hakata H, Hara H, El-Shemy H A, Adu-Gyamfi J, Nguyen N T, Kanai S, Lightfoot D A, Mohapatra P K, Fujita K. 2010. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (L.) genotypes., 48: 124–130.
van den Ende W. 2014. Sugars take a central position in plant growth, development and, stress responses: A focus on apical dominance., 5: 313.
Wang Y L, Zhang Y P, Zeng Y H, Wu H, Xiang J, Chen H Z, Zhang Y K, Zhu D F. 2015. Effect of high temperature stress on rice spikelet differentiation and degeneration during panicle initiation stage., 36(6): 724–731. (in Chinese with English abstract)
Wang Y L, Zhang Y P, Zhu D F, Xiang J, Chen H Z, Zhang Y K. 2016. Response of rice organ morphology and dry matter accumulation to high temperature at different panicle initiation stages., 30(2): 161–169. (in Chinese with English abstract)
Wang Y L, Zhang Y P, Xiang J, Wang L, Chen H Z, Zhang Y K, Zhang W Q, Zhu D F. 2017. Response ofrice spikelet differentiation and degeneration to air temperature and solar radiation of different sowing dates., 28(11): 3571–3580. (in Chinese with English abstract)
Wu C, Cui K H, Wang W C, Li Q, Fahad S, Hu Q Q, Huang J L, Nie L X, Peng S B. 2016. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice., 6: 34978.
Yu J J, Li R, Fan N L, Yang Z M, Huang B R. 2017. Metabolic pathways involved in carbon dioxide enhanced heat tolerance in Bermudagrass., 8: 1506.
Zeng Y H, Zhang Y P, Xiang J, Wang Y L, Chen H Z, Zhu D F. 2015. Effects of low temperature on formation of spikelets and grain filling ofinbred rice during panicle initiation in early-season., 26(7): 2007–2014. (in Chinese with English abstract)
Zhang C X, Feng B H, Chen T T, Fu W M, Li H B, Li G Y, Jin Q Y, Tao L X, Fu G F. 2018. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation., 155: 718–733.
Zhang Y J. 1977. Determination of glucose, fructose, sucrose and starch in fruit and vegetable with anthrone colorimetric method., 5(3): 167–171. (in Chinese)
Copyright © 2020, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.12.005
15 November 2018;
8 February 2019
s:Zhu Defeng (cnrice@qq.com); Zhang Yuping (cnrrizyp@163.com)
(Managing Editor: Wang Caihong)
杂志排行
Rice Science的其它文章
- Cellular Localization of Rice SUMO/SUMO Conjugates and in vitroSumoylation Using Rice Components
- Morpho-Physiological Response of Oryza glaberrima to Gradual Soil Drying
- Drought Stress Impairs Grain Yield and Quality of Rice Genotypes by Impaired Photosynthetic Attributes and K Nutrition
- Differential Expression of Rice Valine-Qlutamine Gene Family in Response to Nitric Oxide and Regulatory Circuit of OsVQ7 and OsWRKY24
- Systematic Characterization of Long Non-Coding RNAs and Their Responses to Drought Stress in Dongxiang Wild Rice
- AssessmentofVariationinMorpho-PhysiologicalTraitsandGeneticDiversityin Relation to Submergence Tolerance of Five Indigenous LowlandRice Landraces
