Morpho-Physiological Response of Oryza glaberrima to Gradual Soil Drying
2020-12-28KartikaKartikaJunIchiSakagamiBenyaminLakitanShinYabutaAndiWijayaSabaruddinKadirLailyIlmanWiduriErnaSiagaYoshihiroNakao
Kartika Kartika, Jun-Ichi Sakagami, Benyamin Lakitan, Shin Yabuta, Andi Wijaya, Sabaruddin Kadir, Laily Ilman Widuri, Erna Siaga, Yoshihiro Nakao
Short Communication
Morpho-Physiological Response ofto Gradual Soil Drying
Kartika Kartika1, Jun-Ichi Sakagami2, Benyamin Lakitan3, 4, Shin Yabuta2, Andi Wijaya3, Sabaruddin Kadir5, Laily Ilman Widuri1, Erna Siaga1, Yoshihiro Nakao6
()
Soil drought occurrence during dry season has been the main constraint, besides prolonged flooding during rainy season, in increasing cropping intensity and rice productivity in tropical riparian wetland. Use of drought tolerant rice genotype might be a suitable option for overcoming such problem. This study focused on the effects of gradual soil drying during early vegetative growth stage on morphological and physiological traits offivegenotypes, namely RAM12, RAM14, RAM59, RAM97 and RAM101, and twosubspgenotypes, i.e. Koshihikari and Minamihatamochi. The plants were subjected to 6 d of gradual soil drying condition from 15 days after transplanting (DAT) to 20 DAT, and were allowed to recover until 22 DAT. Gradual soil drying reduced plant growth as indicated by dry mass accumulation. Drought reduced stomatal conductance and increased leaf rolling score of all the genotypes. All the genotypes showed comparable response on stomatal conductance, butgenotypes performed higher in leaf rolling recovery. Meanwhile,genotypes decreased total leaf area and specific leaf area, but increased specific leaf weight in order to avoid further damages due to drought stress. Drought tolerance mechanisms in RAM101, RAM12, RAM59 and RAM14 were associated with leaf morpho-physiological responses, root traits and dry biomass accumulation.
drought tolerance; leaf rolling; root trait; stomatal conductance; dry matter accumulation
The African species of rice (Steud.) was cultivated long before the Europeans arrived in the continent. However, since yield ofis generally low, this native species has been replaced by introduced Asian species ofL. Nevertheless,is considered as a rich source of genes for tolerance to various biotic and abiotic stresses (Sikirou et al, 2018).has ability to grow in a wide range of harsh environment such as dry rainfed hilly areas, deep flooding conditions and saline coastal areas (Sarla and Swamy, 2005).hasdeveloped adaptive and protective mechanisms for biotic and abiotic stresses caused by weed competition, pest and disease attacks, drought, submergence, acidic and saline soils, Al and Fe toxicity (Sakagami et al, 2009; Rodenburg et al, 2009; Djedatin et al, 2011; Agnoun et al, 2012; Ndjiondjop et al, 2012;Zhang and Wing, 2013).
Among abiotic stress, drought has frequently been reported to cause significant decrease in rice productivity. Drought stress in plantsis characterized by the continuous water loss through transpiration of restricted water uptake due to decrease in soil moisture (Koffler et al, 2014).The earliest leaf physiological responses to drought are the progressive partial closure of stomata which directly restrains leaf-atmosphere gas exchange and decreases the ratio of CO2to O2(Guo et al, 2015). On the other hand, plants have evolved various mechanisms to alleviate water deficit in drought condition, including morphological, physiological, biochemical, cellular and molecular levels (Fang and Xiong, 2015).
Drought stress before or during tillering reduces number of tillers and panicles per hill (Bouman and Toung, 2001). Furthermore, drought stress at the vegetative stage significantly reduces total biomass due to the decrease of photosynthetic rate and dry matter accumulation (Sarvestani et al, 2008). Pantuwan et al (2002) reported that grain yields of some rice varieties are reduced by up to 81% under drought conditions.
Rice varieties response to drought have been extensively studied and many rice lines have been evaluated, but identifying new tolerant lines is still an enormous challenge due to the complexity and the specificity over various environments (Ndjiondjop et al, 2012). At present, farmers in Indonesia, especially at tropical riparian wetland, only cultivate rice once annually (Kartika et al, 2018a, b). Besides prolonged flooding during rainy season, another constraint for higher cropping index is soil drought condition during dry season (Lakitan et al, 2018a, b). The drought stress may occur during reproductive growth stage of the first rice growing season. Most of local farmers hesitate to grow the second rice since they immediately encounter continuous drying soil condition. The currently cultivated varieties are not tolerant to such conditions at tropical riparian in Indonesia.
The objectives of this research wereto highlight how gradual soil drying affects rice growth at early vegetative stage and to evaluate potential of somegenotypes to be selected as donor candidate in rice breeding for developing cultivars tolerant to gradual soil drying conditions.
Materials and Methods
Rice materials
Five genotypes of(RAM12, RAM14, RAM59, RAM97 and RAM101) and two of(a popular lowland rice variety Koshihikari and a drought tolerant upland rice variety Minamihatamochi in Japan)were used. The seeds of Riz Africain du Mali (RAM) series were originated from accessions collected along Niger River in Mali, West Africa and provided by the Institute of Rural Economy of Mali, West Africa.The parent plant is characterized by short ligule (3–4 mm), truncate and membranous. The inflorescence is a terminal, ellipsoid, stiff and compact panicle which is erect at maturity with ascendant racemose branches. Spikelets are ellipsoid and seedsare a laterally compressed caryopsis (grain) and tightly enveloped by lemma and palea. The materials were grown in a growth chamber at the laboratory of Tropical Crop Science at Kagoshima University in Japan.
Growing condition
Seeds were germinated in a nursery bed in an incubator for 3 d with controlled temperature at 28 ºC. Seedlings produced were transferred into a growth chamber for 2 weeks, before being transplanted to boxes (25cm × 37cm × 14cm). Spacing amongst seedlings were 2.7cm × 4.5 cm. Light intensity in the growth chamber was maintained within a range of 200–300μmol/(m2·s) for 12 h daily at center of chamber. Internal air temperature was kept at 28 ºC and relative humidity at 70%–90%.
The experiment was arranged in a split plot design with two soil conditions as the main plots and seven genotypes as the subplots. The 14 treatmentcombinations were replicated three times. Each pot was filled with the same amount of soil to provide standardized soil humidity. Andosol soil type was air dried and weighed evenly. Soil moisture was monitored using Data Logger Em50 Series (Decagon Devices Inc., Pullman, USA). For the control, soil moisture was maintained at 15%–20% and gradual soil drying treatment started at 15 d after transplanting (DAT), commenced at 16% soil moisture and then let gradually dry to 5% soil moisture at 20 DAT. After termination of soil drying treatment, the soil was re-watered to 20% soil moisture for allowing rice plant to recover until 22 DAT (Fig. 1).
Sampling and measurement
The youngest fully expanded leaf was selected for measurement. Leaf chlorophyll content was proxied by the soil and plant analyzer development (SPAD) value (Konica-Minolta, Chlorophyll Meter SPAD-502 Plus) at 15 DAT (start of treatment), 20 DAT (end of treatment) and 22 DAT (after 2 d of recovery). The leaf stomatal conductance (gs) was measured on two plants of each treatment using an AP4 Leaf Porometer (Delta-T Devices,Cambridge, UK). Leaf rolling score (LRS) was indicated from 0 to 9 for drought stress during vegetative stage as described in the Standard Evaluation System for Rice (IRRI, 2002). LRS and gswere determined three times per day (08:00–11:00 AM; 13:00–16:00 PM and 19:00–22:00 PM).
At 23 DAT, two plants were sampled from each treatment and separated into root, stem (culm+sheath) and leaf fractions. Shoot dry weight was obtained by adding leaf dry weight with stem dry weight. The sum of shoot and root dry weight was calculated as the total dry weight. Root morphological parameters were measured using an image analysis system (Regent Instruments Inc., WinRHIZO) to assess the length, surface area and volume. Roots were divided into two types according to the root diameter, i.e. fine (≤0.5 mm) and coarse roots (> 0.5 mm) (Huang et al, 2015). Leaf surface area was calculated using the digital image analysis software (LIA32, developed by Kazukiyo Yamamoto, Nagoya University, Japan). Total leaf surface area (TLA) was measured on the fresh samples, while specific leaf area (SLA) and specific leaf weight (SLW) were calculated after drying. SLA was the ratio of TLA to dry mass, while SLW was the ratio of dry mass to TLA. Per each sampled plant, roots, stems and leaves were oven-dried at 70 ºC for 2 d to determine their dry weights.
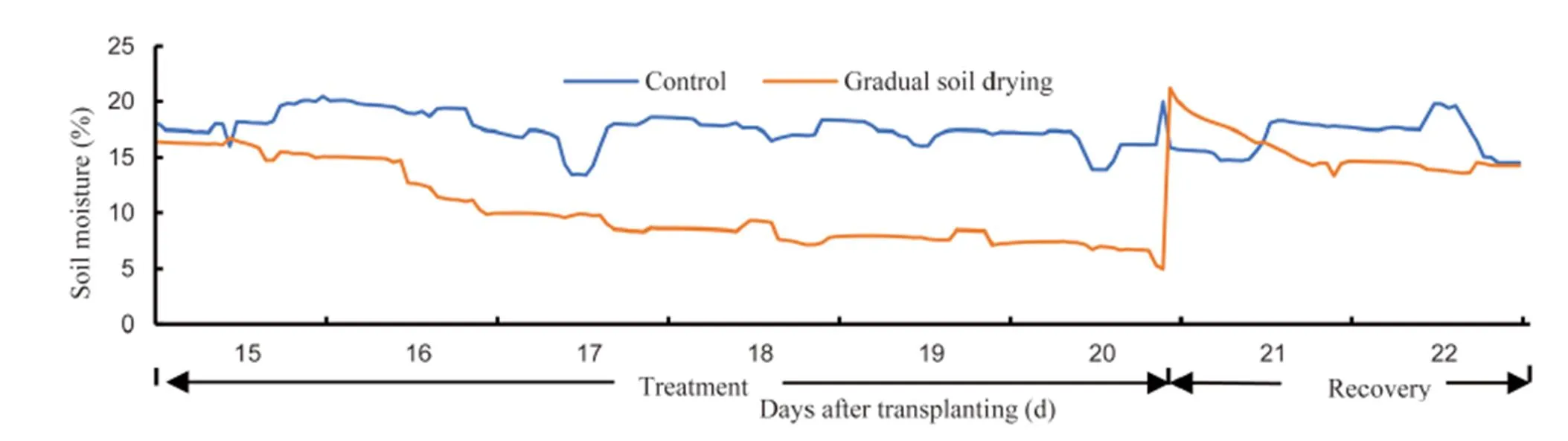
Fig. 1. Soil moisture records during experiment.
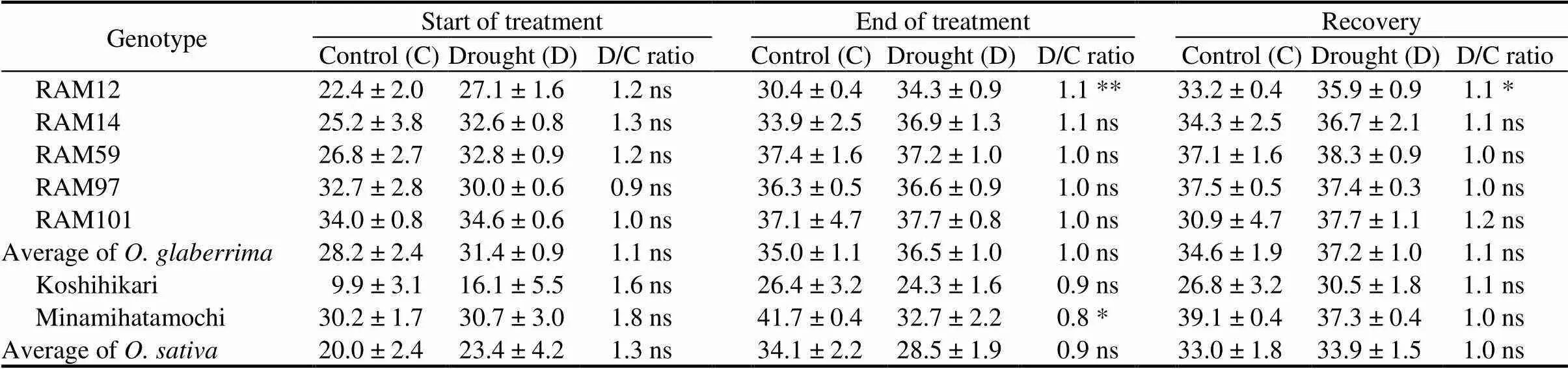
Table 1. Effects of gradual soil drying on soil and plant analyzer development (SPAD) value of seven rice genotypes at different measurement times.
D/C ratio, Ratio between drought-treated and untreated plants.
Data are Mean ±SD (= 3).Means of control and drought plants of each genotype were compared by the Student’s-test (*,< 0.05; **,< 0.01; ns,Non-significant).
Statistical analysis
Data were subjected to the statistical analysis software (SAS 9.0 for Windows). Significant differences between drought and control conditions of each variable were analyzed by the Student’s-test in the R software.< 0.05 was considered to indicate statistical significance. Dry matter accumulation was subjected to the JMP Statistical Discovery 13.0.0 for hierarchical cluster analysis.
Results
Leaf morphological and physiological traits
To explore morphological and physiological responses of rice leaves under gradual soil drying exposure and during recovery period, leaf chlorophyll content, TLA, SLA, SLW, gsand LRS were determined on each sampled plant. There were differences in SPAD values at different measurement times, soil conditions and among genotypes (Table 1). In general, the SPAD values at the beginning of treatment were lower than those at the end of treatment and the recovery period. Drought stress caused no significant SPAD value reduction forgenotypes, but there was a sharp reduction in, especially for Minamihatamochi. It is interesting to note that RAM12 was able to increase the SPAD value in limited water availability. However, all genotypes can increase the SPAD values after recovery period.
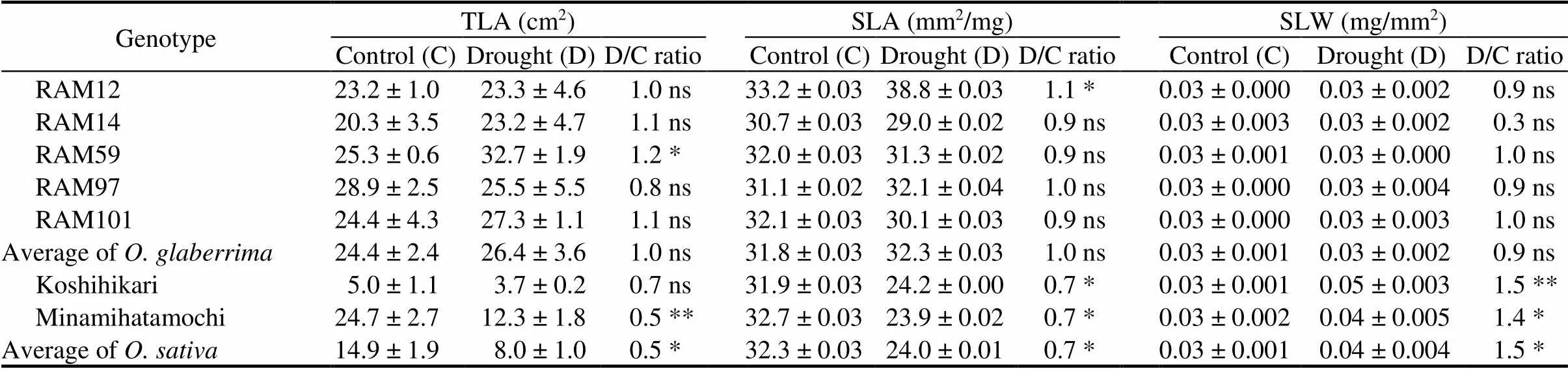
Table 2. Effects of gradual soil drying on total leaf area (TLA), specific leaf area(SLA), and specific leaf weight(SLW) of seven rice genotypes after recovery.
D/C ratio, Ratio between drought-treated and untreated plants.
Data are Mean ±SD (= 3).Means of control and drought plants of each genotype were compared by the Student’s-test (*,< 0.05; **,< 0.01; ns,Non-significant).
Different subspecies showed different responses in leaf growth under the gradual soil drying condition. After drought stress and 2 d recovery, TLA of RAM59 significantly increased while significant decrease in TLA was found in Minamihatamochi.genotypes maintained similar SLA and SLW with an exception of RAM12 that performed significant increment of SLA. In contrary,genotypes reduced SLA and increased SLW (Table 2).
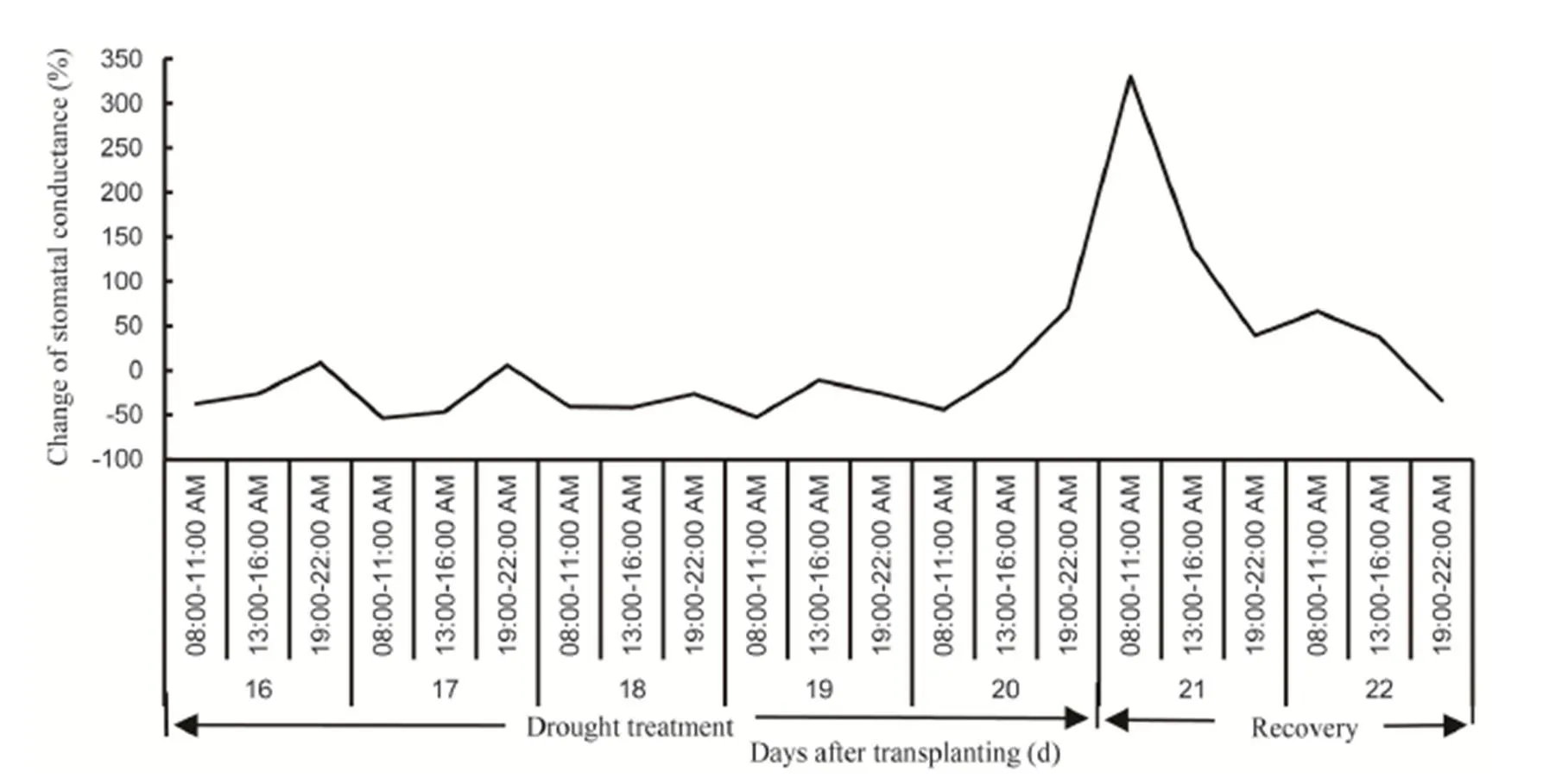
Fig. 2. Stomatal conductance reduction during gradual soil drying conditions and its increment at recovery stage compared to control.
Stomatal conductance mostly decreased in rice plants exposed to the gradual soil drying conditions and the differences with the control plants were more pronounced (up to 50%) at 08:00–11:00 AM than those at 13:00–16:00 PM or 19:00–22:00 PM. Conversely, gssharply increased soon after re-irrigation (Fig. 2). All genotypes showed similar responds to gradual decrease in soil moisture. Within the range of soil moisture during gradual soil drying from 6% to 16%, gsexhibited faster decrease at higher soil moisture range (from 12% to 16%) than at lower range (from 6% to 10%). At pre-stress condition (soil moisture at around 16%), gsofgenotypes was visibly higher than that ofgenotypes, but as soil moisture declining to 12% or lower, gsofandgenotypes was not visibly different (Fig. 3).
The symptom of leaf rolling was not detected in the control plants during the experiment. Generally, rice leaf started to roll at 12% soil moisture (Fig. 4). Leaves of all the genotypes begun to roll at 13:00–16:00 PM of the first day of treatment (16 DAT) but some genotypes reflated their leaves at the following night. Leaf rolling was observed in all the rice genotypes exposed to drought and reached the highest scores at the end of the 4-day drought stress treatment. However,genotypes exhibited significantly higher LRS thangenotypes. Based on individual genotype, the highest score during treatment was observedin Minamihatamochi and the lowest was in RAM59 and RAM97. After the treatment was terminated,genotypes rapidly approached normal LRS (2), butgenotypes were unable to match the score after 2 d of recovery (Fig. 5).
Root structural traits
Drought stress at the early vegetative stage decreased root growth rate and reduced root dry weight.Total root length, surface area and volume were significantly lower in plants experiencing drought stress compared to the control ones (Table 3). Regardless of soil conditions and plant genotypes, the length of fine roots was 2 to 6 times higher than those of coarse roots. Drought stress caused sharp decrease in coarse root length for all the genotypes with an exception in RAM12, which was slightly increased at dry conditions. A significant root length reduction was found in the fine root, especially in RAM97 and Koshihikari. Interestingly, Minamihatamochi was the only genotype that was able to increase fine root length under limited water availability. Root surface area and root volume of all the genotypes were significantly lower at drought conditions than the control. Over all, root growth of Koshihikari was the lowest at both soil water conditions.
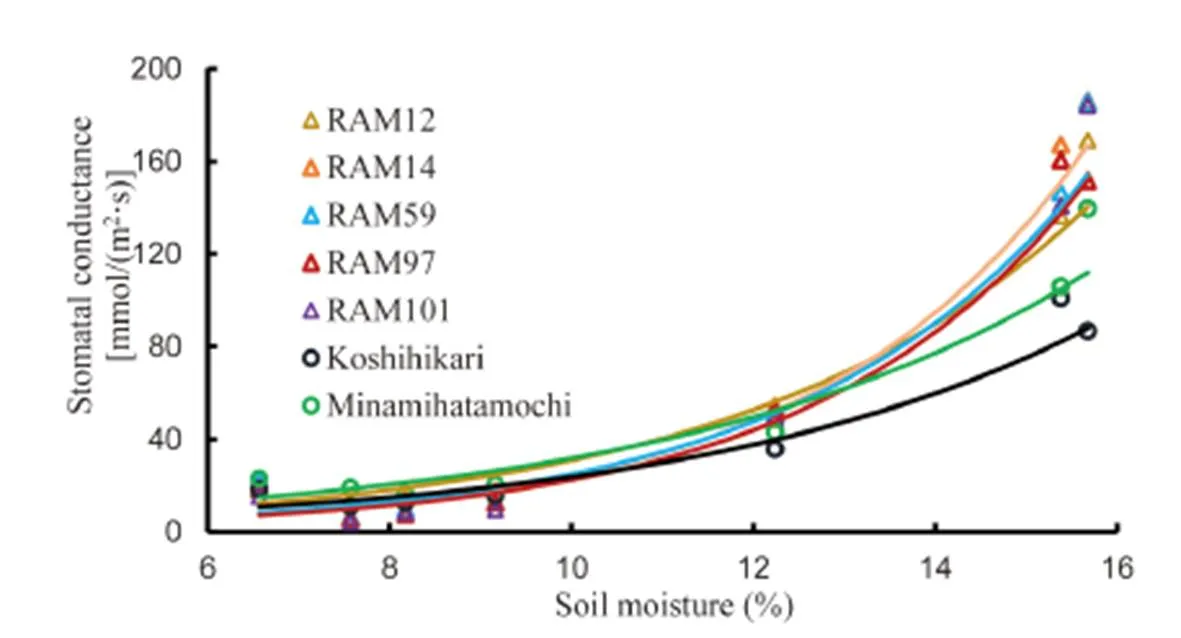
Fig. 3. Trend of relationship between soil moisture content and stomatal conductance in seven rice genotypes.
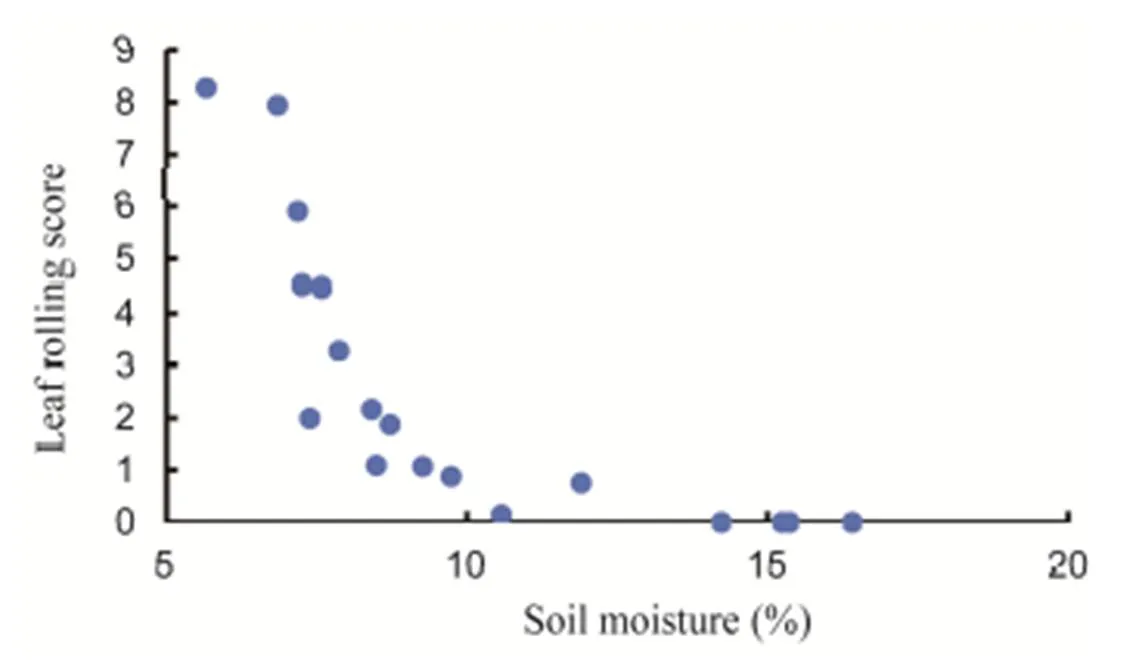
Fig. 4. Trend of relationship between soil moisture content and leaf rolling score in seven rice genotypes.
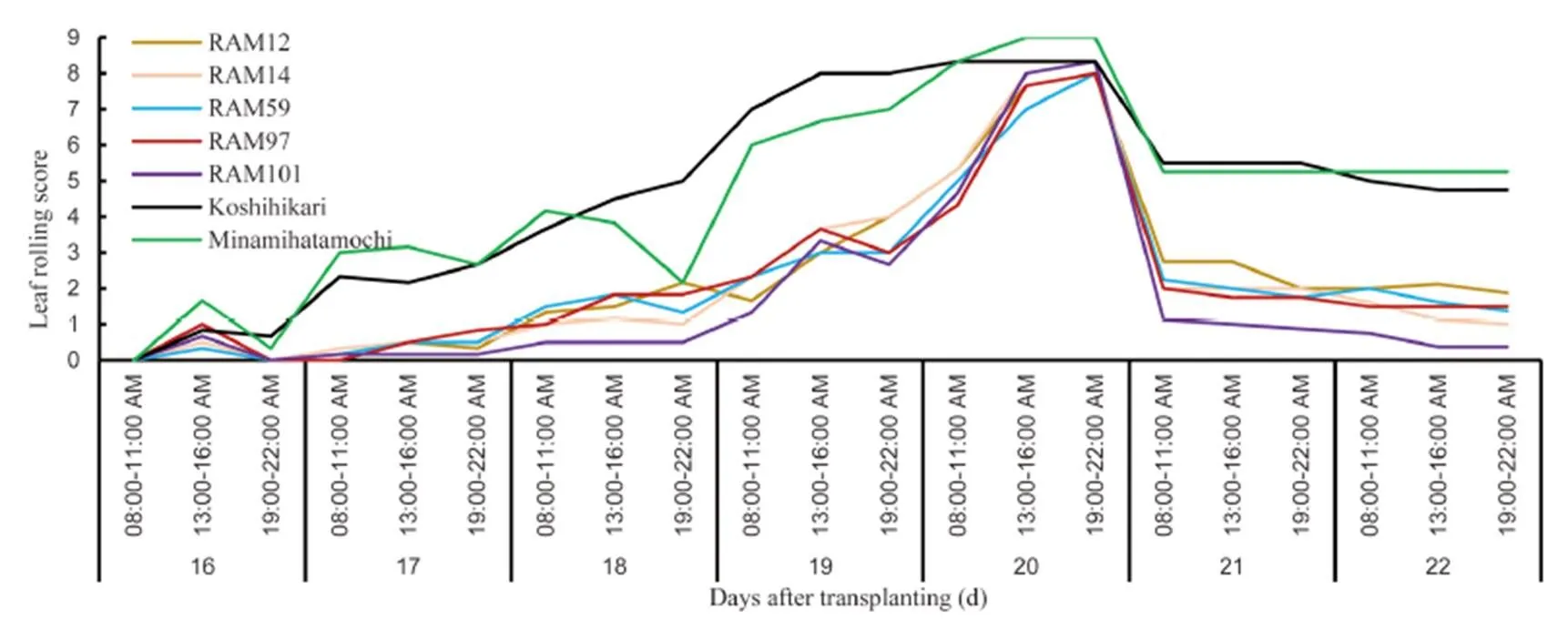
Fig. 5. Leaf rolling score of seven rice genotypes at gradual soil drying and recovery conditions.
Plant dry matter accumulation
Plant dry weight was destructively measured after 2 d of recovery. As affected by genotype, RAM97 had the highest dry matter and Koshihikari had the lowest. In contrary to,genotypes tended to maintain the total dry weight at drought conditions. The highest total dry matter accumulation was produced by RAM59,which was highly contributed by significant increment of shoot as well as the root growth under drought stress conditions. Both Koshihikari and Minamihatamochi showed prodigious decrement of total dry weight (Table 4).
Classification of rice genotypes for drought tolerance
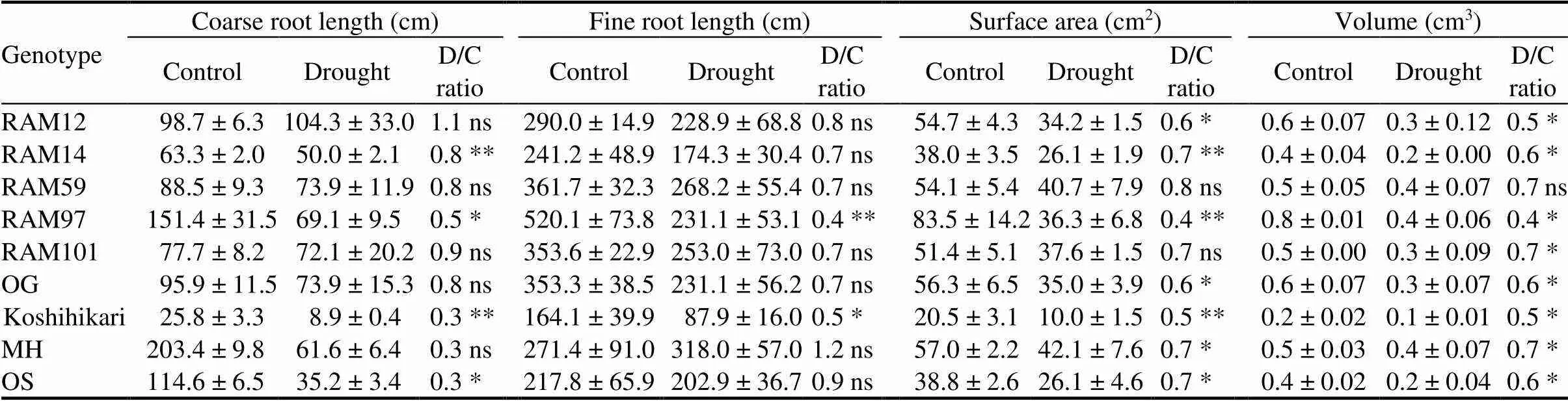
Table 3. Effects of gradual soil drying on length, surface area and volume of rootsin seven rice genotypes.
MH, Minamihatamochi; OG, Mean of; OS, Mean of. D/C ratio, Ratio between drought-treated and control plants.
Coarse root and fine root refer to the roots with diameter > 0.5 mm and0.5 mm, respectively.
Data are Mean ±SD (= 3). Means of control and drought plants of each genotype were compared by the Student’s-test (*< 0.05; **< 0.01; ns,Non-significant).
The seven rice genotypes were classified into four main clusters based onthe hierarchy cluster analysis(Katsura et al, 2016). Cluster I represented the drought-tolerant group. Three genotypes ofincluding RAM12, RAM14 and RAM101 occupied this cluster. RAM59 identified in Cluster II and was considered as moderately tolerant. Cluster III represented sensitive group which consisted of RAM97 and Minamihatamochi. Koshihikari was classified to Cluster IV, which was denoted to highly sensitive genotype. It is interesting to note that clusters I and II were obtained by all thegenotypes. Minamihatamochi is an upland genotype, which is certainly drought tolerance genotype, whileshowed sensitivity on short-term gradual soil drying condition during the vegetative stage (Fig. 6).
Discussion
Drought stress reduces cell water potential and turgor which elevate the concentration of solute in extracellular matrices and cytosol (Lisar et al, 2012). This condition leads to cell enlargement reduction followed by slowing down or stopping growth and reproductive failure. Many studies reported that drought stress affects elongation and expansion growth, and prevent cell enlargement more than its division (Jaleel et al, 2009). However, a slower growth rate during short-term gradual soil drying caused no significant reduction in total dry mass since plants were still able to proceed their metabolism normally during recovery. The capability to maintain plant fresh and dry weight under water limited conditions is desirable characters for drought tolerant. Moreover, a slight decrement growth during vegetative stage is acceptable as long as it does not cause yield reduction (Lakitan et al, 2018a).
Leaves react extremely sensitively to a gradual change of soil moisture. Leaf rolling and stomatal partial closure are the basic mechanisms for reducing damage from water deficit at the vegetative stage (Heinemann et al, 2011). Leaf rolling is the first visual symptom of drought stress to reduce the leaf surface exposure to atmosphere and decrease transpiration (Allah, 2009). The effect of leaf rolling on water vapor varies, it depends on distribution of stomata and the degree and pattern of stomatal opening in rolled leaves (Kadioglu and Terzi, 2007). In our study, leaf rolling may enable partial stomatal conductance under water deficit. This condition allowed plants to alter the microclimate surrounding the leaf by maintaining internal water status and retain photosynthesis and growth. Leaf rolling factor has been used as one of the best parameters for estimating levels of drought tolerance in a large-scale screening (Pandey and Shukla, 2015). In this study,genotypes especially RAM101 expressed the least and slowestleaf rolling with better capability to recover thangenotypes (Fig. 2).
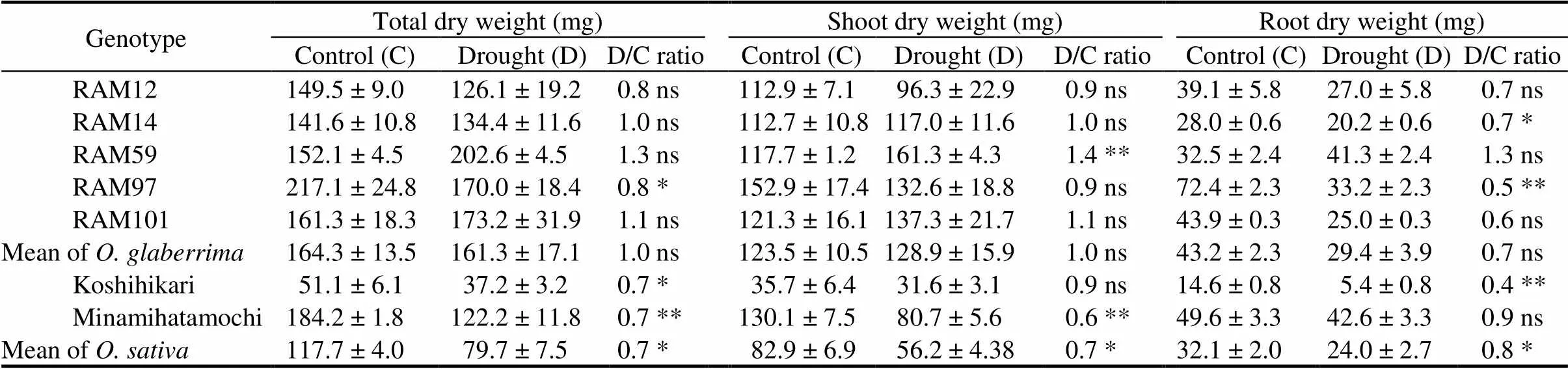
Table 4. Effects of gradual soil drying on dry weight of seven rice genotypes.
D/C ratio, Ratio between drought-treated and untreated plants.
Data are Mean ±SD (= 3). Means of control and drought plants of each genotype were compared by the Student’s-test (*< 0.05, **< 0.01; ns,Non-significant).
Leaf-atmosphere CO2and H2O exchanges are regulated by stomata. Rice utilizing C3 photosynthetic mechanism that has been reported to be more sensitive to drought compared to those involved in C4 and crassulacean acid metabolism (CAM) (Pandey and Shukla, 2015). C3 plants open their stomata at daylight periods for CO2uptake and fixation then close their stomata at night. This mechanism deficient for rice to survive in water deficit condition. Drought stress caused significant decrease ingsof all the rice genotypes used in this study. Stomatal closure related to the changes of leaf water potential and turgor movement in guard cells, with a consequent of reduction in CO2uptake and photosynthetic rate (Fang and Xiong, 2015).
The change of gsvaries with leaf age, previous exposure to stress and environmental conditions (Gimenez et al, 2005). In the response to drought stress, gscan be influenced by leaf anatomical traits, including stomatal density and size. Drought-tolerant types such ashad smaller size of stomata and lower stomatal density (both adaxial and abaxial surfaces) than lowland rice, making it capable to have a faster response to water deficit environment (Ouyang et al, 2017). The results showed that stomatal responses were closely linked to soil moisture (Fig. 4). Dingkuhn et al (1999) found similar results inaccession CG14 that gsis controlled by a soil-moisture-dependent root signal. This suggests that stomata are responding to chemical signals produced by dehydrating roots. Previous study reported that the increase of abscisic acid concentration plays important roles in stomatal closure (Chaves et al, 2002).Beside stomatal closure, plant photosynthesis under water deficit is also affected by changes in photosynthesizing pigments and poor assimilation rates. Reduced photosynthetic metabolites and enzyme activities, low carboxylation efficiency and inhibition of chloroplast activity are co-factors of poor assimilation rate in drought stress (Lisar et al, 2012).
genotypes significantly decreased the leaf area and conversely increased SLW in gradual soil drying conditions (Table 2). The observed SLA reduction and SLW increment indicated that leaves are thicker or have more densely packed mesophyll cells with less intracellular air space (Timung et al, 2017). A reduced SLA most likely reflects that the leaves are conforming their morphology and preventing further damage (Wellstein et al, 2017). This mechanism is profitable for plants to use less water, but generally the plant will become less productive (Fahad et al, 2017). The reduction of leaf area might be due to rapid decline in cell division and leaf elongation. Significant reduction (up to 82%)in total leaf area was previously reported in chili pepper after 12 d of drought stress (Widuri et al, 2017).
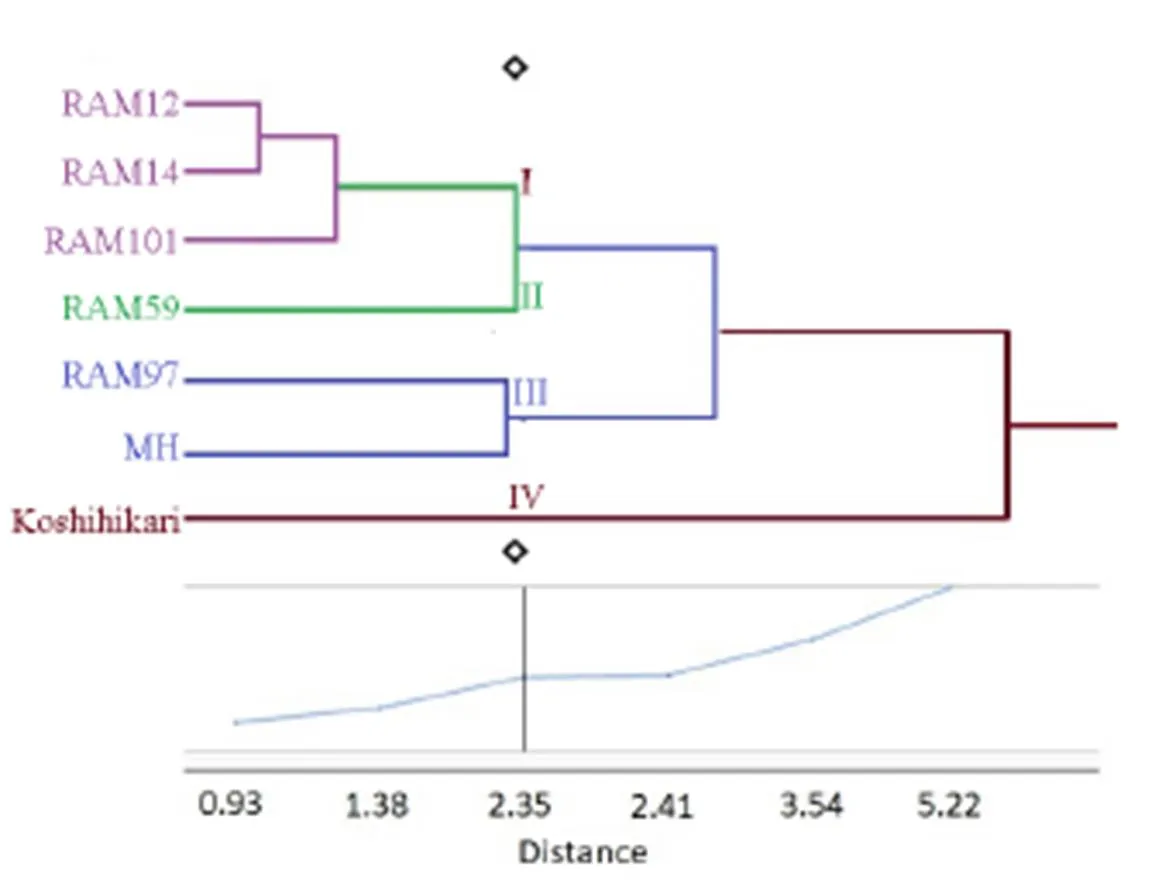
Fig.6. Hierarchical cluster analysis of the seven rice genotypes based on SPAD value, dry matter accumulation, leaf and root traits in gradual soil drying conditions.
Roots are main components of plant adaptation under drought condition. Plants with drought avoidance are characterized by deep root, plenty coarse roots with high branching and penetration ability (Pandey and Shukla, 2015). This type of roots may improve acquisition of water when ample water at deeper soil layer is available. Siaga et al (2018) emphasized that heavily branching roots with high surface area in limited rhizosphere are needed to maximize water uptake. In our study, under drought stress conditions, bothandhad higher small root diameter than the control. Kijoji et al (2013) also reported that drought tolerant rice variety has smaller and denser root, which plays a major role in improving water uptake from deeper soil layer and thus contributing to drought avoidance. Fine root as branched from coarse root also increases hydraulic conductance of plant by increasing surface area and volume of root contact with soil water, increasing root hydraulic conductivity by reducing apoplastic barrier of water absorbsion (Comas et al, 2013).Therefore, root dry weight and length are good predictors of rice yield under drought stress (Feng et al, 2012). In our study, RAM12 and RAM59 most likely to have the characteristics of avoidance water stress ability.
In this study, bothandwere able to recover stomatal conductance but onlygenotypes showed rapid recovery from leaf rolling. Seven rice genotypes were classified into four clusters defining the plants tolerant in drought stress. Three genotypes ofincluding RAM12, RAM14 and RAM101were strongly tolerant and RAM59 was considered as moderately tolerant. Sakagami et al (2013) emphasized thatgenotypes are superior in severely stressful environments. Those genotypes can serve as potential donors for rice breeding for developing rice tolerance to gradual soil drying condition. Further research need to be devoted togenotypes to exploit their potential to drought tolerance.
Acknowledgements
This study was funded by the Program of the United Graduate School of Agricultural Sciences, Kagoshima University, supported by Program Penelitian Unggulan Profesi Universitas Sriwijaya (Grant No. 0006/UN9/SK.LP2M.PT/2018), Program PMDSU (Grant No. 093/SP2H/LT/DRPM/IV/2018) and Enhancing International Publication Program by Directorate of Higher Education of the Ministry of Research, Technology and Higher Education of the Republic of Indonesia.
Agnoun Y, Biaou S S H, Sié M, Vodouhè R S, Ahanchédé A. 2012. The African riceSteud: Knowledge distribution and prospects., 4(3): 158–180.
Allah A A A. 2009.Genetic studies on leaf rolling and some root traits under drought conditions in rice (L.)., 8(22):6241–6248.
Bouman B A M, Tuong T P. 2001. Field water management to save water and increase its productivity in irrigated lowland rice., 49: 11–30.
Chaves M M, Pereira J S, Maroco J, Rodrigues M L, Ricardo C P P, Osório M L, Carvalho I, Faria T, Pinheiro C. 2002. How plants cope with water stress in the field? Photosynthesis and growth., 89(7): 907–916.
Comas L H, Becker S R, Cruz V M, Byrne P F, Dierig D A. 2013. Root traits contributing to plant productivity under drought., 4: 442.
Dingkuhn M, Audebert A Y, Jones M P, Etienne K, Sow A. 1999. Control of stomatal conductance and leaf rolling inandupland rice., 61(3):223–236.
Djedatin G, Ndjiondjop MN, Mathieu T, Cruz CM V, Sanni A, Ghesquière A, Verdier V. 2011. Evaluation of African cultivated ricefor resistance to bacterial blight., 95(4): 441–447.
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D P, Huang J L. 2017. Crop production under drought and heat stress: Plant responses and management options., 8: 1–16.
Fang Y J, Xiong L Z. 2015. General mechanisms of drought response and their application in drought resistance improvement in plants., 72(4): 673–689.
Feng F J, Xu X Y, Du X B, Tong H H, Luo L J, Mei H W. 2012. Assessment of drought resistance among wild rice accessions using a protocol based on single-tiller propagation and PVC-tube cultivation., 6(7): 1204–1211.
Gimenez C, Gallardo M, Thompson R B. 2005. Plant-water relations.: Hillel D. Encyclopedia of Soils in the Environment.Oxford: Elsevier:231–238.
Guo Y Y, Yu H Y, Kong D S, Yan F, Liu D H, Zhang Y J. 2015. Effects of gradual soil drought stress on the growth, biomass partitioning, and chlorophyll fluorescence ofseedlings., 39(4): 532–539.
Heinemann A B, Stone L F, Fageria N K. 2011. Transpiration rate response to water deficit during vegetative and reproductive phases of upland rice cultivars., 68(1): 24–30.
Huang M, Chen J N, Cao F B, Jiang L G, Zou Y B. 2015. Root morphology was improved in a late-stage vigor super rice cultivar., 10(11): e0142977.
International Rice Research Institute (IRRI). 2002. Standard Evaluation System for Rice. Los Banos, the Philippines: IRRI.
Jaleel C A, Manivannan P, Wahid A, Farooq M, Al-Juburi H J, Somasundaram R, Panneerselvam R. 2009. Drought stress in plants: A review on morphological characteristics and pigments composition., 11(1): 100–105.
Kadioglu A, Terzi R. 2007. A dehydration avoidance mechanism: Leaf rolling., 73(4): 290–302.
Kartika K, Lakitan B, Sanjaya N, Wijaya A, Kadir S, Kurnianingsih A, Widuri L I, Siaga E, Meihana M. 2018a. Internal versus edge row comparison in jajar legowo 4:1 rice planting pattern at different frequency of fertilizer applications., 40(2): 222–232.
Kartika K, Lakitan B, Wijaya A, Kadir S, Widuri L I, Siaga E, Meihana M. 2018b. Effects of particle size and application rate of rice-husk biochar on chemical properties of tropical wetland soil, rice growth and yield., 12(5): 817–826.
Katsura K, Tsujimoto Y, Oda M, Matsushima KI, Inusah B, Dogbe W, Sakagami JI. 2016. Genotype-by-environment interaction analysis of rice (spp.) yield in a floodplain ecosystem in West Africa., 73:152–159.
Kijoji A A, Nchimbi-Msolla S, Kanyeka Z L, Klassen S P, Serraj R, Henry A. 2013. Water extraction and root traits in×introgression lines under different soil moisture regimes., 40(1):54–66.
Koffler B E, Luschin-Ebengreuth N, Stabentheiner E, Müller M, Zechmann B. 2014. Compartment specific response of antioxidants to drought stress in., 227:133–144.
Lakitan B, Alberto A, Lindiana L, Kartika K, Herlinda S, Kurnianingsih A. 2018a. The benefits of bichar on growth and yield in tropical riparian wetland, South Sumatera, Indonesia., 17(2): 111–126.
Lakitan B, Hadi B, Herlinda S, Siaga E, Widuri LI, Kartika K, Lindiana L, Yunindyawati Y, Meihana M. 2018b. Recognizing farmers’ practices and constraints for intensifying rice production at riparian wetlands in Indonesia.,84: 10–20.
Lisar S Y S, Motafakkerazad R, Hossain M M, Rahman I M M. 2012. Water Stress in Plants: Causes, Effects and Responses. Rijeka, Croatia: InTech:1–14.
Ndjiondjop M N, Seck P A, Lorieux M, Futakuchi K, Yao K N, Djedatin G, Sow M E, Bocco R, Cisse F, Fatondji B. 2012. Effect of drought onrice accessions andderived-lines., 6(4):144–157.
Ouyang W J, Struik P C, Yin X Y, Yang J C. 2017. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought., 68(18):5191–5205.
Pandey V, Shukla A. 2015. Acclimation and tolerance strategies of rice under drought stress., 22(4): 147–161.
Pantuwan G, Fukai S, Cooper M, Rajatasereekul S, O’Toole JC. 2002. Yield response of rice (L.) genotypes to drought under rainfed lowlands: 2. Selection of drought resistant genotypes., 73:169–180.
Rodenburg J, Saito K, Kakaï RG, Touré A, Mariko M, Kiepe P. 2009. Weed competitiveness of the lowland rice varieties of NERICA in the southern Guinea Savanna., 114(3): 411–418.
Sakagami J I, Joho Y, Ito O. 2009. Contrasting physiological responses by cultivars ofandto prolonged submergence., 103(2): 171–180.
Sakagami J I, Joho Y, Sone C. 2013. Complete submergence escape with shoot elongation ability by underwater photosynthesis in African rice,Steud., 152:17–26.
Sarla N, Swamy B P M. 2005.: A source for the improvement of., 89(6): 955–963.
Sarvestani Z T, Pirdashti H, Sanavy S A M M, Balouchi H. 2008. Study of water stress effects in different growth stages on yield and yield components of different rice (L.) cultivars., 11(10): 1303–1309.
Siaga E, Lakitan B, Hasbi, Bernas S M, Wijaya A, Lisda R, Ramadhani F, Widuri L I, Kartika K, Meihana M. 2018. Application of floating culture system in chili pepper (L.) during prolonged flooding period at riparian wetland in Indonesia., 12(5): 808–816.
Sikirou M, Shittu A, Konaté K A, Maji A T, Ngaujah A S, Sanni K A, Ogunbayo S A, Akintayo I, Saito K, Dramé K N, Ahanchédé A, Venuprasad R. 2018. Screening African rice () for tolerance to abiotic stresses: I. Fe toxicity., 220:3–9.
Timung B, Bharali B, Konwar M J. 2017. Physiological parameters of some upland rice (L.) genotypes under moisture stress condition., 6(6): 1636–1640.
Wellstein C, Poschlod P, Gohlke A, Chelli S, Campetella G, Rosbakh S, Canullo R, Kreyling J, Jentsch A, Beierkuhnlein C. 2017. Effects of extreme drought on specific leaf area of grassland species: A meta-analysis of experimental studies in temperate and sub-Mediterranean systems., 23(6): 2473–2481.
Widuri L I, Lakitan B, Hasmeda M, Sodikin E, Wijaya A, Meihana M, Kartika K, Siaga E. 2017. Relative leaf expansion rate and other leaf-related indicators for detection of drought stress in chili pepper (L.)., 11(12):1617–1625.
Zhang Q F, Wing R A. 2013. Genetics and genomics of rice., 5: 9–25.
Copyright © 2020, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.12.007
16 July 2018;
4 December 2018
s:Jun-Ichi Sakagami (sakagami@agri.kagoshima-u.ac.jp);Benyamin Lakitan (blakitan60@unsri.ac.id)
(Managing Editor: Li Guan)
杂志排行
Rice Science的其它文章
- Cellular Localization of Rice SUMO/SUMO Conjugates and in vitroSumoylation Using Rice Components
- Decrement of Sugar Consumption in Rice Young Panicle Under High Temperature Aggravates Spikelet Number Reduction
- Drought Stress Impairs Grain Yield and Quality of Rice Genotypes by Impaired Photosynthetic Attributes and K Nutrition
- Differential Expression of Rice Valine-Qlutamine Gene Family in Response to Nitric Oxide and Regulatory Circuit of OsVQ7 and OsWRKY24
- Systematic Characterization of Long Non-Coding RNAs and Their Responses to Drought Stress in Dongxiang Wild Rice
- AssessmentofVariationinMorpho-PhysiologicalTraitsandGeneticDiversityin Relation to Submergence Tolerance of Five Indigenous LowlandRice Landraces
