Drought Stress Impairs Grain Yield and Quality of Rice Genotypes by Impaired Photosynthetic Attributes and K Nutrition
2020-12-28MuhammadZahidMUMTAZ,MuhammadSAQIB,GhulamABBAS等
Letter
Drought Stress Impairs Grain Yield and Quality of Rice Genotypes by Impaired Photosynthetic Attributes and K Nutrition
Drought is one of the most prevalent abiotic stresses that adversely affect rice productivity (Petrozza et al, 2014). Rice is very sensitive to drought stress and drought can cause 50% reduction in rice production globally (Yang et al, 2008). To meet the food needs for global population, 63% more agricultural production will be required by the year 2050 than the current production (Alexandratos and Bruinsma,2012; FAO, 2017). Drought stress delays the time of flowering, leading to a reduced number of panicles, number of kernels and ultimately grain yield (Pantuwan et al, 2002; Iseki et al, 2014). The reproductive stage of rice is very sensitive to drought stress, and water limitation at this stage causes a serious reduction in rice yield (Aydinsakir et al, 2013) and biomass (Iseki et al, 2014). It is a major challenge to increase rice production under increasing drought due to changing climate, however, it may be achieved through use of drought-tolerant rice varieties having the ability to produce high yield under drought stress conditions (Luo, 2010).
This study was conducted under a split plot design with three replications at the Research Farm of the Institute of Soil and Environmental Sciences, University of Agriculture Faisalabad, Pakistan. Nursery of 11 rice genotypes (99404, 99417, Super Basmati, KS-282, KSK-434, KSK-133, Basmati-2000, KS-432, Basmati-515, Basmati-385 and Shaheen Basmati) was grown under well-irrigated conditions. Data of rainfall, sunshine, and average temperatures are given in Table 1.A well-irrigated control (non-stressed) was maintained by continuous recommended irrigations from transplanting to a week before maturity. The drought stress treatment was started at 60 d after transplantation and thereafter this field was not irrigated. There was 41.17% less application of water in case of drought treatment as compared to the well-irrigated treatment.
Drought stress affects gas exchange attributes like photosynthetic rate, stomatal conductance and transpiration rate (Serraj et al, 2011). After four weeks of drought stress,the photosynthetic rate, transpiration rate and stomatal conductance of expanded second top leaves were measured according to Mumtaz et al (2018). Drought stress caused a significant reduction in different gas exchange attributes including photosynthetic rate, transpiration rate and stomatal conductance, and significant genotypic variations were observed among different rice genotypes regarding these parameters (Table 2). KS-282 showed significantly higher photosynthetic rate, transpiration rate and stomatal conductance under drought stress as compared to the other genotypes. Drought stress caused 19%, 48%, and 47% decreases in photosynthetic rate, transpiration rate and stomatal conductance of KS-282 over the control, respectively. Meanwhile, 99404 followed by 99417 showed lower photosynthetic rate, transpiration rate and stomatal conductance with 30%, 67% and 68% decreases, respectively, as compared to control.Closure of stomata under drought stress to conserve moisture content may cause a decrease in stomatal conductance (Iseki et al, 2014). Stomatal conductance is associated with turgidity of leaves. Under drought stress, reduction in stomatal conductance also causes decreases in photosynthetic rate and transpiration rate (Rauf et al, 2015). It has been reported that stomatal conductance plays an important role in regulating the water balance of plants (Sinclair et al, 2010). If stomata are closed, the expansion of cell is reduced which leads to limited growth rate, biomass and yield production. Under severe drought conditions, CO2fixation is reduced due to the inhibition of the activities of some key enzymes such as rubisco activase and ribulose-1,5-bisphosphate carboxylase/oxygenase. Drought induced inhibition of enzyme activities results in the generation of reactive oxygen species, which causes photo-oxidation and harm to the photosynthetic membrane proteins, pigments and lipids, and ultimately affects grain quality (Rauf et al, 2015). Drought induced photosynthetic limitations during reproduction phase causes reduction in carbon flux to reproductive organs, triggers ovary abortion, increases pollen sterility, which leads to decreased grain yield and quality (Boyer and Westgate, 2004; Centritto et al, 2009). The results of present work suggested that drought tolerant genotypes have a better ability to maintain their physiological functions under drought stress.
Number of panicles per plant was recorded manually before harvesting. Paddy yield was recorded after air drying the rice grains. One week sun-dried rice straw was weighed and straw yield was noted. Drought stress significantly reduced grain and straw yields as well as number of panicles per plant (Table 3). Under drought stress, KS-282 showed the highest number of panicles per plant with 7% decrease as compared to the control. The lowest number of panicles per plant was shown by 99404 with 29% decrease over the control. KS-282 also differed significantly regarding number of panicles per plant from all the other genotypes whereas 99404 was statistical at par with 99417. Genotypic variations were also observed with regard to straw and grain yields. KS-282 showed the maximum grain yield (38% decrease over the control) but was statistical at par with KS-432, Basmati-385 and Shaheen Basmati under drought stress, whereas 99404 showed the minimum grain yield with 58% decrease over the control. Similarly, KS-282 also produced the highest straw yield (21% decrease over the control) with a significant difference from all the other genotypes under drought conditions. However, the lowest straw yield (33% decrease over the control) under drought conditions was observed in 99404. These findings are supported by Pantuwan et al (2002), Yue et al (2006), Kumar et al (2009), Luo (2010) and Iseki et al (2014). Pantuwan et al (2002) tested 128 genotypes under mild to prolonged severe drought stresses at the grain filling stage and reported flowering time as an important cause of grain yield loss. Reduction in grain yield under drought stress could be due to increased spikelet sterility that reduces fertile panicles and grain weight (Yue et al, 2006). Straw yield is closely related to photosynthesis which is severely reduced under drought stress (Iseki et al, 2014).
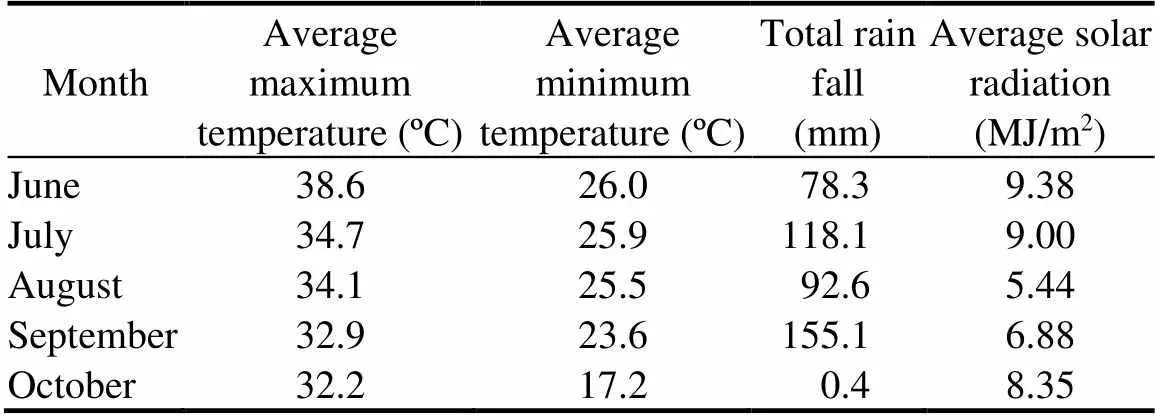
Table 1.Rainfall, solar radiation and average minimum and maximum temperatures for the crop season at the experimental site.

Table 2. Effects of drought stress on photosynthetic rate, transpirational rate and stomatal conductance of different rice genotypes.
Different lowercase letters for each parameter indicate significant difference according to the least significant difference test at≤0.05.
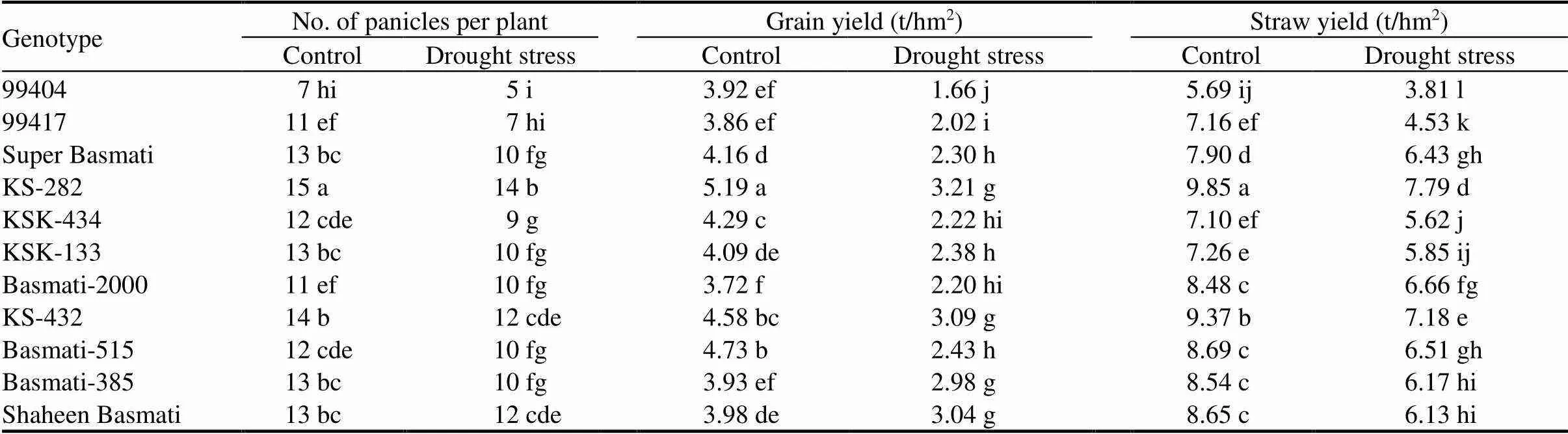
Table 3. Effects of drought stress on number of panicles per plant, grain yield and straw yield of different rice genotypes.
Different lowercase letters for each parameter indicate significant difference according to the least significant difference test at≤0.05.
Plants can maintain the uptake of cations to cope with drought stress (Cakmak, 2005). K+is an important cation in plants, which plays a role in protein synthesis, membrane permeability, cell expansion, enzyme activity and stomatal opening and closing (Hopkins and Huner, 2004). K+content in plantsis positively related to transpiration rate. Drought stress accumulation with K+deficiency causes an increase in reactive oxygen species (ROS) that induced disturbances in stomatal opening and photosynthesis (Mengel and Kirkby, 2001). Severe drought stress increases the demand for K+to avoid oxidative damage and to protect chloroplasts. Plants also need K+under drought stress to maintain photosynthetic CO2fixation, which is reduced as a result of stomatal closure (Egilla et al, 2005).
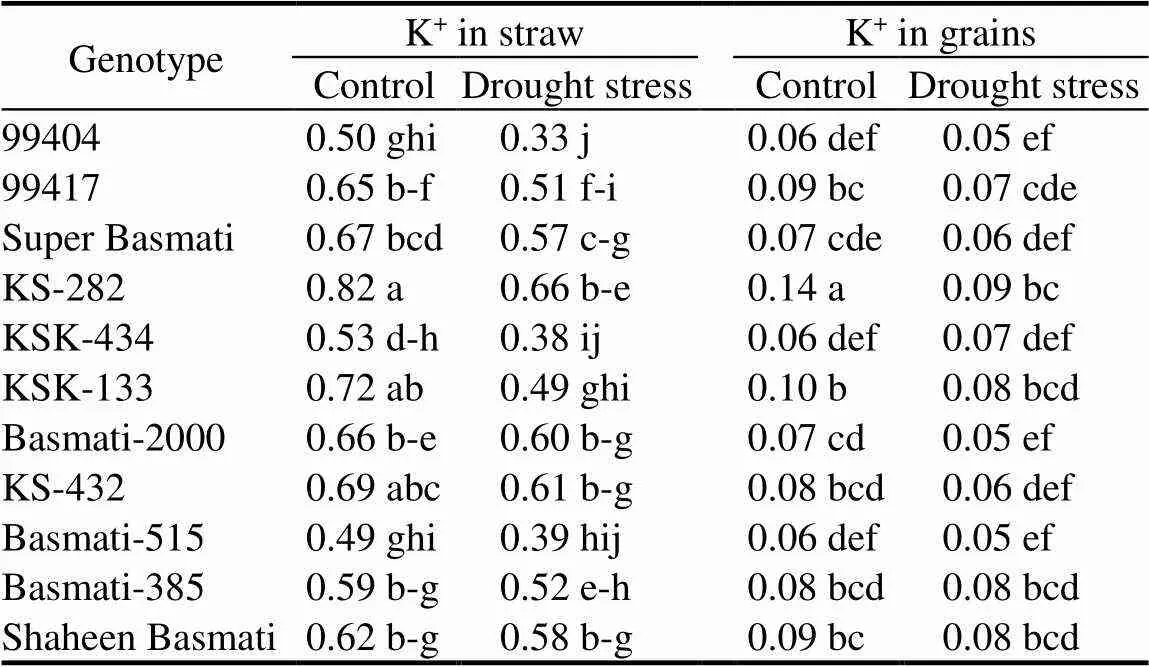
Table 4. Effects of drought stress on K+ concentrations in straw and grains of different rice genotypes. mmol/g
Different lowercase letters for each parameter indicate significant difference according to the least significant difference test at≤0.05.
Determination of K+concentration in straw and grains were conductedby digesting the samples as described by Wolf (1982). There was a significant effect of drought stress on K+concentration in grains and straw with a significant variation among different rice genotypes (Table 4). Significantly higher K+concentrations in straw and grains were observed in KS-282 (20% and 36% decreases, respectively) followed by KS-432 (12% and 25% decreases, respectively) as compared to the control. The lower K+concentrations of straw and grains were shown by 99404 as compared to the rest of the genotypes. This genotype showed 34% and 17% reductions in K+concentrations of straw and grains, respectively, over the control. K+content in drought tolerant genotypes is positively related to transpiration rate, however, K+uptake may vary due to its availability in the soil. Wang et al (2013) concluded that increased K+availability under drought stress improves enzyme activity, cell expansion, stomatal conductance, leaf area index, water use efficiency and nutrient uptake, which may promote dry matter and grain yield production. Wang et al (2004) explored the effects of K application on plant K+uptake, grain yield and quality ofrice,and found that with increasing K application, plant K+uptake is also increased, which considerably increases the number of ear bearing tillers and seed-setting rate. Moreover, high K+content in plants is correlated with a corresponding decrease in chalkiness and amylose content and high grain yield and quality.
Grain quality is important for the acceptance and adaptation of any new cultivars by farmers and consumers (Cooper et al, 2008). Generally, in the Indian subcontinent, people prefer rice grain having medium to long grains. Market price can be estimated by grain size and shape, proportion of chalky and broken rice grain (Cooper et al, 2008). Previous studies regarding drought stress have been restricted to physiological and yield attributes but their effects on grain quality are not well-known.Three genotypes (Super Basmati, KS-282 and 99404) were selected for the determination of grain quality as affected by drought stress. Drought stress caused significant reduction in grain length and width of all the selected rice genotypes (Fig. 1). Under drought conditions, the maximum grain length was showed in Super Basmati (7% reduction) and the minimum grain length (2% reduction) was in KS-282 over control. On the contrast, KS-282 produced the maximum grain width with 6% reduction under drought stress as compared to the control. It was revealed that drought stress significantly increased the broken fraction and reduced the total milling recovery (TMR) of all the three rice genotypes (Fig. 2). The maximum broken fraction was observed in Super Basmati, and KS-282 showed the minimum broken fraction with 25% and 54% increases, respectively, under drought stress as compared to the control. Super Basmati showed the highest TMR with 4% decrease under drought stress whereas the minimum TMR was observed in 99404 with 4% reduction over the control. There were no chalkiness spots observed in any genotype under normal conditions whereas under drought stress, 9, 5 and 5 chalkiness spots were observed on grains of 99404, Super Basmati and KS-282, respectively (Table 5).We measured a negative relation between the grain length and width and these results are in accordance with the findings of Koutroubas et al (2004). Reduction in grain width could be linked with a decrease in average endosperm cell area or with abnormal amyloplast packaging that results in white chalky areas (Ishimaru et al, 2009). Fabre et al (2005) reported that grain dimensions are reduced under stress conditions. These results are also similar to the outcomes of Rao et al (2013). The results of this study also revealed a reduction in the total milling recovery along with an increase in broken fraction of rice grains due to drought stress. Super Basmati showed the minimum total milling recovery with the highest broken fraction and longer grain length. During the milling process, breakdown of longer grains was more serious than that of the shorter to medium grains. There was an inverse relationship between grain length and total milling recovery in this study. Sharifi et al (2009) also reported an interaction between rice genotype and environment with respect to grain length and shape. Similar findings have also been discussed by Adu-Kwarteng et al (2003) and Rao et al (2013).
Chalkiness is an important quality characteristic in the rice grain occurs commonly with the development of numerous air spaces between loosely packed starch granules and environmental stress during grain development (Tashiro and Wardlaw, 1991). In the present study, 99404 showed the greater chalkiness score under drought stress as compared to Super Basmati and KS-282. It could be due to environmental stress (drought in this case) that hindered the normal grain filling (Adu-Kwarteng et al, 2003). Under stress conditions, chalkiness in the grains causes the breakage of grains that fetch a lower price in the market. Chalky spot on grains appears as results of reduced water supply under drought stress. It is particularly evident for 99404 which is a drought-sensitive genotype with greater chalkiness score. Chalkiness degrades the rice appearance, transparency, head rice recovery and consumer acceptability (Graham, 2002; Yoshioka et al, 2007). It is negatively correlated with milling quality as increase in chalkiness caused decrease in head rice recovery (Wassmann et al, 2009; Zhao and Fitzgerald, 2013).
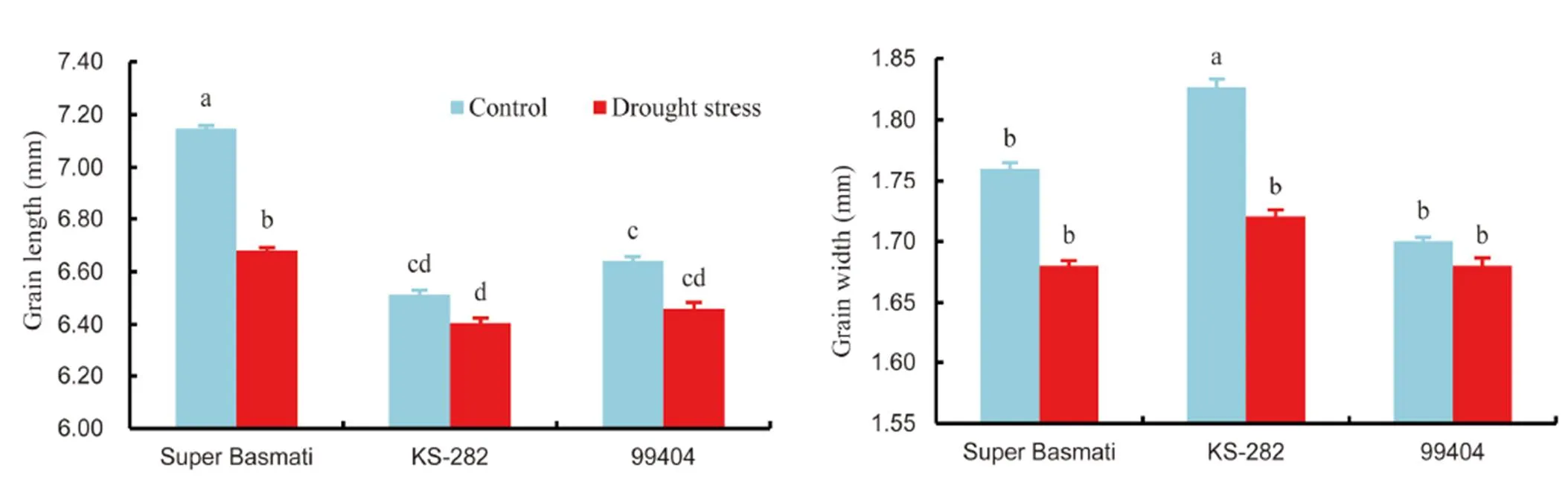
Fig. 1. Effects of drought stress on grain length and grain width of rice genotypes.
Data are Mean ± SE (= 3).Different letters above the bar indicate significant difference according to the least significant difference test at the 0.05 level.
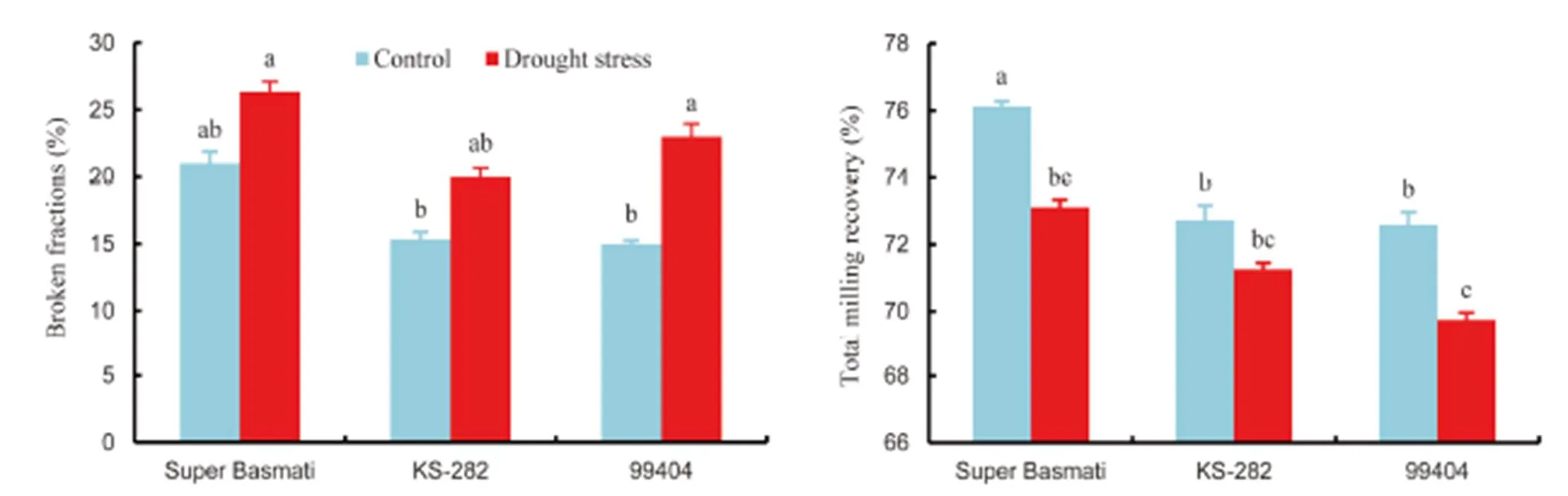
Fig.2. Effects of drought stress on broken fraction and total milling recovery of rice genotypes.
Data are Mean ± SE (= 3).Different letters above the bar indicate significant difference according to the least significant difference test at the 0.05 level.
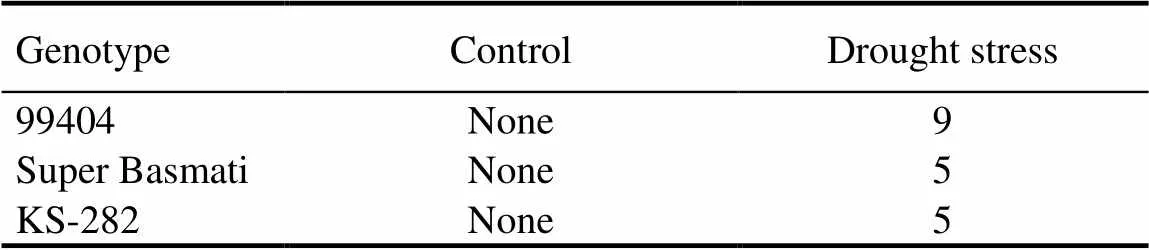
Table 5. Effects of drought stress on chalkiness score on grains of different rice genotypes.
Chalkiness score was rated on a scale from 0 to 9 with respect to increase in chalky area.None,No chalky area; 5, 10% to 20% chalky area; 9, >20% chalky area.
SUPPLEMENTAL DATA
The following material is available in the online version of this article at http://www.sciencedirect.com/science/journal/ 16726308; http://www.ricescience.org.
Supplemental File 1. Materials and methods used in this study.
Adu-Kwarteng E, Ellis W O, Oduro I, Manful J T. 2003. Rice grain quality: A comparison of local varieties with new varieties under study in Ghana., 14(7): 507–514.
Alexandratos N, Bruinsma J. 2012. World agriculture towards 2030/2050: The 2012 revision.: Agricultural Development Economics (ESA) Working Paper No. 12. 3 June, 2012. Rome, Italy: Food and Agriculture Organization of the United Nations.
Aydinsakir K, Erdala S, Buyuktasb D, Bastugb R, Tokera R. 2013. The influence of regular deficit irrigation applications on water use, yield, and quality components of two corn (L.) genotypes., 128: 65–71.
Boyer J S, Westgate M E. 2004. Grain yields with limited water.,55: 2385–2394.
Cakmak I. 2005. The role of potassium in alleviating detrimental effects of abiotic stresses in plants.,168: 521–530.
Centritto M, Lauteri M, Monteverdi M C, Serraj R. 2009. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage.,60: 2325–2339.
Cooper N T W, Siebenmorgen T J, Counce P A. 2008. Effects of nighttime temperature during kernel development on rice physicochemical properties., 85(3): 276–282.
Egilla J N, Davies F T, Boutton T W. 2005. Drought stress influences leaf water content, photosynthesis, and water-use efficiency of hibiscus rosa-sinensis at three potassium concentrations., 43(1): 135–140.
Fabre D, Siband P, Dingkuhn M. 2005. Characterizing stress effects on rice grain development and filling using grain weight and size distribution.,92(1): 11–16.
FAO. 2017. The Future of Food and Agriculture: Trends and Challenges.Rome, Italy: Food and Agriculture Organization of the United Nations.
Graham R. 2002. A proposal for IRRI to establish a grain quality and nutrition research center.: IRRI Discussion Paper Series no. 44. Los Banos, the Phillipine: International Rice Research Institute: 15.
Hopkins W G, Huner N P A. 2004. Responses of plants to environmental stress.:Introduction to Plant Physiology. 4th edn. USA: John Wiley and Sons: 223–239.
Iseki K, Homma K, Shiraiwa T, Jongdee B, Mekwatanakarn P. 2014. The effects of cross-tolerance to oxidative stress and drought stress on rice dry matter production under aerobic conditions., 163: 18–23.
Ishimaru T, Horigane A K, Ida M, Iwasawa N, San-oh Y A, Nakazono M, Nishizawa N K, Masumura T, Kondo M, Yoshida M. 2009. Formation of grain chalkiness and changes in water distribution in developing rice caryopses grown under high- temperature stress., 50(2): 166–174.
Koutroubas S D, Mazzini F, Pons B, Ntanos D A. 2004. Grain quality variation and relationships with morpho-physiological traits in rice (L.) genetic resources in Europe., 86: 115–130.
Kumar A, Verulkar S, Dixit S, Chauhan B, Bernier J, Venuprasad R, Zhao D, Shrivastava M N. 2009. Yield and yield-attributing traits of rice (L.) under lowland drought and suitability of early vigor as a selection criterion., 114(1): 99–107.
Luo L J. 2010. Breeding for water-saving and drought-resistance rice (WDR) in China., 61(13): 3509–3517.
Mengel K, Kirkby E A.2001. Principles of Plant Nutrition. 5th edn. Dordrecht: Kluwer Academic Publishers.
Mumtaz M Z, Saqib M, Abbas G, Akhtar J, Qamar Z U. 2018. Genotypic variation in rice for grain yield and quality as affected by salt-affected field conditions., 41(2): 233–242.
Pantuwan G, Fukai S, Cooper M, Rajatasereekul S, O’Toole J C. 2002. Yield response of rice (L.) genotypes to drought under rainfed lowlands: 2. Selection of drought resistant genotypes., 73: 169–180.
Petrozza A, Santaniello A, Summerer S, Tommaso G D, Tommaso D D, Paparelli E, Piaggesi A, Perata P, Cellini F. 2014. Physiological responses to Megafol treatments in tomato plants under drought stress: A phenomic and molecular approach., 174: 185–192.
Rao P S, Mishra B, Gupta S R. 2013. Effects of soil salinity and alkalinity on grain quality of tolerant, semi-tolerant and sensitive rice genotypes., 20(4): 284–291.
Rauf S, Al-Khayri J M, Zaharieva M, Monneveux P, Khalil F. 2016. Breeding strategies to enhance drought tolerance in crops.: Al-Khayri J M. Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits.Switzerland: Springer International Publishing: 397–445.
Serraj R, McNally K L, Slamet-Loedin I, Kohli A, Haefele S M, Atlin G, Kumar A. 2011. Drought resistance improvement in rice: An integrated genetic and resource management strategy.,14: 1–14.
Sharifi P, Dehghani H, Mumeni A, Moghaddam M. 2009. Genetic and genotype × environment interaction effects for appearance quality of rice.,8(8): 891–901.
Sinclair T R, Messina C D, Beatty A P, Samples M. 2010. Assessment across the United States of the benefits of altered soybean drought traits., 102(2): 475–482.
Tashiro T, Wardlaw I F. 1991. The effect of high temperature on kernel dimensions and the type and occurrence of kernel damage in rice., 42:485–496.
Wang M, Zheng Q S, Shen Q R, Guo S W. 2013. The critical role of potassium in plant stress response.,14(4): 7370–7390.
Wang Q S, Zhen R H, Ding Y F, Ji Z J, Cao W X, Huang P S. 2004. Effects of potassium fertilizer application rates on plant potassium accumulation and grain quality ofrice., 37: 1444–1450. (in Chinese with English abstract)
Wassmann R, Jagadish S V K, Heuer S, Ismail A, Redona E, Serraj R, Singh R K, Howell G, Pathak H, Sumfleth K. 2009. Climate change affecting rice production: The physiological and agronomic basis for possible adaptation strategies., 101: 59–122.
Wolf B. 1982. A comprehensive system of leaf analysis and its use for diagnosing crop nutrient status., 13(12): 1035–1059.
Yang JC, Liu K, Zhang SF, Wang XM, Wang ZQ, Liu LJ. 2008. Hormones in rice spikelets in responses to water stress during meiosis., 34(1): 111–118. (in Chinese with English abstract)
Yoshioka Y, Iwata H, Tabata M, Ninomiya S, Ohsawa R. 2007. Chalkiness in rice: Potential for evaluation with image analysis.,47(5): 2113–2120.
Yue B, Xue W Y, Xiong L Z, Yu X Q, Luo L J, Cui K H, Jin D M, Xing Y Z, Zhang Q F. 2006. Genetic basis of drought resistance at reproductive stage in rice: Separation of drought tolerance from drought avoidance., 172(2): 1213–1228.
Zhao X, Fitzgerald M A. 2013. Climate change: Implications for the yield of edible rice., 8(6): e66218.
Muhammad Zahid Mumtaz1, 2, Muhammad Saqib1, Ghulam Abbas1, 3, Javaid Akhtar1, Zia Ul-Qamar4
(; Department of Environmental Sciences, 61100, Pakistan; Plant Breeding and Genetics Division, Nuclear Institute for Agriculture and Biology, Faisalabad 38000, Pakistan)
Copyright © 2020, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.12.001
Muhammad Zahid Mumtaz (zahidses@gmail.com)
7 August 2018;
30 October 2018
杂志排行
Rice Science的其它文章
- Cellular Localization of Rice SUMO/SUMO Conjugates and in vitroSumoylation Using Rice Components
- Decrement of Sugar Consumption in Rice Young Panicle Under High Temperature Aggravates Spikelet Number Reduction
- Morpho-Physiological Response of Oryza glaberrima to Gradual Soil Drying
- Differential Expression of Rice Valine-Qlutamine Gene Family in Response to Nitric Oxide and Regulatory Circuit of OsVQ7 and OsWRKY24
- Systematic Characterization of Long Non-Coding RNAs and Their Responses to Drought Stress in Dongxiang Wild Rice
- AssessmentofVariationinMorpho-PhysiologicalTraitsandGeneticDiversityin Relation to Submergence Tolerance of Five Indigenous LowlandRice Landraces
