Cellular Localization of Rice SUMO/SUMO Conjugates and in vitroSumoylation Using Rice Components
2020-12-28JoungsuJOO,DongHeeCHOI,SangHyonKIM等
Letter
Cellular Localization of Rice SUMO/SUMO Conjugates andSumoylation Using Rice Components
Many proteins are regulated by post-translational modifications, such as the reversible covalent attachment of ubiquitin and ubiquitin-like proteins in eukaryotes (Kerscher et al, 2006). Post-translational modification of proteins by the SUMO protein family is involved in diverse cellular processes, including development, hormonal responses, and biotic and abiotic stress signaling (Park et al, 2011). SUMO modification can modulate protein-protein interactions, intracellular localization or the activities of the protein (Gareau and Lima, 2010). Sumoylation occurs through a series of biochemical steps similar to ubiquitylation (Kerscher et al, 2006). The SUMO conjugation to substrates occurs through sequential enzyme action, including SUMO activating enzyme (E1), SUMO conjugating enzyme (E2) and SUMO ligase (E3). Many SUMO-modified proteins identified contain ψKX(D/E) consensus motifs (ψ, Large hydrophobic amino acid; K, Acceptor lysine; X, Any amino acid; D/E, aspartate or glutamate) (Rodriguez et al, 2001).
In many eukaryotes, SUMO is encoded by a single-copy gene (Flotho and Melchior, 2013). In contrast, mammals and plants encode multi-copy SUMO genes. Functionally, the mammalian SUMO2/3 can form SUMO chains, whereas SUMO1 cannot (Hay, 2013). SUMO1 and SUMO2/3 also interact with different proteins noncovalently (Hecker et al, 2006). The mammalian SUMO2 is essential for embryonic development, whereas SUMO3 is dispensable. The functional difference between SUMO2 and SUMO3 appears to be caused by differences in their expression levels, with SUMO2 being the predominant transcript (Wang et al, 2014). Rice encodes four SUMO genes, but their functional differences are not clear. In this study, we have characterized the rice OsSUMO1, OsSUMO2, OsSUMO3and OsSUMO4, and analyzed their expression patterns and subcellular localizations.
To identify rice SUMO orthologs, we performed blast searches againstdatabases of the National Center for BiotechnologyInformation usingSUMOs (Novatchkova et al, 2004) as a query. Four rice SUMO orthologs wereidentified with significant sequence identity to AtSUMOs. OsSUMO1 (LOC_4324360), OsSUMO2 (LOC_4324359), OsSUMO3 (LOC_4343692) and OsSUMO4 (LOC_107276773) shared 89%, 86%, 41% and46% amino acid sequence identity with AtSUMO1, respectively. OsSUMO2, OsSUMO3 and OsSUMO4 shared 89%, 43% and 48% of amino acid sequence identity with OsSUMO1, respectively. OsSUMO3 and OsSUMO4 shared 77% of amino acid sequence identity (Fig. 1-A).
We identified the consensus SUMO attachment site (ψKxE) (Melchior, 2000) within the N-terminal extensions of OsSUMO3 and OsSUMO4, VKVE sequence (Fig. 1-A). This suggested that both OsSUMO3 and OsSUMO4 can form poly-SUMOchains, using the lysine as the connection site. AtSUMO4 and AtSUMO6 also contained the consensus SUMO attachment site (VKME) within the N-terminal extensions (Kurepa et al, 2003). However, it is not clear whether AtSUMO4 and AtSUMO6are active members of the SUMO family (Lois et al, 2003). Interestingly,fourloci (andon chromosome 1,andon chromosome 7) are arranged as tandempairs in the genome without other genes between them such as(,,andon chromosome 5) (Fig. 1-B). These results suggested that the diversification of rice SUMO genes is driven by tandem duplications which is similar in(Hammoudi et al, 2016). A phylogenetic analysis showed that the four riceSUMO proteins clustered into two sub-families: OsSUMO1/2 and OsSUMO3/4 (Fig. 1-C). The results of amino acid sequence, gene structure and phylogenetic analyses suggested that gene duplications drive diversification of SUMO genes in rice.
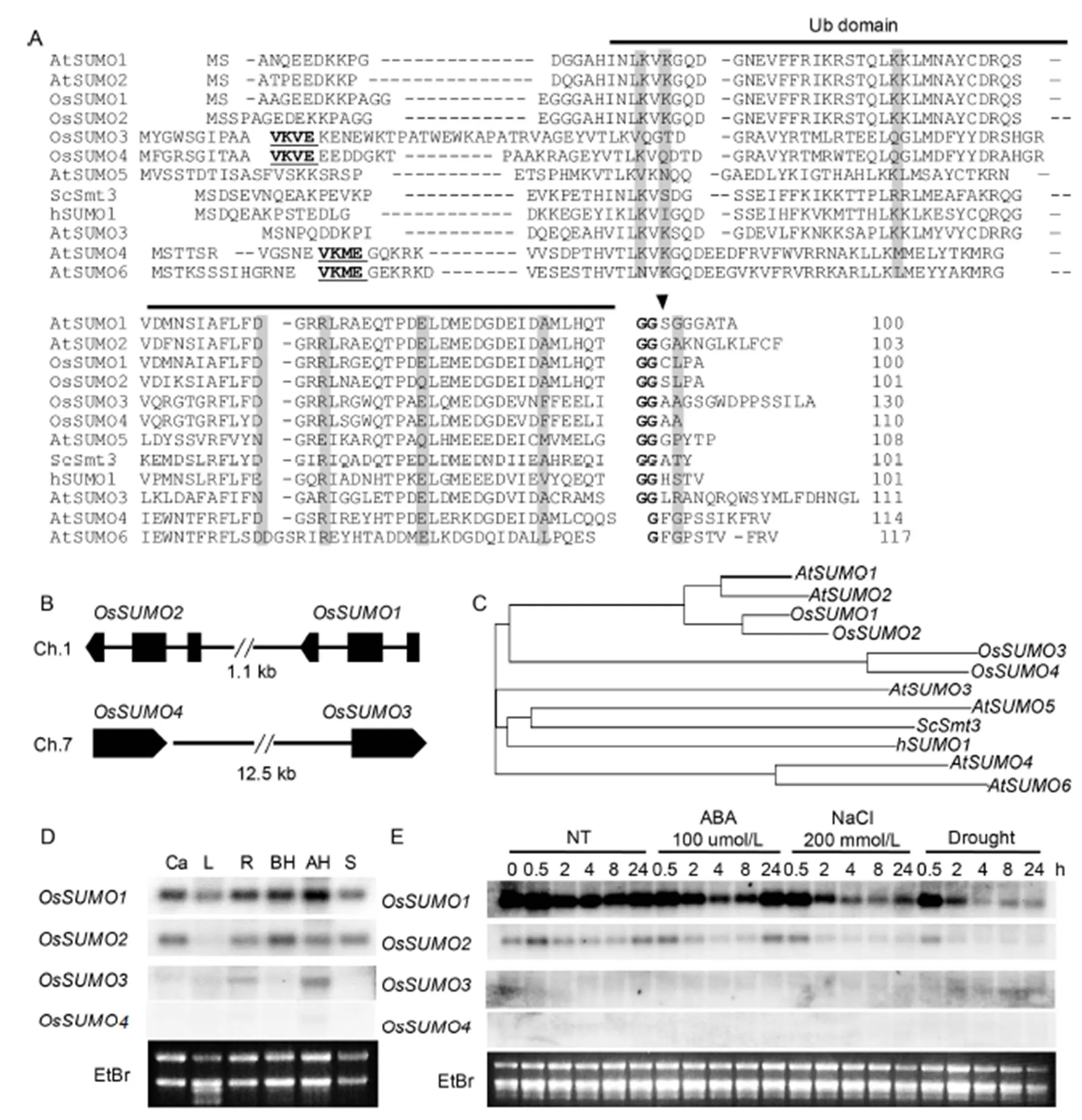
Fig. 1. Four SUMO members (OsSUMO1, OsSUMO2, OsSUMO3 and OsSUMO4) in rice.
To investigate the expression of rice SUMO genes in various organs, an RNA gel blot analysis was performed (Fig. 1-D). Theandtranscripts were detected in all the tested organs. However, the expression level ofwas higher than that of thegene. This result indicated that the genes have functions throughout the whole plant body. Thetranscripts were detected only in root and panicle tissues. The transcript ofwas not detected by the RNA gel blot analysis. We detected transcripts,andcDNA in the riceexpressed sequence tag databases, but not incDNA. These results suggested that thegene is expressed too minimally to be detected by an RNA gel blot analysis or may not be expressed.
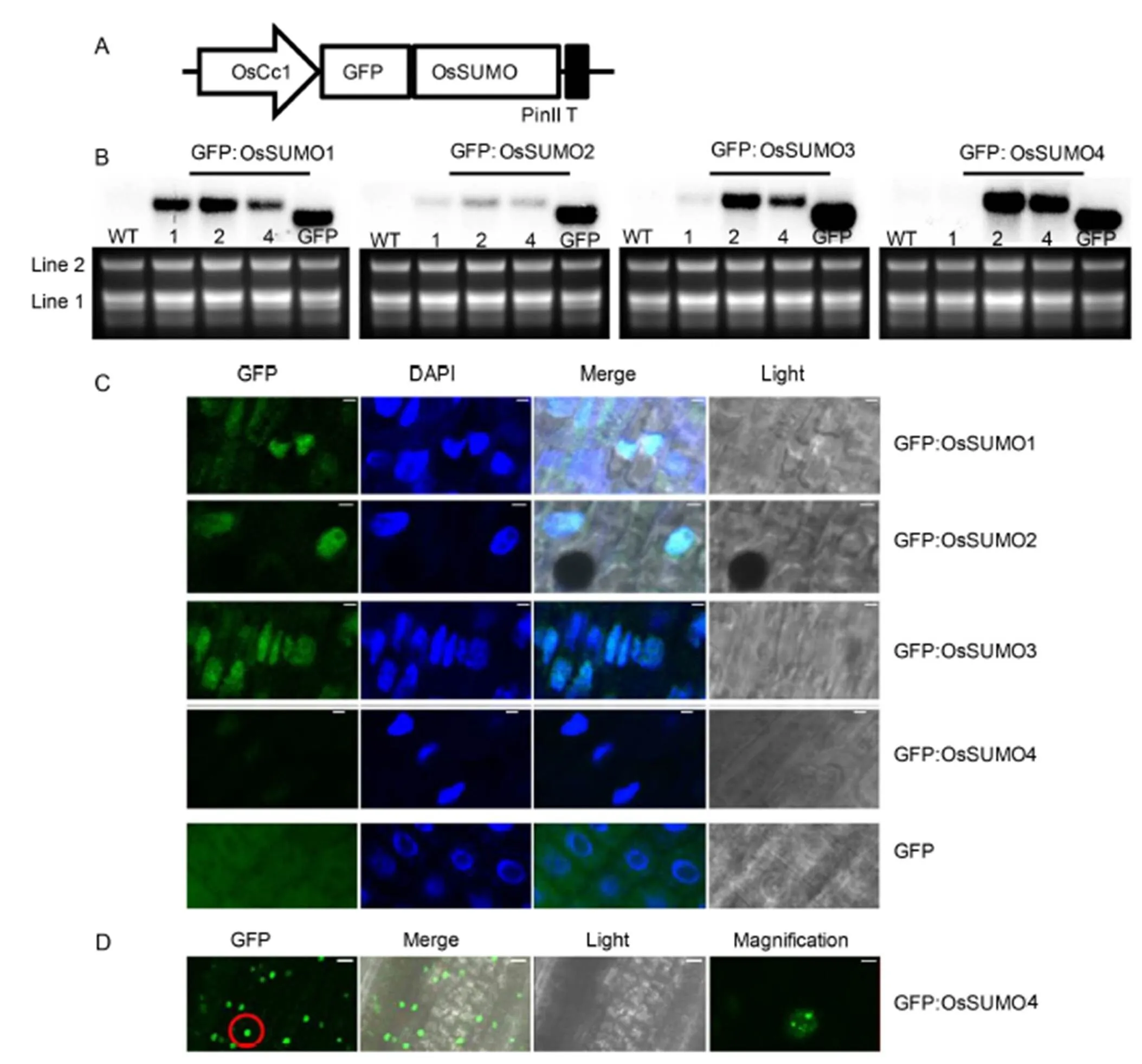
Fig. 2. Subcellular localization analysis of GFP:OsSUMO fusion proteins.
The plant sumoylation system has been reported to be involved in abscisic acid (ABA) signaling and abiotic stress response. The expression levels of OsSUMO genes were analyzed in response to ABA and stress conditions in rice (Fig. 1-E). The expression levels ofandwere not detected.andgene expression levels decreased in response to ABA and stress treatment. Interestingly, thetranscript was very rapidly decreased by drought and high-salinity treatments.
To characterize the localization of OsSUMOs in rice cells, green fluorescent protein (GFP) was fused in the frame of translation to the OsSUMO’s N-terminus (Fig. 2-A). Subsequently, the GFP:OsSUMO fusion proteins were expressed in the rice plants. The ectopic expression of the transgenes in transgenic rice plants was confirmed using RNA gel blot analysis (Fig. 2-B). Line 2 of each construct that showed the highest expression level was subjected to further analysis. The epidermal cells of root tissues were analyzed using confocal laser scanning microscopy (Fig. 2-C). The GFP:OsSUMO1, GFP:OsSUMO2 and GFP:OsSUMO3 fusion proteins were detected in root tissues. All of these proteins were localized mainly in the nucleus. In contrast, the negative control GFP protein was evenly distributed in all areas of the cells. The GFP:OsSUMO4 fusion protein was not detected in root tissue, whereas it was detected in leaf tissue (Fig. 2-C and -D). The GFP:OsSUMO4 fusion protein was localized mainly in the nucleus. Interestingly, the GFP:OsSUMO4 fusion protein localized to specific compartments of the nucleus. Even though the GFP protein alone does not have a specific distribution in the cells (Fig. 2-C), the GFP fused with SUMO could affect protein sumoylation, cellular localization or interaction with other proteins. Thus, the results only represent the fused proteins. The levels of SUMO conjugation are increased by abiotic stress. It suggested that sumoylation plays a role in stress protection. Furthermore, sumoylation of transcription factorslocalizes into nuclear bodies (Geiss-Friedlander and Melchior, 2007), and SUMO E3 ligase (SIZ1) is localized to nuclear loci (Miura et al, 2005). Thus,conjugates facilitate recruitment into nuclear bodies in the leaves.
To performsumoylation, several recombinant plasmids were constructed: Glutathione S-transferase (GST)-taggedand histidine-tagged other sumoylation components (Fig. 3-A). All the tagged recombinant proteins were expressed instrain BL21. After 1.0 mmol/L isopropyl β-D-1-thiogalactopyranoside(IPTG) induction, GST- and histidine-tagged proteins were purified using nickel and glutathione resins, respectively. In thesumoylation experiments, putative substrate OsRanGAP1 was incubated with purified OsSUMO1, E1 (OsSAE1-OsSAE2), E2 (OsSCE2a) and E3 (OsSIZ1) together with adenosine triphosphate (ATP). Sumoylation of OsRanGAP1 substrate was examined by Western blotting using anti-His antibody (Fig. 3-B). Although sumoylated OsRanGAP1 was not detected in low concentration of His-OsRanGAP1, it was detected in five and ten times concentration conditions. In addition, the reduction of non-conjugated GST-OsSUMO1 was detected using anti-GST antibody. Many SUMO-modified proteins identified contain ψKX(D/E) consensus motifs. The predicted amino acid sequence of OsRanGAP1 contained two putative sumoylation sites LKDD (425–428) and LKVE (539–542). Two bands of sumoylated OsRanGAP1 proteins were detected as expected. The lower band may be single sumoylated and upper band double sumoylated OsRanGAP1 protein.
Yeast and invertebrates have a single SUMO encoding gene, whereas vertebrate and plant genomes have several SUMO isoforms (Gareau and Lima, 2010; Novatchkova et al, 2012). Rice encodes four SUMO genes, and gene duplications drive diversification of SUMO genes in rice. However, the function of SUMO isoforms is not clear. In humans, the functional difference between SUMO isoforms appears to be caused by differences in their expression levels (Wang et al, 2014). Therefore, expression and cellular localization analyses of rice SUMO isoforms are important in determining the functional differences between SUMO isoforms. In conclusion, the genome of rice encodes four SUMO genes that represent two distinct types,/and/. The transcript level of thegene is higher than that ofgene. Their expression levels were repressed through ABA and stress treatments in leaves. The transcript level of thegene was very low, and the transcript level of thegene was not detected by our assay system. The GFP:OsSUMO1, GFP:OsSUMO2and GFP:OsSUMO3 fusion proteins were localized mainly in the nucleus in roots. In contrast, the GFP:OsSUMO4 fusion protein was not detected in root tissue, whereas it was expressed and localized to specific compartments of the nucleus in leaves.
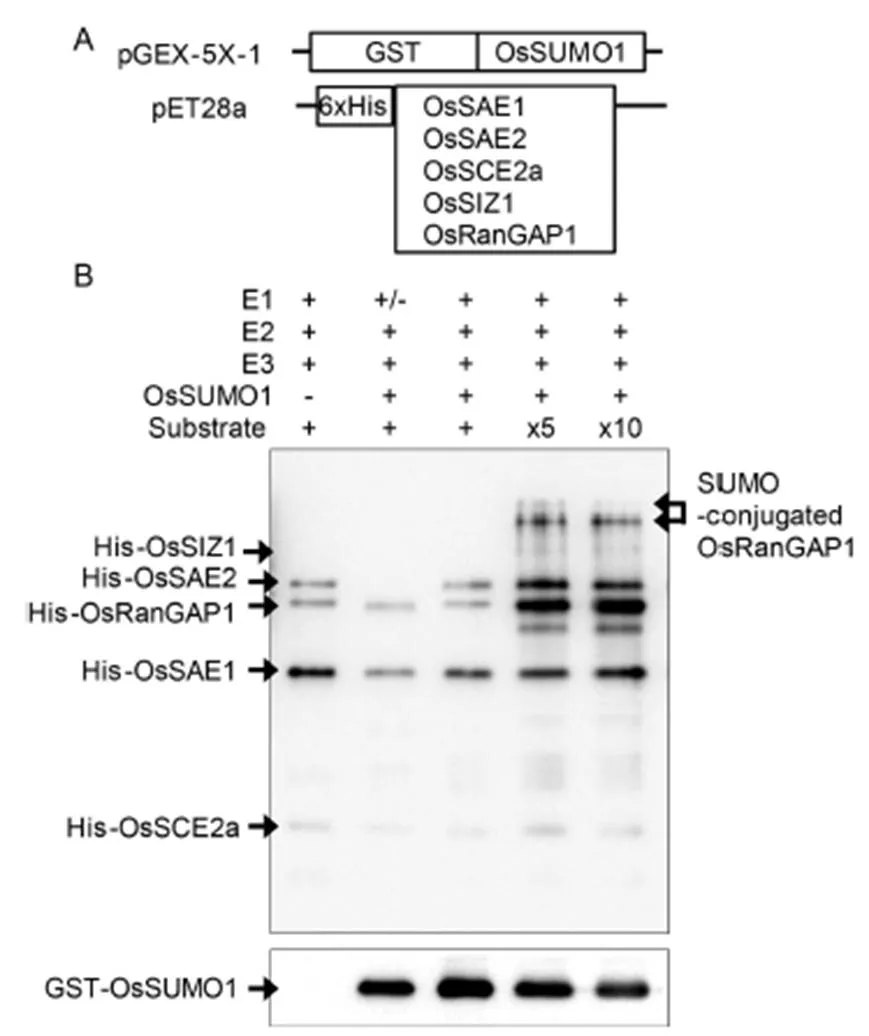
Fig. 3. in vitro sumoylation assays.
ACKNOWLEDGEMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (Grant No. 2017R1D1A1B03030725).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/journal/ 16726308; http://www.ricescience.org.
Supplemental Table 1. List of gene-specific primers used in this study.
Supplemental File 1. Materials and methods used in this study.
Flotho A, Melchior F. 2013. Sumoylation: A regulatory protein modification in health and disease., 82:357–385.
Gareau J R, Lima C D. 2010. The SUMO pathway: Emerging mechanisms that shape specificity, conjugation and recognition., 11(12):861–871.
Geiss-Friedlander R, Melchior F. 2007. Concepts in sumoylation: A decade on., 8:947–956.
Hammoudi V, Vlachakis G, Schranz M E, van den Burg H A. 2016. Whole-genome duplications followed by tandem duplications drive diversification of the protein modifier SUMO in angiosperms., 211(1):172–185.
Hay R T. 2013. Decoding the SUMO signal., 41:463–473.
Hecker C M, Rabiller M, Haglund K, Bayer P, Dikic I. 2006 Specification of SUMO1- and SUMO2-interacting motifs., 281:16117–16127.
Kerscher O, Felberbaum R, Hochstrasser M. 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins., 22:159–180.
Kurepa J, Walker J M, Smalle J, Gosink M M, Davis S J, Durham T L, Sung D Y, Vierstra R D. 2003. The small ubiquitin-like modifier (SUMO) protein modification system in: Accumulation of SUMO-1 and -2 conjugates is increased by stress., 278(9):6862–6872.
Lois L M, Lima C D, Chua N H. 2003. Small ubiquitin-like modifier modulates abscisic acid signaling in., 15(6):1347–1359.
Melchior F. 2000. SUMO-nonclassical ubiquitin., 16:591–626.
Miura K, Rus A, Sharkhuu A, Yokoi S, Karthikeyan A S, Raghothama K G, Baek D, Koo Y D, Jin J B, Bressan RA, Yun D J, Hasegawa P M. 2005. TheSUMO E3 ligase SIZ1 controls phosphate deficiency responses., 102:7760–7765.
Novatchkova M, Budhiraja R, Coupland G, Eisenhaber F, Bachmair A. 2004. SUMO conjugation in plants., 220(1):1–8.
Novatchkova M, Tomanov K, Hofmann K, Stuible H P, Bachmair A. 2012. Update on sumoylation: Defining core components of the plant SUMO conjugation system by phylogenetic comparison., 195(1):23–31.
Park H J, Kim W Y, Park H C, Lee S Y, Bohnert H J, Yun D J. 2011. SUMO and SUMOylation in plants., 32(4):305–316.
Rodriguez M S, Dargemont C, Hay R T. 2001. SUMO-1 conjugationrequires both a consensus modification motif and nuclear targeting., 276:12654–12659.
Wang L L, Wansleeben C, Zhao S L, Miao P, Paschen W, Yang W. 2014. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development., 15(8):878–885.
Joungsu Joo, Dong Hee Choi, SangHyonKim, Sang Ik Song
()
Copyright © 2020, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.02.003
Sang Ik Song (sisong@mju.ac.kr)
28 September 2018;
20 February 2019
杂志排行
Rice Science的其它文章
- Decrement of Sugar Consumption in Rice Young Panicle Under High Temperature Aggravates Spikelet Number Reduction
- Morpho-Physiological Response of Oryza glaberrima to Gradual Soil Drying
- Drought Stress Impairs Grain Yield and Quality of Rice Genotypes by Impaired Photosynthetic Attributes and K Nutrition
- Differential Expression of Rice Valine-Qlutamine Gene Family in Response to Nitric Oxide and Regulatory Circuit of OsVQ7 and OsWRKY24
- Systematic Characterization of Long Non-Coding RNAs and Their Responses to Drought Stress in Dongxiang Wild Rice
- AssessmentofVariationinMorpho-PhysiologicalTraitsandGeneticDiversityin Relation to Submergence Tolerance of Five Indigenous LowlandRice Landraces
