热结合Nisin处理对枯草杆菌芽孢的杀灭效果
2020-12-25郭家俊尚彬玲
陈 乐,章 中,郭家俊,申 瑾,陈 翔,尚彬玲
热结合Nisin处理对枯草杆菌芽孢的杀灭效果
陈 乐,章 中※,郭家俊,申 瑾,陈 翔,尚彬玲
(宁夏大学食品与葡萄酒学院,银川 750021)
芽孢是最难被杀灭的微生物,会造成食品腐败和食物中毒。食品工业上常采用100 ℃以上的高温来杀灭食品中的芽孢,但高温热处理会大大影响食品的营养和感官品质。为找到在较低温度下杀灭芽孢的方法,该研究采用5个不同处理的芽孢悬浮液(单独80 ℃热处理、100 mg/L Nisin处理、500 mg/L Nisin处理、80 ℃结合100 mg/L Nisin处理、80 ℃结合500 mg/L Nisin处理)对芽孢的杀灭效果,研究并探讨了杀菌机理。采用平板计数法、荧光偏振法、分光光度法和流式细胞术对Nisin协同较低温度的热处理后枯草芽孢杆菌芽孢的存活率、内膜流动性、吸光度值及内膜通透性进行了研究。研究发现:单独80 ℃热处理和单独使用Nisin均无法杀灭芽孢,但是80 ℃热处理与Nisin结合时能够对芽孢产生杀灭作用。80 ℃结合500 mg/L Nisin处理20 min后,芽孢存活浓度下降约1.4 lg(CFU/mL)。80 ℃结合500 mg/L Nisin处理20 min后,芽孢悬浮液荧光偏振度显著降低(< 0.05),表明芽孢内膜流动性大幅增加;在此处理条件下芽孢的内容物释放程度最大,直观表现为吸光度值显著降低(< 0.05)。80 ℃结合Nisin处理后,芽孢内膜通透性显著增加(< 0.05),并且Nisin浓度越高,芽孢内膜通透性越强。试验结果表明:80 ℃结合不同浓度Nisin处理能提高芽孢内膜流动性和通透性,能有效杀灭细菌芽孢。Nisin能降低细菌芽孢耐热性,有利于减少热杀菌处理对食品的负面影响。
耐热性;杀菌;内膜;膜流动性;膜通透性;乳酸链球菌素;芽孢
0 引 言
芽孢是一些细菌在极端恶劣的环境下形成的休眠体,具有极强的抗逆性,可以存活数年甚至数百万年[1-4]。食品杀菌过程难以彻底将芽孢杀灭,导致食品腐败及食物中毒的事件时常发生[5-7]。芽孢含水率极低,高度脱水及芽孢内膜的极端不通透性使其具有极强抗逆性[8-11]。研究表明,水分子透过芽孢内膜,与芽孢内核水合是杀灭芽孢的重要途径之一[12-15]。水分子的跨膜运输方式主要是自由扩散,芽孢内膜的流动性及通透性直接影响水分子通过内膜的难易程度,因此研究其内膜流动性及通透性十分重要[16]。
磷脂是构成芽孢内膜的主要成分,不同条件下磷脂存在不同的状态。研究表明[17-18]温度升高能够加强磷脂分子的侧向热运动,改变磷脂间氢键的作用,同时使磷脂的相态发生转变,宏观表现为膜流动性及通透性的增强,因此温度是影响芽孢内膜流动性及通透性的重要原因之一。
乳酸链球菌素(Nisin)是一种有效的天然生物防腐剂,是一种天然生物活性抗菌肽,也被称为尼生素或乳链菌肽,具有较强的热稳定性和耐酸性。中国已允许Nisin作为食品防腐剂在多种食品中使用,根据GB2760《食品添加剂使用卫生标准》对食品添加剂的要求,Nisin在食品中的最大添加量为500 mg/L。Nisin主要作用于细胞膜的表面,造成细菌膜的损伤[19-21]。Marta等[22]研究表明Nisin能够抑制牛奶中梭状芽胞杆菌营养细胞和芽孢的生长。通常温度70~80 ℃就能够杀灭食品中的细菌营养体,但是细菌芽孢能够耐受更高的温度[23]。本文将热处理与Nisin结合,采用平板计数法、荧光偏振法、分光光度法和流式细胞术测定了芽孢的存活率、荧光偏振度、吸光度值及内膜通透性,研究了Nisin协同较低温度的热处理对枯草杆菌芽孢的杀灭效果并探讨了杀菌机理。本文通过研究芽孢的内膜变化,以期降低杀灭芽孢所需的温度,增强杀灭芽孢的效果,为食品杀菌提供一定的理论依据并有一定应用价值。
1 材料与方法
1.1 材料与试剂
枯草芽孢杆菌(),中国普通微生物菌种保藏管理中心(CGMCC),编号As 1.433;营养琼脂,天津市大茂化学试剂厂;硫酸锰(分析纯),天津市大茂化学试剂厂;1,6-二苯基-1,3,5-己三烯(1,6-Diphenyl-1,3,5-hexatriene,DPH),上海阿拉丁生化科技股份有限公司;Nisin(分析纯),上海阿拉丁生化科技股份有限公司;碘化丙啶(Propidium Iodide,PI),北京索莱宝科技有限公司;TSA-YE培养基,北京索莱宝科技有限公司。
1.2 仪器与设备
DSX-280B型高压灭菌锅,上海申安医疗器械厂;LRH系列生化培养箱,上海一恒科学仪器有限公司;722型可见分光光度计,上海驰唐电子有限公司;TGL-10B型离心机,上海安亭科学仪器厂;电子恒温不锈钢水浴锅,上海宜昌仪器纱筛厂;F-7000荧光分光光度计,日本日立有限公司;CyFlow Cube 8流式细胞仪,日本SYSMEX(希森美康)株式会社。
1.3 方 法
1.3.1 培养基的配制
促芽孢生长锰盐营养琼脂培养基:取普通营养琼脂培养基33 g加入硫酸锰0.153 8 g,蒸馏水1 000 mL,pH值为7.0,加热煮沸3次,分装试管内,121 ℃灭菌15 min,待用。
1.3.2 枯草芽孢杆菌芽孢的培养及菌悬液制备
枯草芽孢杆菌(As 1.433)菌种在营养琼脂培养基活化后划线接种到试管斜面促芽孢生长培养基上,在37 ℃培养7 d,洗涤离心3次(9 000 r/min,15 min),菌悬液浓度大约调整在1.5×109CFU/mL左右,在4 ℃保存[24]。
1.3.3 DPH(1,6-Diphenyl-1,3,5-hexatriene)配制
以四氢呋喃作溶剂,配制浓度为2×10-3mol/L的DPH储备液,4 ℃密闭保存在棕色瓶中,使用前用0.02 mol/L 磷酸盐缓冲液(pH值为6.8)将储备液稀释至所需要的浓度[25]。
1.3.4 DPH标记芽孢内膜
DPH标记芽孢内膜参考钱静亚等[25]的方法并进行了优化。取8 mL芽孢悬浮液,离心(4℃,9 000 r/min,15 min),用PBS洗涤1次后离心(4 ℃,9 000 r/min,15 min)弃上清,加入8 mL浓度为3×10-6mol/L的DPH溶液,在50 ℃下孵育30 min;再用PBS洗涤2次,离心后菌体悬浮在适量的PBS缓冲液中,使菌体浓度在1×106~1×107CFU/mL。
1.3.5 芽孢悬浮液的热结合Nisin处理
取标记后的芽孢悬浮液,离心去上清液后,分别加入100和500 mg/L Nisin溶液,在80 ℃下处理20 min。即处理组:80 ℃、100 mg/L Nisin、500 mg/L Nisin、80 ℃结合100 mg/L Nisin、80 ℃结合500 mg/L Nisin温度均为25 ℃;对照组:不加Nisin,25 ℃。
500 mg/L是国家食品添加剂标准中Nisin的最大添加量,100 mg/L是与最大添加量相比,较低的添加量。通常70~80 ℃温度就能够杀灭食品中的细菌营养体,但是对细菌芽孢产生杀灭作用就需要更高的温度,将温度与Nisin结合处理,预期在80℃条件下能够对细菌芽孢产生杀灭作用。
1.3.6 芽孢悬浮液荧光偏振度的测定
荧光偏振度的测定,参照Voss等[26]的方法,在360 nm激发波长和430 nm发射波长下,采用激发与发射缝宽10 nm,测定水平荧光强度和垂直荧光强度并计算荧光偏振度,计算公式为


式中I为起偏器和检偏器光轴同为垂直方向时测得的荧光强度,I为起偏器和检偏器光轴分别为垂直和水平方向时测得的荧光强度,为光栅校正因子,I为起偏器和检偏器光轴分别为水平和垂直方向时测得的荧光强度,I为起偏器和检偏器光轴同为水平方向时测得的荧光强度。荧光偏振度值越小,流动性越大,反之则相反。
1.3.7 枯草芽孢杆菌的平板计数
将处理后的芽孢悬浮液进行梯度稀释,吸取1 mL稀释液,用TSA-YE培养基倾注平板计数,每个平板中倒入1 mL稀释菌液和15~20 mL TSA-YE培养基,在37 ℃下培养24~48 h,进行计数并计算存活芽孢浓度,计算公式为

式中为不同处理后的菌落数。
1.3.8 芽孢悬浮液吸光度值的测定
取处理后的枯草芽孢杆菌芽孢悬浮液,在600 nm下测定吸光度值,该吸光度值常用来估计芽孢内容物的释放情况[27]。测定前将芽孢悬浮液摇晃均匀。
1.3.9 流式细胞仪检测枯草芽孢杆菌芽孢内膜通透性
取处理前后的枯草芽孢杆菌芽孢悬浮液,稀释到菌液浓度为106~107CFU/mL。使用PI染色后,用流式细胞仪检测前向散射光(Forward Scatter,FS)、侧向散射光(Side Scatter,SS)、荧光通道FL2和FL3[28]。数据采集后用FCS Express Version 3.0软件(De Novo software,Canada)分析。
1.3.10 统计分析
所有的试验至少重复3次,数据通过SPSS 17.0进行分析,以<0.05表示差异性显著,采用Origin 2018软件作图。
2 结果与分析
2.1 热结合Nisin处理对枯草芽孢杆菌芽孢的杀灭作用
如图1所示,与未经任何处理的对照相比,单独使用80 ℃热处理或Nisin处理都无法对芽孢产生杀灭作用。热结合Nisin能够杀灭芽孢,80 ℃结合100 mg/L Nisin处理20 min后,芽孢存活浓度下降了0.5 lg(CFU/mL),80 ℃结合500 mg/L Nisin处理20 min后,芽孢存活浓度下降程度最大,下降了约1.4 lg(CFU/mL),并且Nisin浓度越高,对芽孢造成的杀灭作用越强。温度能够对内膜磷脂产生一定影响,同时Nisin能够作用于芽孢内膜,热结合Nisin对芽孢内膜的影响可能存在协同作用,导致芽孢内膜通透性增加,水分子进入芽孢内部,导致其耐热性下降,从而被杀灭。因此,以下试验以芽孢内膜为研究对象,采用荧光偏振法、分光光度法及流式细胞术对芽孢悬浮液荧光偏振度、吸光度值和芽孢内膜通透性进行研究,探究芽孢内膜流动性及通透性的变化是否是导致其被杀灭的重要原因之一。
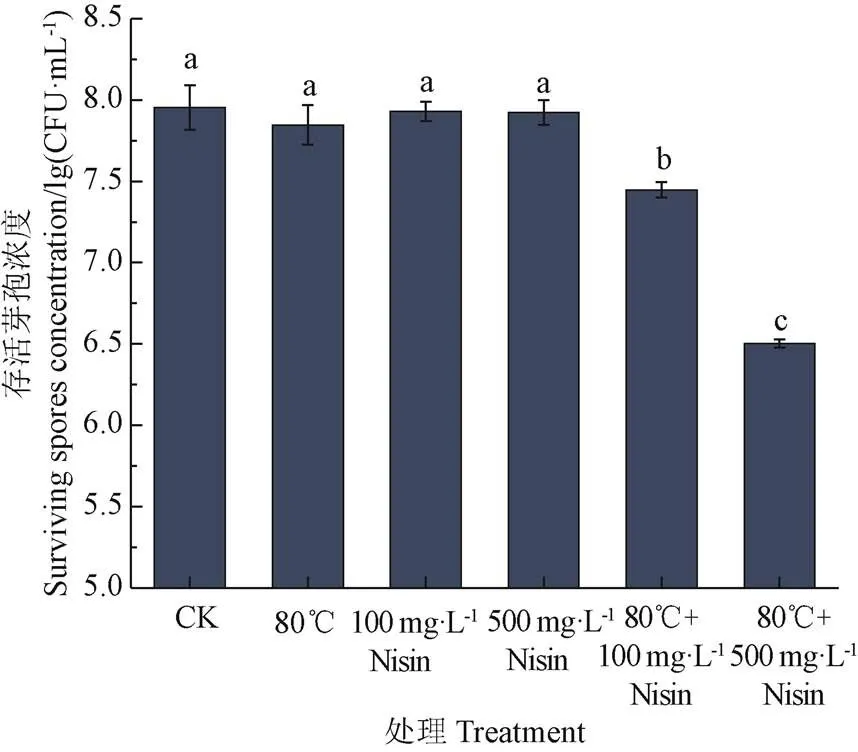
注:标有不同字母的处理之间具有显著性差异(P<0.05),下同。
2.2 热结合Nisin处理对枯草芽孢杆菌芽孢悬浮液荧光偏振度的影响
作为一种较为敏感的荧光探针,DPH常用于研究生物膜的流动性,通常标记于磷脂双分子层内,外界环境改变时,磷脂特性的改变会使荧光偏振度发生变化,宏观表现为膜流动性的改变,荧光偏振度越低,膜流动性越强[26,29-30]。热结合Nisin处理后,芽孢悬浮液荧光偏振度如图2。从图中可以看出,80 ℃热处理或Nisin处理都能够增加芽孢内膜的流动性,但80 ℃、100 mg/L Nisin和80 ℃结合100 mg/L Nisin处理间,荧光偏振度没有显著差异(>0.05)。500 mg/L Nisin处理和80 ℃结合500 mg/L Nisin处理后,荧光偏振度下降程度最大,但两者间没有显著差异(>0.05)。Nisin可直接作用芽孢内膜,影响其荧光偏振度,并且Nisin浓度越高,对芽孢悬浮液荧光偏振度影响越大,芽孢内膜流动性越强。而80 ℃的热处理对芽孢内膜的影响较小,500 mg/L浓度的Nisin对芽孢悬浮液荧光偏振度的影响远大于80 ℃热处理对芽孢悬浮液荧光偏振度的影响。
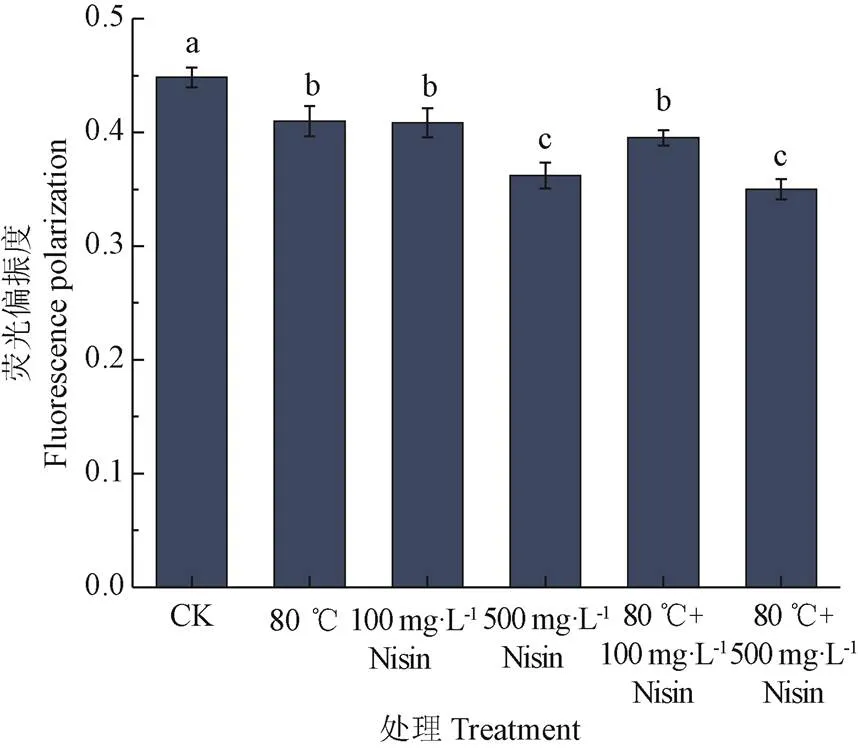
图2 热结合Nisin处理对枯草芽孢杆菌芽孢悬浮液荧光偏振度的影响
2.3 热结合Nisin处理对枯草芽孢杆菌芽孢悬浮液吸光度值的影响
芽孢内容物的释放与芽孢内膜通透性密切相关,芽孢悬浮液的吸光度值常用来估计芽孢内容物的释放情况,芽孢内容物释放程度越大,吸光度越低[27,31-32]。热结合Nisin处理后,芽孢悬浮液吸光度值变化如图3。不同处理后,芽孢悬浮液吸光度值显著降低(<0.05)。Nisin单独处理后,芽孢悬浮液吸光度值显著低于单独的80 ℃热处理(<0.05),并且Nisin浓度越高,吸光度值下降程度越大。80 ℃结合500 mg/L Nisin处理后,吸光度值下降程度最大。Nisin能够作用于芽孢内膜,在膜的表面形成通透的孔道,导致芽孢内容物的释放。

图3 热结合Nisin处理对枯草芽孢杆菌芽孢悬浮液吸光度值的影响
2.4 热结合Nisin处理后枯草芽孢杆菌芽孢内膜通透性的变化
进一步采用流式细胞术研究芽孢内膜损伤情况。当膜受到损伤时,PI能够透过受损的细胞膜进入到细胞内部,结合DNA后发出强烈的荧光。将热结合Nisin处理后的芽孢用PI染色,流式细胞术检测结果如图4,以对照芽孢为标准将流式细胞术直方图分为M1阴性和M2阳性区域,对照芽孢未经任何处理,芽孢内膜完整,无损伤情况,其DNA无法被PI染色,其荧光分布基本在M1阴性区域。经过杀菌处理后,芽孢内膜遭到破坏,PI透过受损的芽孢内膜进入芽孢内部,与芽孢DNA结合,发出强烈荧光,流式细胞术直方图表现为荧光区域分布均从M1阴性区域向M2阳性区域移动。80 ℃处理后,阳性区域占比34.78%。100 mg/L Nisin处理后,阳性区域占比35.45%,500 mg/L Nisin处理后,阳性区域占比62.74%。80 ℃结合100 mg/L Nisin处理后,阳性区域占比64.51%,80 ℃结合500 mg/L Nisin处理后,阳性区域占比81.18%。结果表明,热结合Nisin处理,会使芽孢内膜破损,增加芽孢内膜的通透性。相比热处理或Nisin单独作用,热结合Nisin处理后,芽孢内膜通透性显著增加(<0.05),Nisin浓度越高,芽孢内膜通透性越强。温度对芽孢内膜的影响及Nisin对芽孢内膜的作用可能是导致芽孢内膜通透性变化的主要原因。

注:M1表示荧光强度较低的阴性区域,M2表示荧光强度较高的阳性区域。
热结合Nisin处理后,芽孢内膜流动性、芽孢悬浮液吸光度值及芽孢流式细胞仪检测结果是一致的,均表明热结合Nisin处理下芽孢死亡与芽孢内膜通透性的变化相关。
3 结 论
1)采用不同的处理方法(单独80 ℃、100 mg/L Nisin、500 mg/L Nisin、80℃结合100 mg/L Nisin、80℃结合500 mg/L Nisin)对芽孢悬浮液进行处理,平板计数结果表明,单独使用80 ℃热处理或Nisin处理都无法对芽孢产生杀灭作用。80 ℃结合500 mg/L Nisin处理20 min后,芽孢存活浓度下降程度最大。
2)对不同处理的芽孢悬浮液的荧光偏振度分析发现,与对照组比较,单独80℃、不同浓度的Nisin、温度结合不同浓度Nisin处理后,荧光偏振度均显著下降(<0.05),但500 mg/L浓度的Nisin对芽孢悬浮液荧光偏振度最大,并远大于80 ℃热处理对芽孢内膜的影响。
3)对芽孢悬浮液的吸光度值及内膜通透性分析发现,不同处理后吸光度值显著降低(<0.05),并且Nisin浓度越高,吸光度值下降程度越大。80 ℃结合500 mg/L Nisin处理后,吸光度值下降程度最大。相比热处理或Nisin单独作用,热结合Nisin处理后,芽孢内膜通透性显著增加(<0.05),Nisin浓度越高,芽孢内膜通透性越强。
综上所述,芽孢内膜是有关芽孢高杀菌抗性的关键结构,本试验针对芽孢内膜展开研究,发现Nisin和80 ℃热处理两者结合处理可有效破坏芽孢内膜的水分子通透屏障,推测水分子更易通过芽孢内膜进入到芽孢核心。随着芽孢核心水分含量的增加,芽孢耐热性大大降低,80 ℃热结合Nisin处理就能有效杀灭芽孢,为保证食品安全提供了理论依据和新的技术手段。
[1] Trunet C, Ngo H, Coroller L. Quantifying permeabilization and activity recovery ofspores in adverse conditions for growth[J]. Food Microbiology, 2019, 81: 115-120.
[2] Fan Lihua, Ismail Balarabe Bilyaminu, Hou Furong, et al. Thermosonication damages the inner membrane ofspores and impels their inactivation[J]. Food Research International, 2019, 125: 108514. 1-108514. 8.
[3] 朱瑶迪,张佳烨,李苗云,等. 肽聚糖对肉制品中产气荚膜梭菌芽孢萌发率影响及预测[J]. 农业工程学报,2020,36(4):287-293. Zhu Yaodi, Zhang Jiaye, Li Miaoyun, et al. Effect of different Peptidoglycan onspore germination and quantitative prediction[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(4): 287-293. (in Chinese with English abstract)
[4] Paul Christophe, Filippidou Sevasti, Jamil Isha, et al. Bacterial spores, from ecology to biotechnology[J]. Advances in Applied Microbiology, 2018, 106: 79-111.
[5] Voundi Steve Olugu, Nyegue M, Bougnom Blaise Pascal, et al. The Problem of Spore-forming Bacteria in Food Preservation and Tentative Solutions[M]. New Jersey: John Wiley & Sons, Inc, 2017: 139-151.
[6] 梁栋,陈芳,胡小松. 芽孢萌发研究进展[J]. 中国食品学报,2018,18(6):221-228. Liang Dong, Chen Fang, Hu Xiaosong. Research progress on the spore germination[J]. Journal of Chinese Institute of Food Science and Technology, 2018, 18(6): 221-228. (in Chinese with English abstract)
[7] 何树祥. 运用光学新技术探究理化因子对苏云金芽孢杆菌芽孢萌发的影响[D]. 桂林:广西师范大学,2017. He Shuxiang. Exploring the Effects of Physical and Chemical Factors on Spore ofby Using New Optical Technology[D]. Guilin: Guangxi Normal University, 2017. (in Chinese with English abstract)
[8] Leggett M J, McDonnell G, Denyer S P, et al. Bacterial spore structures and their protective role in biocideresistance[J]. Journal of Applied Microbiology, 2012, 113(3): 485-498.
[9] Cowan Ann E, Olivastro Elizabeth M, Koppel Dennis E, et al. Lipids in the inner membrane of dormant spores ofspecies are largely immobile[J]. Proceedings of the National Academy of ences of the United States of America, 2004, 101(20): 7733-7738.
[10] Genest P C, Barbara S, Elizabeth M, et al. Killing of spores ofby peroxynitrite appears to be caused by membrane damage[J]. Microbiology, 2002, 148: 307-314.
[11] Setlow P. Spores of: Their resistance to and killing by radiation, heat and chemicals[J]. Journal of Applied Microbiology, 2006, 101(3): 514-25.
[12] Dong W, Green J, Korza G, et al. Killing of spores ofspecies by cetyltrimethylammonium bromide[J]. Journal of Applied Microbiology, 2019, 126: 1391-1401.
[13] Lee S Y, Chung H J, Kang D H. Combined treatment of high pressure and heat on killing spores ofacidoterrestris in apple juice concentrate[J]. Journal of Food Protection, 2006, 69(5): 1056-1060.
[14] Aldrete-Tapia J A, Torres J. Antonio. Enhancing the inactivation ofspores during pressure-assisted thermal processing[J]. Food Engineering Reviews, 2020.
[15] Zhang Zhong, Jiang Bin, Liao Xiaojun, et al. Inactivation ofspores by combining high-pressure thermal sterilization and ethanol[J]. International Journal of Food Microbiology, 2012, 160(2): 99-104.
[16] Takuya Inokuchi, Noriyoshi Arai. Relationship between water permeation and flip-flop motion in a bilayer membrane[J]. Physical Chemistry Chemical Physics Pccp, 2018, 20(44): 28155-28161.
[17] 张良. 高静压与温度协同杀灭芽孢的效果与机制研究[D]. 北京:中国农业大学,2015. Zhang Liang. Research on Effectiveness and Mechanism of Spore Inactivation by High Hydrostatic Pressure Combined with Heat[D]. Beijing: China Agricultural University, 2015. (in Chinese with English abstract)
[18] E. 西姆. 膜生物化学[M]. 北京:科学出版社,1985.
[19] 廖涵. 乳酸链球菌素(Nisin)与超高压结合对的协同杀菌效应[J]. 食品工业科技,2019,40(20): 82-87. Liao Han. Synergistic effects of Nisin and HPP on the inactivation of[J]. Science and Technology of Food Industry, 2019, 40(20): 82-87. (in Chinese with English abstract)
[20] 刘洪霞. ε-聚赖氨酸、Nisin和纳他霉素的抑菌特性及协同抑菌机理研究[D]. 泰安:山东农业大学,2013. Liu Hongxia The Inhibition Activity and Synergistic Mechanism of ε - Polylysine、Nisin and Natamycin[D]. Taian: Shandong Agricultural University, 2013. (in Chinese with English abstract)
[21] Modugno Chloe, Kmiha Souhir, Simonin Helene, et al. High pressure sensitization of heat-resistant and pathogenic foodborne spores to nisin[J]. Food Microbiology, 2019, 84: 103244.
[22] Marta Ávila, Natalia Gómez-Torres, Marta Hernández, et al. Inhibitory activity of reuterin, nisin, lysozyme and nitrite against vegetative cells and spores of dairy-related Clostridium species[J]. International Journal of Food Microbiology, 2014, 172: 70-75.
[23] 胡长利,向新华,韩晓旭,等. 耐热芽孢杆菌()的研究进展概述[J]. 食品安全质量检测学报,2015,6(7): 2795-2801. Hu Changli, Xiang Xinhua, Han Xiaoxu, et al. Research and development of[J]. Journal of Food Safety & Quality, 2015, 6(7): 2795-2801. (in Chinese with English abstract)
[24] 章中,孙静,张津瑜,等. 高压热杀菌处理对枯草杆菌芽孢皮层裂解酶活力的影响[J]. 食品工业科技. 2018,39(15):90-95.
Zhang Zhong, Sun Jing, Zhang Jinyu, et al. Effects of high pressure thermal sterilization on the activity of cortex-lytic enzyme extracted fromspores[J]. Science and Technology of Food Industry. 2018,39(15):90-95. (in Chinese with English abstract)
[25] 钱静亚,马海乐,李树君,等. 脉冲磁场对枯草芽孢杆菌细胞膜流动性的影响[J]. 农业机械学报,2013,44(11): 202-207. Qian Jingya, Ma Haile, Li Shujun, et al. Effects of the pulsed magnetic field on membrane fluidity of[J]. Transactions of the Chinese Society for Agricultural Machinery. 2013, 44(11): 202-207. (in Chinese with English abstract)
[26] Voss Dnielle, Montville Thomas J. 1, 6-Diphenyl-1, 3, 5-hexatrine as a reporter of inner spore membrane fluidity inand Alicyclobacillus acidoterrestris[J]. Journal of Microbiological Methods, 2014, 96: 101-103.
[27] Hue Nguyen Thi Minh, Dantigny Philippe, Gervais Patrick, et al. Germination and inactivation ofspores induced by moderate hydrostatic pressure[J]. Biotechnology and Bioengineering, 2010, 107: 876-83.
[28] Amor Kaouther Ben, Breeuwer Pieter, Verbaarschot Patrick, et al. Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and deadcells during bile salt stress[J]. Applied and Environmental Microbiology, 2002, 68(11): 5209-5216.
[29] Trevors Jack. Fluorescent probes for bacterial cytoplasmic membrane research[J]. Journal of Biochemical and Biophysical Methods, 2003, 57(2): 87-103.
[30] Gharib Riham, Fourmentin Sophie, Charcosset Catherine, et al. Effect of hydroxypropyl-β–cyclodextrin on lipid membrane fluidity, stability and freeze-drying of liposomes[J]. Journal of Drug Delivery ence and Technology, 2018, 44: 101-107.
[31] Wuytack E Y, Soons J, Poschet F, et al. Comparative study of pressure and nutrient induced germination ofspores[J]. Applied and Environmental Microbiology, 2000, 66(1): 257-61.
[32] Farkas J, Andrassy E, Simon A, et al. Effecte of pasteurizing levels of high hydrostatic pressure onluxAB spores[J]. Acta Alimentaria, 2003, 32(4): 373-381.
Effects of heat combining with Nisin treatment on the sterilization ofspores
Chen Le, Zhang Zhong※, Guo Jiajun, Shen Jin, Chen Xiang, Shang Binling
(,750021,))
Bacterial spores are the most difficult microorganisms to be inactivated, which can cause food spoilage and poisoning in food production. High-temperature treatment above 100 ℃ is often used to inactivate bacterial spores in food, but the heat treatment at high temperature inevitably affects the nutrition and sensory quality of products. In this study, a lower-temperature heat treatment combined with Nisin on bacterial spores was proposed to explore the inactivation effects and mechanism, in order to find a feasible way to inactivate bacterial spores with lower-temperature. A plate-counting method, fluorescence polarization method, spectrophotometry and flow cytometry were used to determine the survival rate, fluorescence polarization degree, the absorbance value and inner membrane permeability of the spore samples. The results showed that: 1) The 80 ℃ treatment or Nisin alone cannot inactivate bacterial spores, but the 80℃ heat treatment combined with Nisin can effectively inactivate bacterial spores. After treated at 80 ℃ with 500 mg/L Nisin for 20 min, the survival concentration of bacterial spores decreased by about 1.4 lg (CFU/mL). 2) The fluorescent probe DPH was used to mark the inner membrane of bacterial spores, and further to analyze the fluidity of bacterial spores in the inner membrane. The results showed that the heat treatment combined with Nisin can significantly improve the fluidity of the inner membrane. Nisin can directly affect the degree of fluorescence polarization in the inner membrane. With higher Nisin concentration, the influence of Nisin on the degree of fluorescence polarization of inner membrane was stronger, whereas, the influence was smaller in the heat treatment at 80 ℃. The effects of 500 mg/L Nisin on the bacterial spores were much stronger than that of heat at 80 ℃. 3) The content release of spore was closely related to the inner membrane permeability of bacterial spores, and the absorbance value of spore suspension was often used to estimate the release of spore’s content. The higher release degree of spore’s content caused more significant decrease of the absorbance value of spore suspension. After 80℃ thermal treatment combined with Nisin, the absorbance value of spore suspension was significantly lower than that of thermal or Nisin treatment alone. After the treatment with 80 ℃ heat and 500 mg/L Nisin for 20 min, the absorbance value of spore suspension was the lowest. 4) The spores treated with heat combined with Nisin were stained with PI (Propidium Iodide), whereas, the changes of inner membrane permeability were studied by flow cytometry. The 80℃ thermal treatment combined with Nisin can significantly increase the permeability of inner membrane. Compared with heat or Nisin used alone, the permeability of inner membrane increased significantly after thermal treatment combined with Nisin. The higher Nisin concentration caused the higher permeability of inner membrane. It was found that Nisin had significant effects on the fluidity and permeability of inner membrane for the spore, where the 80 ℃ thermal treatment combined with different concentrations of Nisin had synergistic effect to improve the permeability of inner membrane. With the increase in the permeability of inner membrane, water was easier to enter the core of bacterial spores, which reduced the heat resistance of bacterial spores. The 80 ℃ thermal treatment combined with 500 mg/L Nisin can effectively inactivate bacterial spores, while the addition of Nisin greatly reduced the temperature required for inactivation of bacterial spore. The findings can contribute to reduce the damages of thermal sterilization on the nutritional and sensory quality in food production.
heat resistance; sterilization; inner membrane; membrane fluidity; membrane permeability; Nisin; spores
陈乐,章中,郭家俊,等. 热结合Nisin处理对枯草杆菌芽孢的杀灭效果[J]. 农业工程学报,2020,36(20):320-325.doi:10.11975/j.issn.1002-6819.2020.20.037 http://www.tcsae.org
Chen Le, Zhang Zhong, Guo Jiajun, et al. Effects of heat combining with Nisin treatment on the sterilization ofspores[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(20): 320-325. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2020.20.037 http://www.tcsae.org
2020-07-12
2020-10-10
国家自然科学基金项目(31760474);国家自然科学基金项目(31460410)
陈乐,主要研究方向为非热加工技术。Email:chenlechengle@163.com
章中,博士,副教授,主要研究方向为非热加工技术。Email:zhangzhong99@126.com
10.11975/j.issn.1002-6819.2020.20.037
TS201.3
A
1002-6819(2020)-20-0320-06
