Fatty Acid Composition and Digestive Enzyme Activities of Rainbow Trout in Response to Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) During Salinity Acclimation
2020-11-30HUANGMingZHOUYangenGEJianAGUSTSSONThorleifurLILiGAOQinfengandDONGShuanglin
HUANG Ming, ZHOU Yangen, *, GE Jian, AGUSTSSON Thorleifur,LI Li, GAO Qinfeng, 4), and DONG Shuanglin, 4)
Fatty Acid Composition and Digestive Enzyme Activities of Rainbow Trout in Response to Dietary Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) During Salinity Acclimation
HUANG Ming1), ZHOU Yangen1), *, GE Jian1), AGUSTSSON Thorleifur2), 3),LI Li1), GAO Qinfeng1), 4), and DONG Shuanglin1), 4)
1),,266100,2),12, 4072,,3)5, 107,,4),,266235,
This physiological study aimed to evaluate the effects of dietary docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the fatty acid composition and digestive enzyme activities of rainbow trout () during salinity acclima- tion. Rainbow trout with an average initial weight of 90.61g±9.25g were fed diets with the quantities of DHA and EPA equaling to 0.54%, 0.95%, 1.40% and 1.79% (abbreviated as DE-0.54, DE-0.95, DE-1.40, and DE-1.79, respectively) for eight weeks, after whichthe gastric and intestinal fatty acids composition were analyzed. Subsequently, the fish underwent salinity acclimation. On days 1, 4, 7, and 14 after the freshwater was replaced by seawater and at the end of the 8-week period, gastric and intestinal digestive enzyme activi- ties were determined. The results showed that the gastric and intestinal DHA and EPA contents of the fish were positively correlated to their dietary DHA and EPA levels. Low dietary DHA and EPA levels inhibited the protease activity of rainbow trout. Fish in the DE-0.54 group increased the lipase activity to enhance the utilization of lipids maybe due to the inadequate essential fatty acids for fish in this group. Hence, rainbow trout in the DE-0.54 group failed to maintain suitable activities of digestive enzymes after salinity acclimation. Therefore, a diet with minimum 0.95% DHA and EPA levels is necessary for rainbow trout during salinity acclimation.
digestive enzymes; docosahexaenoic acid; eicosapentaenoic acid; rainbow trout
1 Introduction
Rainbow trout (), which is classi- fied as salmonids, is one of the world’s most important aquaculture species and is farmed in many countries (Es- maeili., 2017). Due to the increasing demand for rainbow trout, Chinese researchers are establishing mari- culture system using deep-sea cages far offshore in the Yellow Sea Cold Water Mass of China (Evans, 2018; Dong, 2019). The ‘mountain-sea transfer’ culturing method is used, in which juvenile fish are hatched and cultivated in freshwater in mountainous areas before being transferred to seawater cages for growth until harvest.
The parr-smolt transformation process of salmonids (smoltification) requires up to 50% of the total available energy (Moser., 1994; Boeuf., 2001). Fishes take energy mainly from the proteins and lipids and the de- mand for essential fatty acids varies among different spe- cies (Glencross, 2009; NRC, 2011). It has been reported that the demand for polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA, C22:6n3) and eicosapentaenoic acid (EPA, C20:5n3), in salmonids in- crease during smoltification (Ackman., 1986; Tocher., 2000). Furthermore, previous studies have revealed that adequate accumulation of DHA and EPA is a prereq- uisite for salmonids before being transferred to the sea (Sheridan, 1985; Li, 1992; Bell, 1997).
It has been suggested that digestive enzyme activities can be regulated by dietary composition (Fountoulaki., 2005; Santigosa., 2008; Castro., 2013; Sivara- makrishnan., 2017; Fuentes-Quesada., 2018). Xie(2018) reported that the activities of protease, lipase and α-amylase in the digestive tract of rabbitfish () changed depending on the levels of dietary proteins, lipids, and carbohydrates, respectively. The digestive ability of fish changes in response to the replacement of fish oil with vegetable oil in the diet, and this replacement causes a decrease in the dietary DHA and EPA levels. You(2019) reported that dietary soybean oil significantly decreases the amylase, trypsin, and lipase activities in the intestine of golden pompano (). However, Bowyer(2012)found that dietary coconut oil decreases trypsin and lipase ac- tivities but had no effect on amylase activity in yellowtail kingfish ().
The exposure of fish to a hypersaline environment ac- celerates the drinking rate and thereby changes the ion content and pH in the gastrointestinal tract of fish, in- fluencing the activities of digestive enzyme (Noda., 1981; Squires., 1986a; Usher., 1988; Moutou., 2004). The effects of salinity on digestive enzyme activities have been widely reported in many species, such as turbot () (Chen., 2006), Caspian kutum (, Kamensky, 1901)(Gheisvandi., 2015), Japanese flounder () (Bolasina., 2007), and fat snook () (Tsuzuki., 2007).
Interactions between osmoregulation and digestion have been reported in fish (Psochiou., 2007). Digestive enzyme activities are indicators of digestive processes and nutritional conditions that can be easily and reliably ana-lyzed (Barman., 2005; Bolasina., 2006; Run- gruangsak-Torrissen., 2006; Nikolopoulou., 2011; Sutthinon., 2015; Liu., 2017). However, previ- ous studies have mainly focused either on the effects of dietary components or salinity acclimation on the diges- tive enzyme activities, while few studies have considered these two factors together at the same time. The objective of this study was to explore the effects of dietary DHA and EPA levels on the gastric and intestinal digestive en- zyme activities in rainbow trout during salinity acclima- tion.
2 Materials and Methods
2.1 Experimental Diets
The basal diet was formulated to be both isoproteic (45% proteins) and isolipidic (16% lipids) using fish meal, soybean meal, chicken meal, and corn gluten meal as the primary protein sources and fish oil and soybean oil as the lipid sources. With the adjustment of the proportions of fish oil and soybean oil, four different practical diets with DHA+EPA levels equal to 0.54%, 0.95%, 1.40%, or 1.79% were designed (Table 1). The fatty acid composi- tion of the experimental diets is presented in Table 2.
2.2 Experimental Design and Sample Collection
The experiment was conducted at the Key Laboratory of Mariculture, Ocean University of China (Qingdao, Shan- dong, China). Juvenile rainbow trouts were obtained from the Linqu Cold Water Fish Farm (Linqu, Shandong, Chi- na). Prior to the experiment, stomachs and intestines of three fish were collected as initial samples, after which fish were acclimated by being stocked in circular tanks (vol- ume, 180L; height, 0.60m; upper diameter, 0.65m; lower diameter, 0.60m) for two weeks and fed experimental diets (mixed by the four aforementioned diets). After the ac- climation period, fish with an average weight of (90.61±9.25)g were randomly assigned to four groups, and the fish in the various groups were fed diets with DHA and EPA levels equaling to 0.54%, 0.95%, 1.40% and 1.79% (DE-0.54, DE-0.95, DE-1.40 and DE-1.79 groups, respec- tively) of the total diets, respectively. Three replicates were included in each group, and each tank contained 12 fish. The experimental diets were offered twice daily at 8:30 a.m. and 4:00 p.m. for an 8-week period, and a 12-h light: 12-h dark photoperiod was maintained during this period. Approximately 70% of the water was changed daily at 12:00 a.m. During this period, the following water quality pa- rameters (mean±standard deviation) were maintained: tem- perature, (16±1.2)℃; pH, 7.89±0.09; dissolved oxygen (DO), (8.32±0.34)mgL−1; ammonia nitrogen, (0.04±0.01)mgL−1; and nitrite nitrogen, (0.02±0.01)mgL−1. Every two weeks, the experimental fish were starved for one day before being anesthetized by a moderate level of meth- anesulfonate (MS-222) and then weighed.
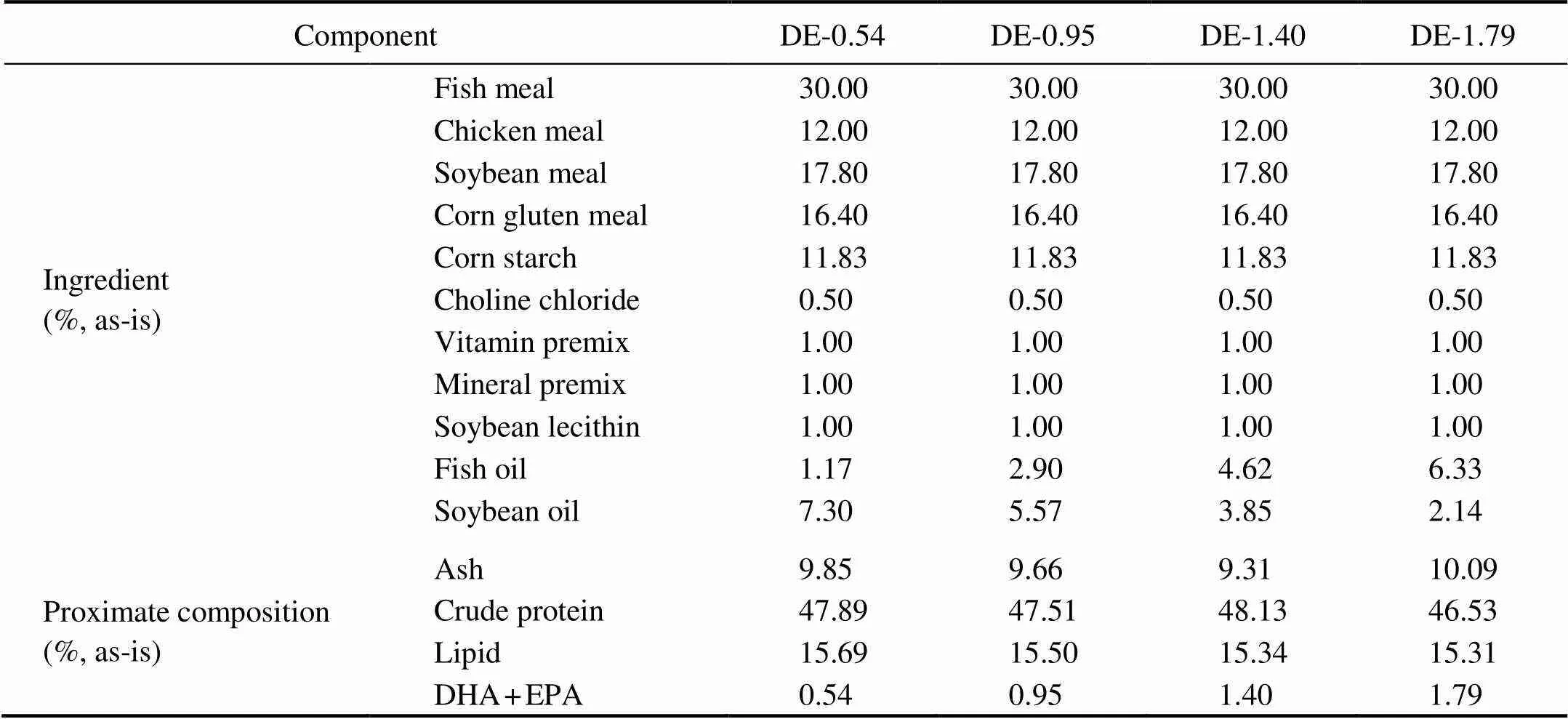
Table 1 Composition of the four diets (45% protein and 16% lipid) formulated with 0.54%, 0.95%, 1.40%,and 1.79% oftotal dietary DHA and EPA
Notes: Fish meal, crude protein 63.83%, crude lipid 10.0%; Chicken meal, crude protein 59.51%, crude lipid 15.47%; Soy- bean meal, crude protein 42.17%, crude lipid 4.42%; Corn starch, crude protein 67.06%, crude lipid 3.37%. Ingredients were obtained from Great Seven Bio-Tech (Qingdao, Shandong, China). Vitamin premix (mg(kgdiet)−1): 5mg vitamin D; 10mg vitamin K; 10mg vitamin B12; 20mg vitamin B6; 20mg folic acid; 25mg vitamin B1; 32mg vitamin A; 45mg vitamin B2; 60mg pantothenic acid; 60mg biotin; 200mg niacin acid; 240mg α-tocopherol; 800mg inositol; 2000mg ascorbic acid. Mineral premix (mg(kgdiet)−1): 10mg CuSO4·5H2O; 25mg Na2SeO3(1%); 50mg ZnSO4·H2O; 50mg CoC12·6H2O (1%); 60mg MnSO4·H2O; 80mg FeSO4·H2O; 180mg Ca(IO3)2; 1200mg MgSO4·7H2O; 8345mg zeolite. DHA, docosahexaenoic acid, C22:6n3. EPA, eicosapentaenoic acid, C20:5n3.
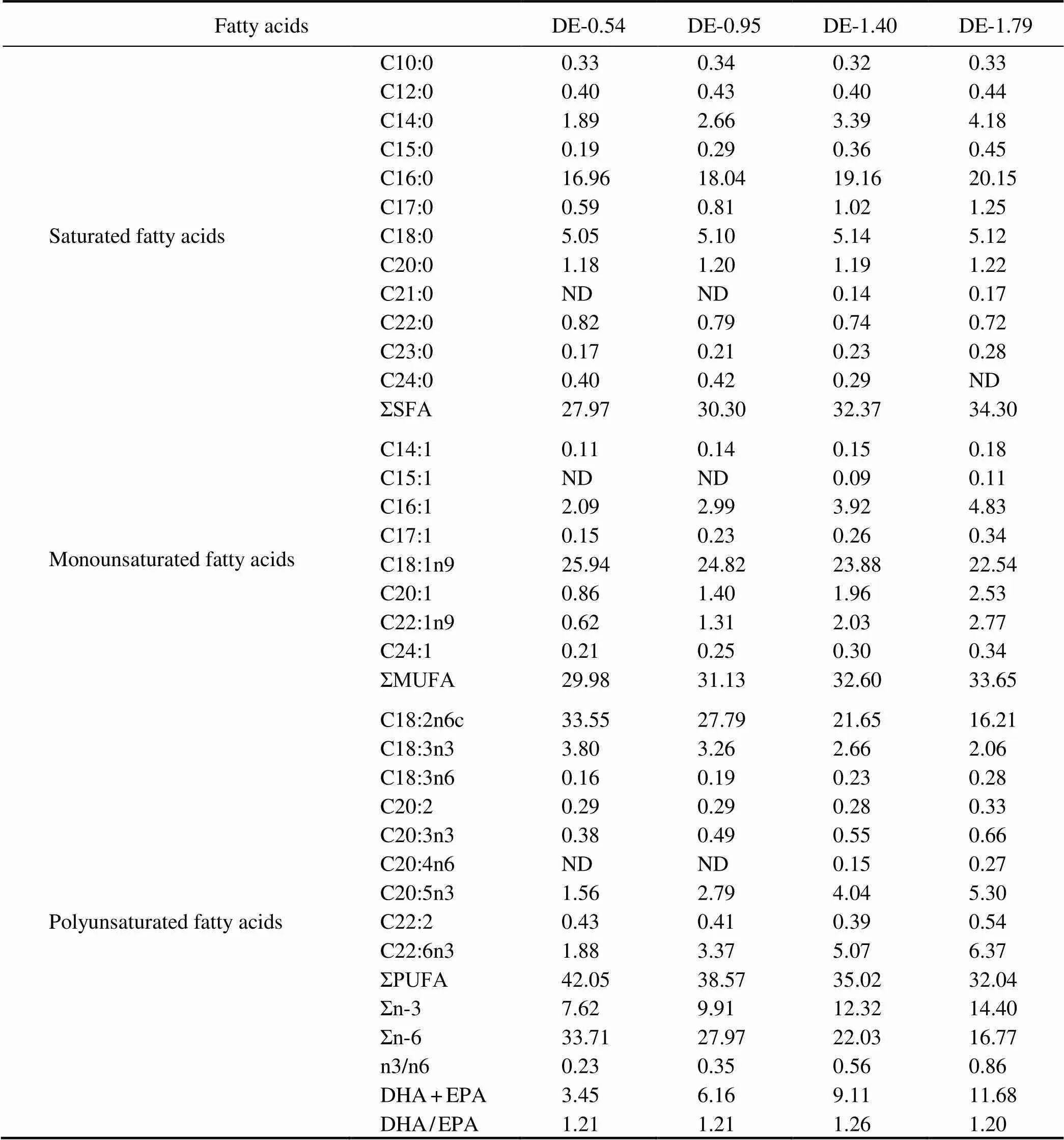
Table 2 Fatty acid composition of the experimental diets (% of total fatty acids)
Notes: Values are the means of three replicates. n-3, omega-3 series polyunsaturated fatty acids; n-6, omega-6 series poly- unsaturated fatty acids; DHA, docosahexaenoic acid, C22:6n3; EPA, eicosapentaenoic acid, C20:5n3; ND, not detected.
At the end of the 8-week period, three fish per treat- ment were anesthetized using MS-222 (70mgL−1), and their stomachs and proximal intestines were dissected out, after which the gastric and intestinal contents were squeezed out by sterile tweezers. Sampled stomachs and proximal intestines were kept at −80℃ for fatty acid and digestive enzyme activities determination.
Salinity acclimation was performed in all tanks by mix- ing freshwater with natural seawater. Salinity was directly increased to 14gL−1and then increased to final 30gL−1at a rate of 4gL−1per day, after which it was kept constant for an additional period of 14 days. A hand-held salino- meter (Dedu DT-Y100, Changzhou, China) was used to measure the salinity. On days 1, 4, 7, and 14 after salinity acclimation, stomachs and proximal intestines of three fishper treatment were collected following the method discuss- ed above. All sampling was performed at 24h post-feed- ing. The stomachs were used for determination of the gas- tric amylase, pepsin and lipase activities, and the intestines were used for determination of the intestinal amylase, trypsin and lipase activities.
2.3 Measurements of Indicators and Analytical Methods
Fatty acids were extracted according to the method de- scribed by Bligh(1959). They were esterified into methyl esters using methyl esterification reagent (hydro- chloric acid/methanol, 1:5) and analyzed by gas chroma- tography (Shimadzu GC-2010 plus, Kyoto, Japan). The re- lated parameters and instruments used in this experiment were described by Liu. (2018).
All digestive enzyme activities were measured using commercial kits (Nanjing Jiancheng Bioengineering Insti- tute, Nanjing, China) following the manufacturer’s ins- tructions. The stomachs and intestines were weighed and homogenized together with cold saline (0.75% NaCl, pH 7.0). The homogenates were immediately centrifuged at 800×for 10min at 4℃, after which the supernatants were collected and used for determination of enzyme ac- tivities after appropriate dilution.
2.4 Statistical Analysis
All statistics were performed using SAS 9.4 (SAS Ins- titute Inc., Cary, NC). Data were analyzed using a one-way analysis of variance (ANOVA) followed by the Student- Newman-Keuls (SNK) multiple comparisons test to iden- tify significant differences among different treatment groups. Additionally, interactions between dietary DHA and EPA le- vels and culture time on digestive enzyme activities were evaluated by the two-way ANOVA among treatment means. All statistical tests for significance were set at<0.05. All figures were constructed by GraphPad Prism 7 (GraphPad Software, San Diego, CA, www.graphpad.com).
3 Results
3.1 Growth
The survivals of rainbow trout in all treatment groups were 100%. No significant differences of final mean weight, final biomass, weight gain percentage and feed conver- sion ratio (FCR) of fish were observed among different treatments (Table 3).
3.2 Fatty Acid Composition
The gastric and intestinal DHA and EPA contents of rainbow trout were positively correlated to their dietary DHA and EPA levels (Tables 4 and 5). At the end of 8- week period, the gastric DHA and EPA contents in the DE-1.40 and DE-1.79 groups were significantly higher than in DE-0.54 and DE-0.95 groups. The intestinal DHA content was the lowest in the DE-0.54 group and the high- est in the DE-1.79 group. The intestinal EPA in the DE- 1.40 and DE-1.79 groups were significantly higher than in DE-0.54 and DE-0.95 groups. Moreover, the linoleic (C18:2n6) contents in the DE-1.40 and DE-1.79 groups were significantly lower than in DE-0.54 and DE-0.95 groups according to the dietary content of soybean oil.
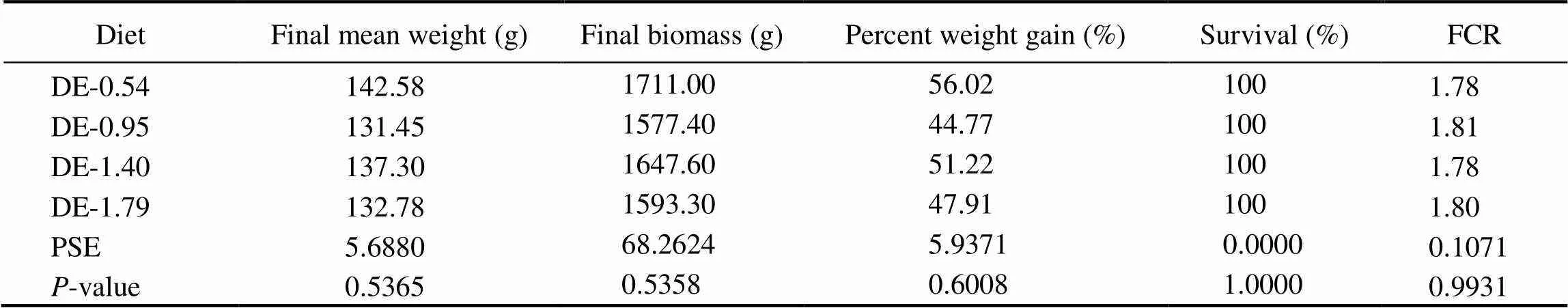
Table 3 Growth performance of rainbow trout fed four experimental diets (initial weight of 90.61g±9.25g, 8 weeks)
Notes: The initial weight was presented as mean±standard deviation. Values are the means of three replicates. Means with different superscripts indicate significant differences (0.05) based on one-way ANOVA and the Student-Newman-Keuls (SNK) test. PSE, pooled standard error; FCR, feed conversion rate. Final biomass was measured by weighting 12 fish from each tank, FCR=(Dry feed offered)/(Wet weight gain). Percent weight gain=100×(Final body weight−Initial body weight)/(Initial body weight).
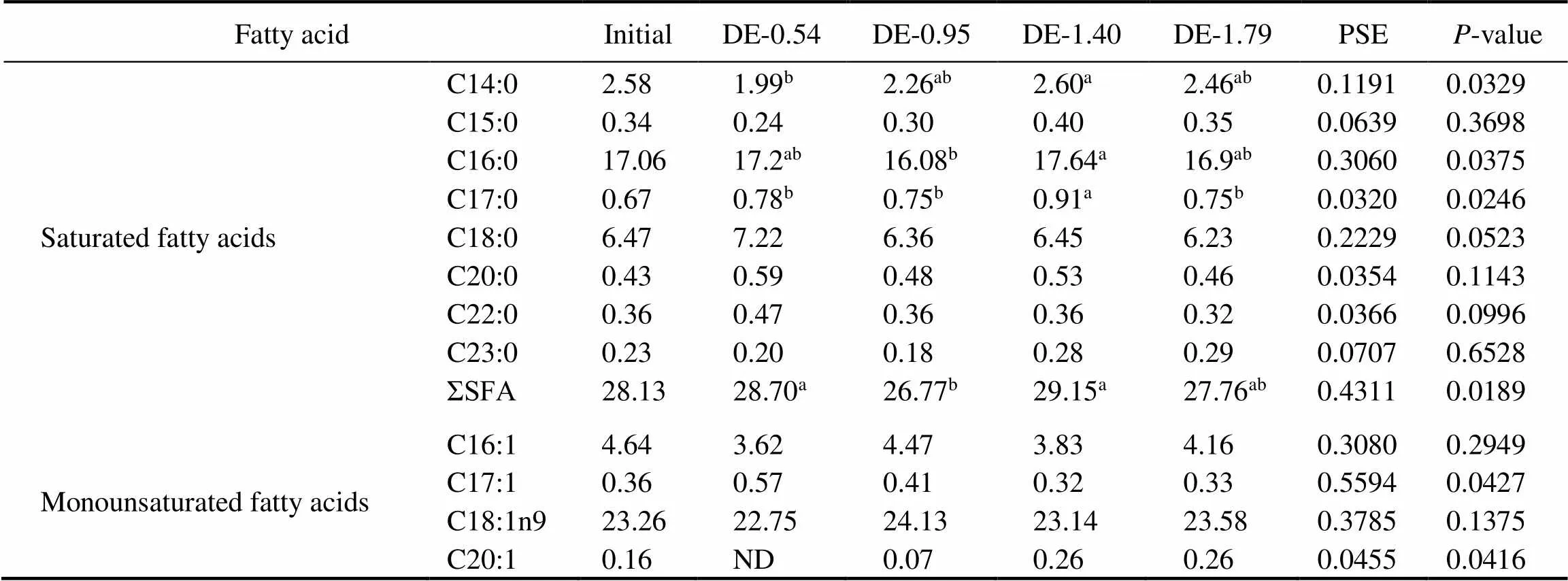
Table 4 Fatty acid composition of the stomach fat of rainbow trout fed the experimental diets for 8 weeks (% of total fatty acids)
()
()
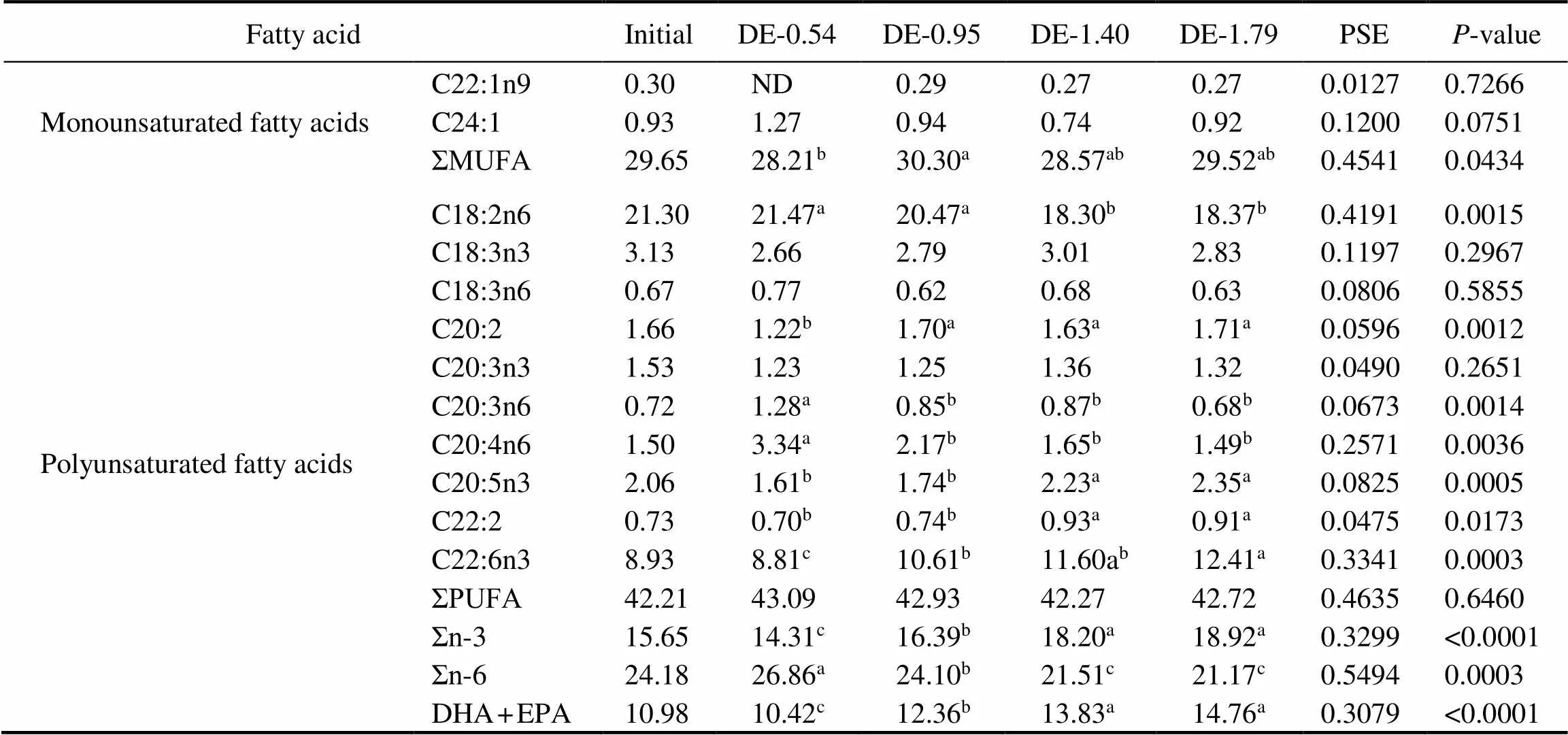
Fatty acidInitialDE-0.54DE-0.95DE-1.40DE-1.79PSEP-value Monounsaturated fatty acidsC22:1n90.30 ND0.29 0.27 0.27 0.0127 0.7266 C24:10.93 1.27 0.94 0.74 0.92 0.1200 0.0751 ΣMUFA29.65 28.21b30.30a28.57ab29.52ab0.4541 0.0434 C18:2n621.30 21.47a20.47a18.30b18.37b0.4191 0.0015 C18:3n33.13 2.66 2.79 3.01 2.83 0.1197 0.2967 C18:3n60.67 0.77 0.62 0.68 0.63 0.0806 0.5855 C20:21.66 1.22b1.70a1.63a1.71a0.0596 0.0012 C20:3n31.53 1.23 1.25 1.36 1.32 0.0490 0.2651 Polyunsaturated fatty acidsC20:3n60.72 1.28a0.85b0.87b0.68b0.0673 0.0014 C20:4n61.50 3.34a2.17b1.65b1.49b0.2571 0.0036 C20:5n32.06 1.61b1.74b2.23a2.35a0.0825 0.0005 C22:20.73 0.70b0.74b0.93a0.91a0.0475 0.0173 C22:6n38.93 8.81c10.61b11.60ab12.41a0.3341 0.0003 ΣPUFA42.21 43.09 42.93 42.27 42.72 0.4635 0.6460 Σn-315.65 14.31c16.39b18.20a18.92a0.3299 <0.0001 Σn-624.18 26.86a24.10b21.51c21.17c0.5494 0.0003 DHA+EPA10.98 10.42c12.36b13.83a14.76a0.3079 <0.0001
Notes: The values represent the means from three replicates. Means with different superscripts indicate significant differences (0.05) based on one-way ANOVA and the Student-Newman-Keuls (SNK) test. PSE, pooled standard error; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; n-3, omega-3 series polyunsaturated fatty acid; n-6, omega-6 series polyunsaturated fatty acid; DHA, docosahexaenoic acid, C22:6n3; EPA, eicosapentaenoic acid, C20:5n3; ND, not detected.
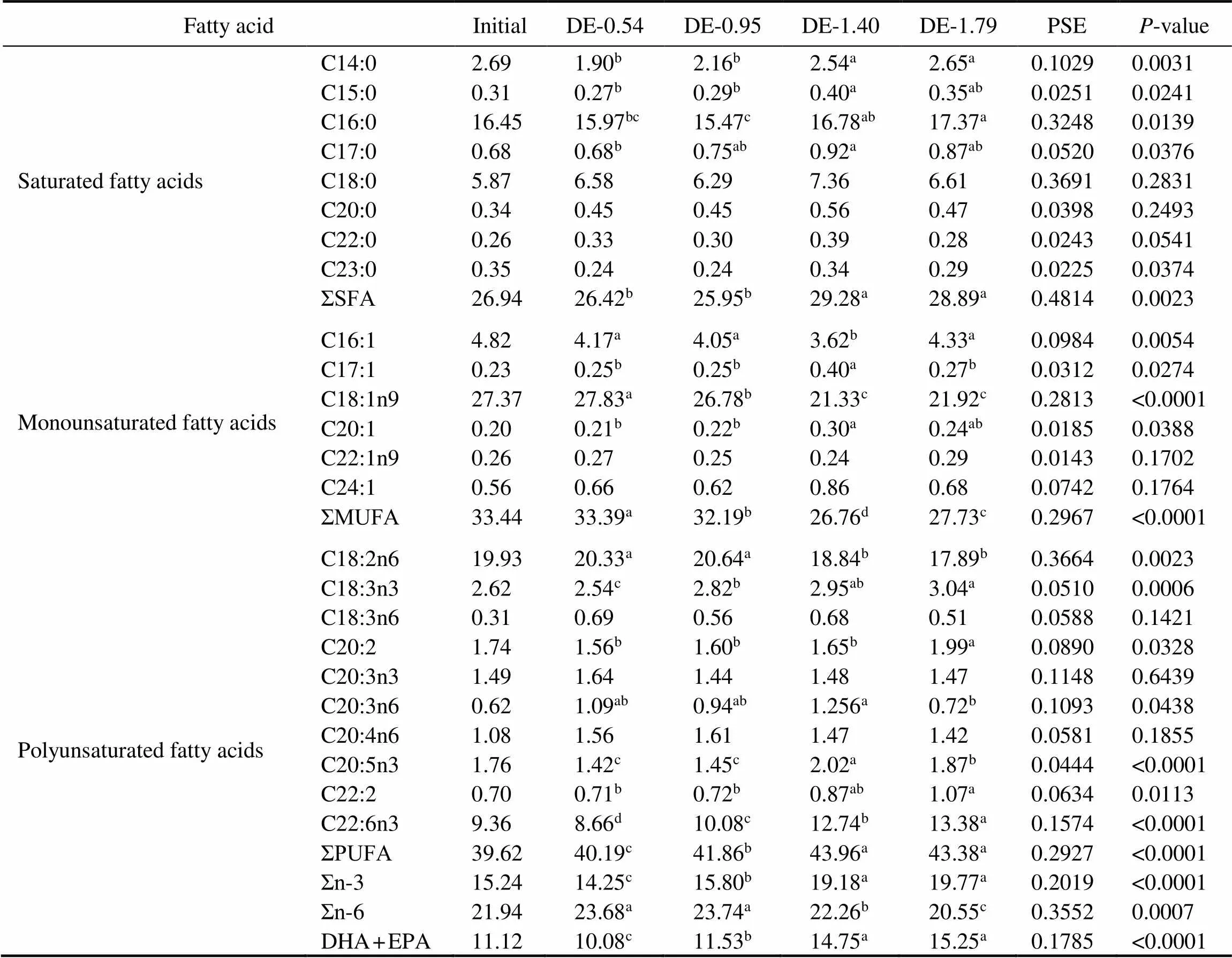
Table 5 Fatty acid composition of the intestine fat in rainbow trout fed the experimental diets for 8 weeks (% of total fatty acids)
Notes: The values represent the means from three replicates. Means with different superscripts indicate significant differences (0.05) based on one-way ANOVA and the Student-Newman-Keuls (SNK) test. PSE, pooled standard error. SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; n-3, omega-3 series polyunsaturated fatty acid; n-6, omega-6 series polyunsaturated fatty acid; DHA, docosahexaenoic acid, C22:6n3; EPA, eicosapentaenoic acid, C20:5n3.
3.3 Gastric Amylase, Pepsin and Lipase Activities in Rainbow Trout
No significant difference of gastric amylase activity in rainbow trout was observed with different dietary DHA and EPA levels before salinity acclimation (Fig.1A). Gas- tric amylase activity in rainbow trout in the DE-1.40 and DE-1.79 groups continually increased following salinity increase, reaching maximum values on day 7, and subse- quently recovered to freshwater values on day 14. More- over, gastric amylase activity in the DE-0.54 group was significantly lower compared to the other three groups on day 4 after salinity acclimation.
No significant difference of gastric pepsin activity in rainbow trout was observed with different dietary DHA and EPA levels before salinity acclimation (Fig.1B). Gas- tric pepsin activity of rainbow trout in the DE-0.54 group peaked on day 1 after salinity acclimation, and then tended to decrease, reaching a minimum value on day 7. The acti- vity increased again on day 14, but failed to return to the freshwater value. Salinity stress increased the gastric pep- sin activity of rainbow trout in the DE-1.79 group before fully recovering on day 14.
Gastric lipase activity of rainbow trout in the DE-1.79 group was significantly lower compared to other groups before salinity acclimation (Fig.1C). Gastric lipase activi- ty of rainbow trout in the DE-0.54 group decreased to the lowest value on day 7 and increased to the highest value on day 14. In contrast, gastric lipase activity in the DE-0.95 and DE-1.79 groups decreased to their freshwater values following an increasing trend, peaking on days 4 and 7, re- spectively. The gastric lipase activity of rainbow trout in the DE-0.54 group was significantly lower than that in the other groups on day 7, and the gastric lipase activity in theDE-0.95 group was significantly lower than that in the DE- 0.54 and DE-1.40 groups on day 14.
3.4 Intestinal Amylase, Trypsin and Lipase Activities in Rainbow Trout
No significant difference of intestinal amylase activity in rainbow trout was observed with different dietary DHA and EPA levels prior to salinity acclimation (Fig.2A). In- testinal amylase activity of rainbow trout was inhibited by salinity stress on day 4. The activities in DE-0.54 andDE-0.95 groups turned to freshwater values on day 14, whereas the activity in DE-1.40 and DE-1.79 groups re- covered to high values on day 7, before decreasing sig- nificantly again on day 14. The intestinal amylase activi- ties in rainbow trout were significantly lower on days 4 and 14, but significantly higher on day 7 in the DE-1.40 and DE-1.79 groups compared to DE-0.54 and DE-0.95 groups.
No significant difference of intestinal trypsin activity in rainbow trout was observed with different dietary DHA and EPA levels before salinity acclimation (Fig.2B). The intestinal trypsin activity of rainbow trout in all groups decreased immediately on day 1 after salinity acclimation, followed by continuous increase before reaching thefresh- water values on day 14. At the end of the experiment, intestinal trypsin activity of rainbow trout in the DE-1.40 and DE-1.79 groups were significantly higher compared to the DE-0.54 and DE-0.95 groups.
No significant difference of intestinal lipase activity in rainbow trout was observed with different dietary DHA and EPA levels during freshwater stage (Fig.2C). Intesti- nal lipase activity of rainbow trout in the DE-0.54 group decreased to the minimum value on day 4 of salinity ac- climation, and then increased significantly on day 7, and the values on day 7 and 14 were significantly higher com- pared to the freshwater value. Intestinal lipase activity of rainbow trout in the DE-1.40 group decreased significant- ly after salinity acclimation. Although it recovered to its freshwater value on day 7, it declined again on day 14. In- testinal lipase activity of rainbow trout in the DE-1.79 group decreased significantly on day 1 after salinity acclimation before leveling off. On days 7 and 14, the intestinal lipase activity of rainbow trout in the DE-0.54 group was signi- ficantly higher than in other groups.
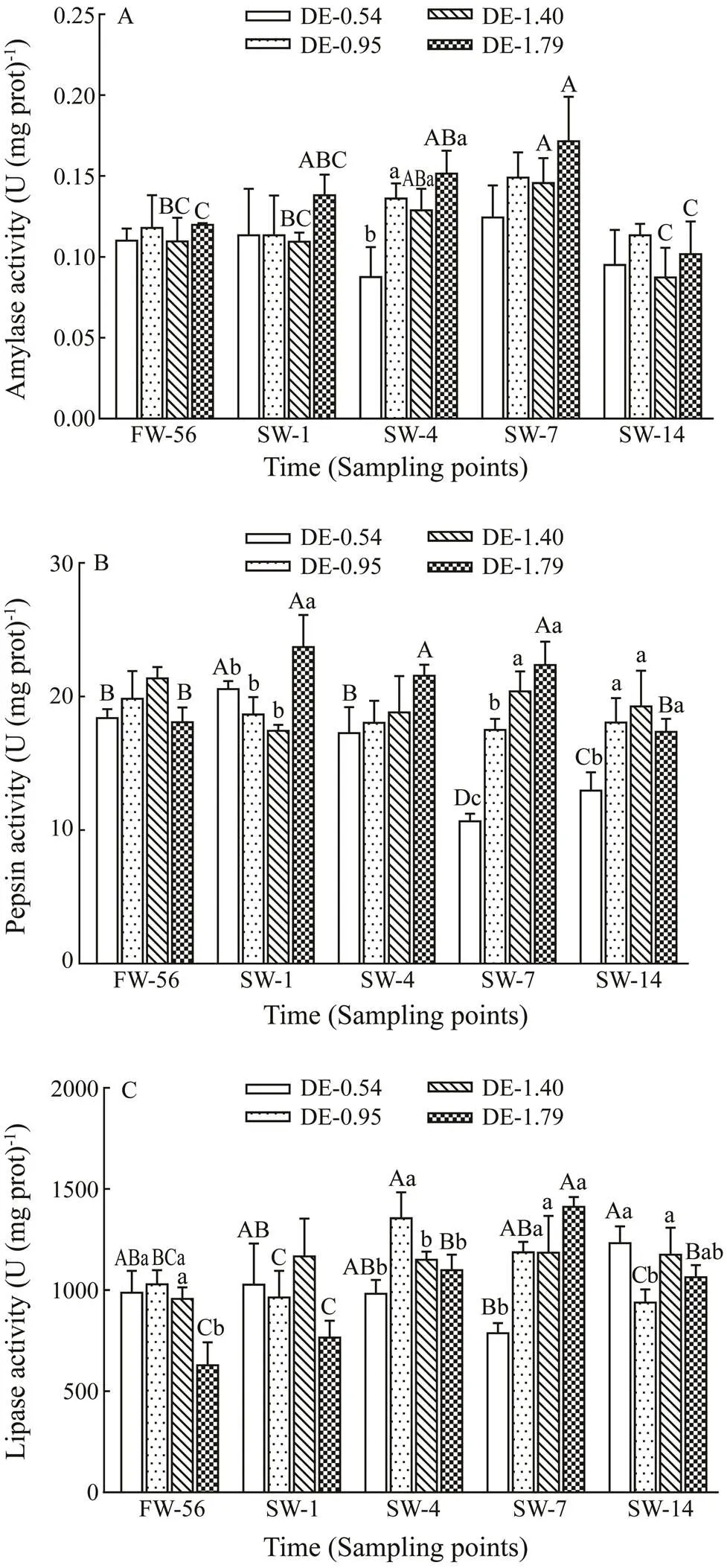
Fig.1 Variations in the gastric amylase activity (A), pep- sin activity (B) and lipase activity (C) in rainbow troutfed different levels of dietary DHA and EPA after salinity ac- climation. The values represent the means of three repli- cates. The different lowercase letters indicate significant differences (P<0.05) among different dietary treatments at the same time point, and the different capital letters in- dicate significant differences (P<0.05) at different time points in the same dietary treatment based on two-way ANOVA and the Student-Newman-Keuls (SNK) test. FW- 56, before salinity acclimation; SW-1, one day after sali- nity reached 30gL−1; SW-4, four days after salinity reached 30gL−1; SW-7, seven days after salinity reached 30gL−1; SW-14, fourteen days after salinity reached 30gL−1.
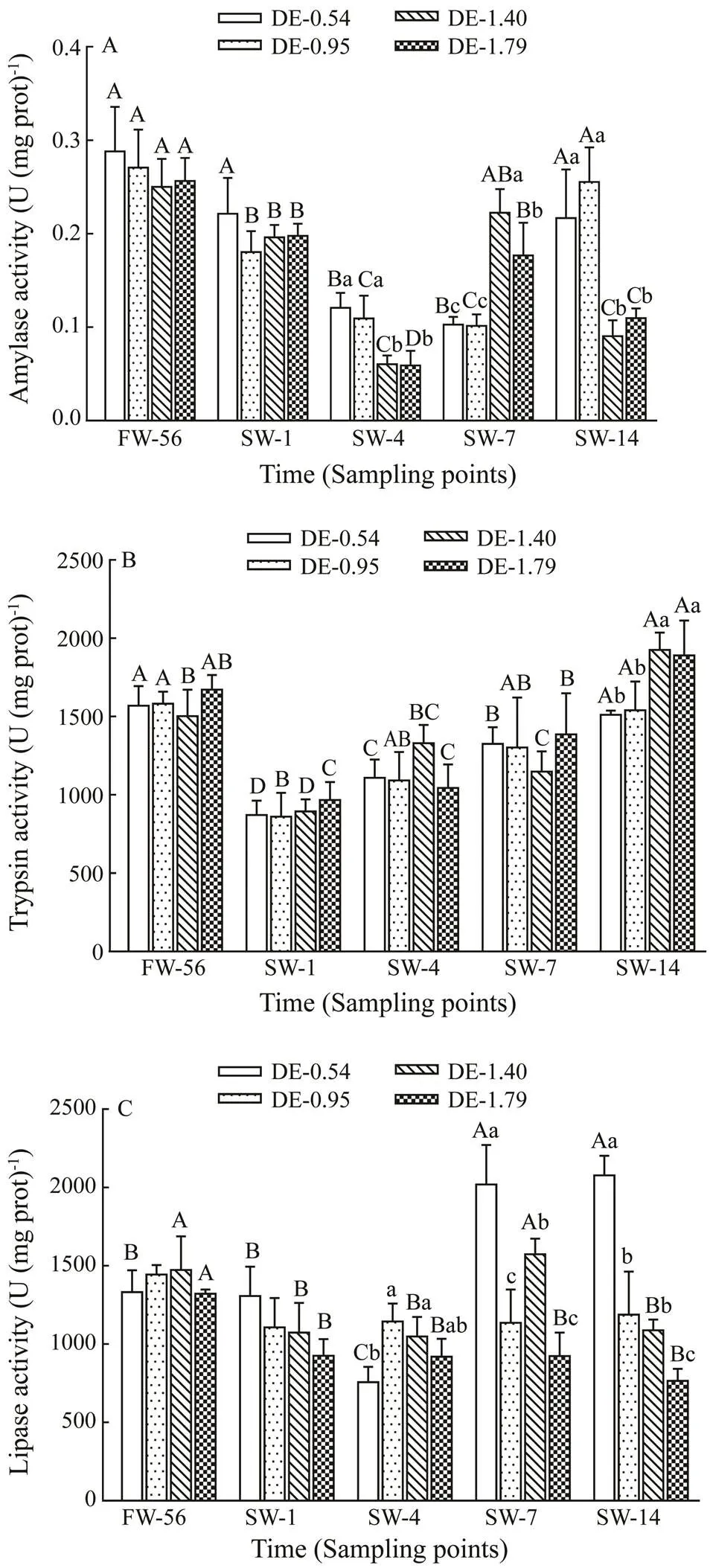
Fig.2 Same as those in Fig.1 but for variations in the in- testinal amylase activity (A), trypsin activity (B) and li- pase activity (C).
3.5 Interaction Effect of Culture Time and Dietary DHA and EPA Level on Digestive Enzyme Activities
Two-way ANOVA showed a significant interaction ef- fect of culture time and dietary DHA and EPA on gastric pepsin and lipase activities in rainbow trout. This interac- tion effect was also found for intestinal amylase, trypsin and lipase activities in rainbow trout (Table 6).
4 Discussion
The present study found that dietary DHA and EPA sup- plementation (0.54%–1.79%) did not significantly improve the final biomass, survival and FCR of rainbow trout rear- ed in freshwater for 8 weeks, which is consistent with the results of several previous studies on rainbow trout (Guler., 2011; Thanuthong., 2011) or Atlantic salmon () (Bell., 1997; Tocher., 2000).
DHA and EPA play important roles in the anti-stress re- sponse, salinity acclimation, and digestive ability (Cornet., 2018; Fuentes-Quesada., 2018). Fish need to obtain these essential fatty acids from food, and food is the main source of deposited DHA in fish tissues (Van Anholt., 2012). In general, high dietary DHA and EPA levels can improve the DHA and EPA contents in fish tissues (Guler., 2011; Hixson., 2014; Wijekoon., 2014). Similarly, our results showed that the gastric and intestinal DHA and EPA contents were positively cor- related to the dietary DHA and EPA levels. In addition, high levels of DHA and EPA in membrane can increase membrane fluidity and change the microenvironment of the membrane, which will further affect the activity of attach- ed enzymes. Thus, it is reasonable to speculate that diges- tive enzyme activities are influenced by the changes in the gastric and intestinal fatty acid composition in rain- bow trout.
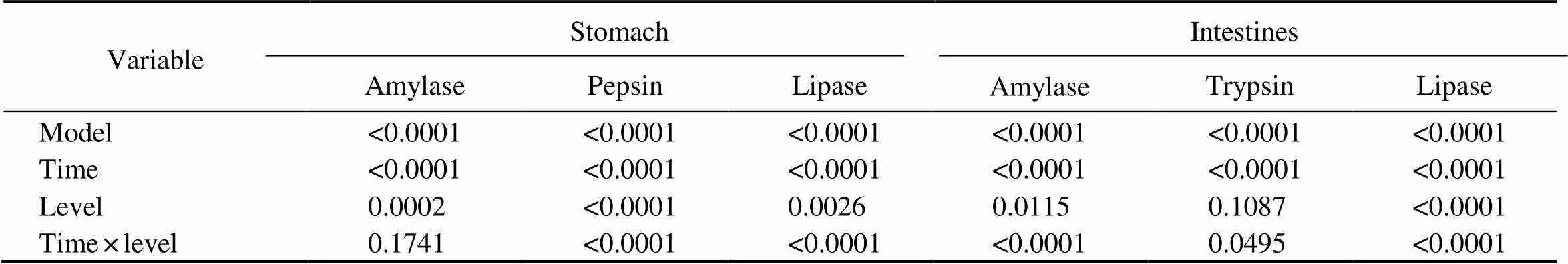
Table 6 The P-values of two-way ANOVAs for the effects of culture time and dietary DHA and EPA levels on the gastric and intestinal digestive enzyme activities in rainbow trout after salinity acclimation
Carbohydrate is hydrolyzed by amylase to glucose ab- sorbed by fish. In our results, the gastric and intestinal amylase activities of rainbow trout have not been signifi- cantly influenced by dietary DHA and EPA levels before salinity acclimation, which is consistent with the results of several previous studies. Murashita(2008) found that α-amylase activity in the digestive tract was not af- fected by dietary proteins, lipids or starch in yellowtail. Santigosa(2011) reported that amylase activity in the proximal intestine of gilthead sea bream (L.) was not affected when the fish oil in diets was replaced by vegetable oil causing low dietary DHA and EPA level. In addition, our findings indicate that gastric amylase activity was lower and more stable than intesti- nal amylase activity. A possible reason for this finding is that amylase is synthesized by the hepatopancreas and directly secreted into the intestines. Moreover, because the optimal pH for amylase is 7.0–8.0 (Ugwumba, 1993; Mu- nilla-Morán., 1996; Fernandez., 2001), the low pH in the stomach inhibits the activity of amylase.
In this study, short-term exposure to salinity stress in- hibited intestinal amylase activity of rainbow trout, and this effect might be due to the high concentration of Cl−in seawater, which negatively affects amylase activity (Po- doler., 1971). Although the intestinal amylase acti- vity of rainbow trout in the DE-1.40 and DE-1.79 groups could recover to the freshwater values on day 7 after sali- nity acclimation, the activities significantly decreased again on day 14.This result may indicate that high dietary DHA and EPA inhibits carbohydrate digestion in rainbow trout during long-term mariculture; however, its underlying me- chanism still needs further study.
Proteases are important components of the digestive en- zymes and are responsible for the digestion of dietary proteins (Psochiou., 2007). In the current study, no significant differences in the gastric pepsin and intestinal trypsin activities were observed among the groups of rain- bow trout prior to salinity acclimation. Similarly, Castro(2016) found that the dietary replacement of fish oil by vegetable oil had no effect on trypsin activity in Euro- pean sea bass (). However, You(2019) and Bowyer(2012) found decreased trypsin activity in golden pompano and yellowtail kingfish with low dietary DHA and EPA levels, respectively. Santigosa(2011) observed increased and decreased alkaline pro- tease activities in the pyloric caeca and proximal intestine, respectively, in gilthead sea bream fed diets including a vegetable oil blend. Therefore, it can be speculated that different effects of dietary DHA and EPA on protease acti- vity might be species- and/or tissue-specific.
Our findings show that gastric pepsin activity in rain- bow trout was significantly lower in the DE-0.54 group compared with the other groups on day 14 after seawater acclimation. A possible reason is that fish in this group can- not adapt to the seawater environment (data from this ex- periment) (Huang., 2020), and consequently, they need to take in large volumes of alkaline seawater to compen- sate for the water loss of tissues, resulting in an increase in pH that exceeds the optimal pH range for pepsin in the stomach (Usher., 1990; Boeuf., 2001; Gheisvan- di., 2015).
Intestinal trypsin activity was inhibited by short-term salinity stress, which is consistent with the results of se- veral previous studies (Woo., 1995; Moutou., 2004; Silva-Brito., 2019). In our study, the trypsin activity in rainbow trout recovered to the freshwater va- lues on day 14, which indicated that trypsin can regain its normal function after the fish adapt to the hypersaline environment. In addition, our results indicate that the try- psin activities in the DE-1.40 and DE-1.79 groups were significantly higher than those in the DE-0.54 and DE- 0.95 groups on day 14 after salinity acclimation. One pos- sible explanation is that rainbow trouts in the DE-1.40 and DE-1.79 groups exhibited higher intestinal DHA and EPA levels, resulting in improved membrane fluidity and material exchange ability (Huster., 1997; Hishikawa., 2017; Cornet., 2018). As a result, redundant ions can be excreted over time after the fish drink large volume of seawater, and the trypsin activity can thus be maintained at a high level because high concentrations of Cl−, Na+and K+inhibit the activity of protease (Squires., 1986b). Hence, a certain level of dietary DHA and EPA is necessary for the maintenance of high protease ac- tivity in rainbow trout after salinity acclimation.
Lipase plays important role in lipid absorption, which can hydrolyze dietary lipids into partial glycerides and free fatty acids (Hamosh., 1973). In our results, the low- est gastric lipase activity in rainbow trout was found in the DE-1.79 group before salinity acclimation, which is in agreement with the results of Morais(2004), who re- ported lower lipase specific activity in larval sea bass fed fish oil than in larvae fed coconut oil. However, the gas- tric lipase in the DE-1.79 group could increase to similar value as the other groups after salinity acclimation, indi- cating that gastric lipase activity of rainbow trout in the DE-1.79 group was only temporarily inhibited and can re- cover if needed.
The results of this study showed that intestinal lipase activity in rainbow trout was inhibited by short-term sa- linity stress. Similarly, Liu(2017) found that lipase activity in juvenile American shad () de- creased after exposure to increased salinity. Our findings showed that on day 14, the highest and lowest intestinal lipase activities were detected in the DE-0.54 and DE- 1.79 group, respectively. A possible explanation for this finding is that lipase specificity can change with the un- saturated degree and the chain length of dietary fatty ac- ids (Castro., 2016). Thus, low lipase activity is de- tected in fish if the demand for dietary essential fatty ac- ids has been met. An adequate supply of DHA and EPA is an indispensable factor for achieving suitable growth, sur- vival and anti-stress responses in fish (Furuita., 1998; Hamre., 2013; Pinto., 2016; Fuentes-Quesada.,2018). In the current study, the intestinal lipase ac- tivity of rainbow trout in the DE-0.54 group was higher than those in the other groups on days 7 and 14 because the fish in this group need to increase their lipase activity to enhance their utilization of lipids to meet their demands for DHA and EPA, since rainbow trout needs 1.40% die- tary DHA and EPA during salinity acclimation (Huang.,2020).
5 Conclusions
In this study, the gastric and intestinal DHA and EPA contents of rainbow trout were positively correlated to the dietary DHA and EPA levels. Both dietary DHA and EPA levels together with salinity acclimation play important roles in digestion in rainbow trout. Intestinal amylase, tryp- sin, and lipase activities of rainbow trout were inhibited by short-term exposure to salinity stress, while the gastric digestive enzyme activities were more stable. In the long term of salinity acclimation, high dietary DHA and EPA levels (DE-1.40 and DE-1.79) improved intestinal trypsin activities of rainbow trout, while rainbow trout in low die- tary DHA and EPA group (DE-0.54 group) cannot main- tain their activity of gastric pepsin. In addition, fish in the DE-0.54 group kept extremely high intestinal lipase acti- vity. According to the results, rainbow trout fed the DE- 0.54 diet cannot maintain an appropriate digestive capa- city after entering the seawater. Thus, a diet with a mini- mum DHA and EPA level equaling to 0.95% is necessary for rainbow trout during salinity acclimation.
Acknowledgements
The authors would like to express their gratitude to eve- ryone who critically reviewed this manuscript and helped with the sample collection. This publication was support- ed by the National Key Research and Development Pro- gram of China (No. 2019YFD0901005), the National Na- tural Science Foundation of China (Nos. 31702364 and31572634), and the Primary Research and Development Program of Shandong Province (No. 2018CXGC0101).
Ackman, R. G., and Takeuchi, T., 1986. Comparison of fatty acids and lipids of smolting hatchery-fed and wild Atlantic salmon ().,21 (2): 117-120, DOI: 10.1007/BF02534431.
Barman, U. K., Jana, S., Garg, S., Bhatnagar, A., and Arasu, A., 2005. Effect of inland water salinity on growth, feed conver- sion efficiency and intestinal enzyme activity in growing grey mullet,(Linn.): Field and laboratory studies.,13 (3): 241-256, DOI: 10.1007/s10499-004-2479-5.
Bell, J. G., Tocher, D. R., Farndale, B. M., Cox, D. I., McKinney, R. W., and Sargent, J. R., 1997. The effect of dietary lipid on polyunsaturated fatty acid metabolism in Atlantic salmon () undergoing Parr-Smolt transformation.,32 (5): 515-525, DOI: 10.1007/s11745-997-0066-4.
Bligh, E. G., and Dyer, W. J., 1959. A rapid method of total lipid extraction and purification.,37 (8): 911-917.
Boeuf, G., and Payan, P., 2001. How should salinity influence fish growth?,130 (4): 411-423, DOI: 10.1016/S1532-0456(01)00268-X.
Bolasina, S. N., Tagawa, M., and Yamashita, Y., 2007. Changes on cortisol level and digestive enzyme activity in juveniles of Japanese flounder,, exposed to diffe- rent salinity regimes.,266 (1-4): 255-261, DOI: 10.1016/j.aquaculture.2007.01.046.
Bolasina, S., Pérez, A., and Yamashita, Y., 2006. Digestive en- zymes activity during ontogenetic development and effect of starvation in Japanese flounder,.,252 (2-4): 503-515, DOI: 10.1016/j.aquaculture.2005.07.015.
Bowyer, J. N., Qin, J. G., Adams, L. R., Thomson, M. J., and Stone, D. A., 2012. The response of digestive enzyme acti- vities and gut histology in yellowtail kingfish () to dietary fish oil substitution at different temperatures.,368: 19-28, DOI: 10.1016/j.aquaculture.2012.09.012.
Castro, C., Couto, A., Perez-Jimenez, A., Serra, C. R., Diaz-Ro- sales, P., Fernandes, R., Corraze, G., Panserat, S., and Oliva-Teles, A., 2016. Effects of fish oil replacement by vegetable oil blend on digestive enzymes and tissue histomorphology of European sea bass () juveniles.,42 (1): 203-217, DOI: 10.1007/s10695-015-0130-1.
Castro, C., Pérez-Jiménez, A., Coutinho, F., Pousão-Ferreira, P., Brandão, T. M., Oliva-Teles, A., and Peres, H., 2013. Digestive enzymes of meagre () and white seabream (). Effects of dietary brewer’s spent yeast supple-mentation.,416: 322-327, DOI: 10.1016/j.aquaculture.2013.09.042.
Chen, M. Y., Zhang, X. M., Gao, T. X., and Chen, C., 2006. Ef- fects of temperature, pH and NaCl on protease activity in di- gestive tract of young turbot,.,24 (3): 300-306, DOI: 10.1007/PL00020776.
Cornet, V., Ouaach, A., Mandiki, S. N. M., Flamion, E., Ferain, A., Van Larebeke, M., Lemaire, B., Lopez, F. E. R., Tort, L., Larondelle, Y., and Kestemont, P., 2018. Environmentally-rea-listic concentration of cadmium combined with polyunsatu- rated fatty acids enriched diets modulated non-specific immu- nity in rainbow trout.,196: 104-116, DOI: 10.1016/j.aquatox.2018.01.012.
Dong, S., 2019. Research progress and prospect on large Sal- monidae at the areas of Cold Water Mass in Yellow Sea.,49: 1-6, DOI: 10.16441/j.cnki.hdxb.20180303.
Esmaeili, M., Kenari, A. A., and Rombenso, A. N., 2017. Effects of fish meal replacement with meat and bone meal using garlic () powder on growth, feeding, digestive enzymes and apparent digestibility of nutrients and fatty acids in juvenile rainbow trout (Walbaum, 1792).,23 (6): 1225-1234, DOI: 10.1111/anu.12491.
Evans, O., 2018. China gets ready to harvest first batch of farm- ed salmon from huge, deep sea fully-submersible fish cage. Salmon Business News. Retrieved from https://salmonbusi- ness.com/chinas-gets-ready-to-harvest-first-batch-of-farmed-salmon-from-huge-deep-sea-fully-submersible-fish-cage/.
Fernandez, I., Moyano, F., Dıaz, M., and Martınez, T., 2001. Cha- racterization of α-amylase activity in five species of Mediter- ranean sparid fishes ().,262 (1): 1-12, DOI: 10.1016/S0022-0981(01)00228-3.
Fountoulaki, E., Alexis, M. N., Nengas, I., and Venou, B., 2005. Effect of diet composition on nutrient digestibility and diges- tive enzyme levels of gilthead sea bream (L.).,36 (13): 1243-1251, DOI: 10.1111/j.1365-2109.2005.01232.x.
Fuentes-Quesada, J. P., and Lazo, J. P., 2018. The effect of lipid type on lipid digestion enzymes during larval development of the California halibut,.,488: 49-60, DOI: 10.1016/j.aquaculture.2018.01.018.
Furuita, H., Takeuchi, T., and Uematsu, K., 1998. Effects of ei- cosapentaenoic and docosahexaenoic acids on growth, survi- val and brain development of larval Japanese flounder ().,161 (1-4): 269-279, DOI: 10.1016/S0044-8486(97)00275-5.
Gheisvandi, N., Hajimoradloo, A., Ghorbani, R., and Hoseinifar, S. H., 2015. The effects of gradual or abrupt changes in sa- linity on digestive enzymes activity of Caspian kutum,(Kamensky, 1901) larvae.,31 (6): 1107-1112, DOI: 10.1111/jai.12891.
Glencross, B. D., 2009. Exploring the nutritional demand for essential fatty acids by aquaculture species.,1 (2): 71-124, DOI: 10.1111/j.1753-5131.2009.01006.x.
Guler, M., and Yildiz, M., 2011. Effects of dietary fish oil re- placement by cottonseed oil on growth performance and fatty acid composition of rainbow trout ().,35 (3): 157-167, DOI: 10.3906/vet-1002-252.
Hamosh, M., and Scow, R. O., 1973. Lingual lipase and its role in the digestion of dietary lipid.,52 (1): 88-95, DOI: 10.1172/JCI107177.
Hamre, K., Yúfera, M., Rønnestad, I., Boglione, C., Conceição, L. E., and Izquierdo, M., 2013. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing.,5: S26-S58, DOI: 10.1111/j.1753-5131.2012.01086.x.
Hishikawa, D., Valentine, W. J., Iizuka-Hishikawa, Y., Shindou, H., and Shimizu, T., 2017. Metabolism and functions of doco- sahexaenoic acid-containing membrane glycerophospholipids.,591 (18): 2730-2744, DOI: 10.1002/1873-3468.12825.
Hixson, S. M., Parrish, C. C., and Anderson, D. M., 2014. Changes in tissue lipid and fatty acid composition of farmed rainbow trout in response to dietary camelina oil as a replacement offish oil.,49 (1): 97-111, DOI: 10.1007/s11745-013-3862-7.
Huang, M., Zhou, Y., Liu, C., Davis, D. A., Li, L., Gao, Q., and Dong, S., 2020. Fatty acid composition, osmolality, Na+, K+- ATPase activity, cortisol content and antioxidant status of rain- bow trout () in response to various die- tary levels of eicosapentaenoic acid and docosahexaenoic acid., 51 (7): 2777-2789, DOI: 10.1111/are.14617.
Huster, D., Jin, A. J., Arnold, K., and Gawrisch, K., 1997. Water permeability of polyunsaturated lipid membranes measured by O-17 NMR.,73 (2): 855-864, DOI: 10.1016/S0006-3495(97)78118-9.
Li, H. O., and Yamada, J., 1992. Changes of the fatty-acid com- position in smolts of masu salmon (), associated with desmoltification and sea-water transfer.,103 (1): 221-226, DOI: 10.1016/0300-9629(92)90266-S.
Liu, C. Y., Zhou, Y. G., Dong, K., Sun, D. J., Gao, Q. F., and Dong, S. L., 2018. Differences in fatty acid composition of gill and liver phospholipids between steelhead trout () and Atlantic salmon () under de- clining temperatures.,495: 815-822, DOI: 10.1016/j.aquaculture.2018.06.045.
Liu, Z. F., Gao, X. Q., Yu, J. X., Qian, X. M., Xue, G. P., Zhang, Q. Y., Liu, B. L., and Hong, L., 2017. Effects of different sa- linities on growth performance, survival, digestive enzyme ac-tivity, immune response, and muscle fatty acid composition in juvenile American shad ().,43 (3): 761-773, DOI: 10.1007/s10695-016-0330-3.
Morais, S., Cahu, C., Zambonino-Infante, J. L., Robin, J., Ron- nestad, I., Dinis, M. T., and Conceicao, L. E. C., 2004. Die- tary TAG source and level affect performance and lipase ex- pression in larval sea bass ().,39 (5): 449-458, DOI: 10.1007/s11745-004-1250-2.
Moser, M., and Miller, J., 1994. Effects of salinity fluctuation on routine metabolism of juvenile spot,.,45 (2): 335-340, DOI: 10.1111/j.1095-8649.1994.tb01312.x.
Moutou, K. A., Panagiotaki, P., and Mamuris, Z., 2004. Effects of salinity on digestive protease activity in the euryhaline sparidL.: A preliminary study.,35 (9): 912-914, DOI: 10.1111/j.1365-2109.2004.01068.x.
Munilla-Morán, R., and Saborido-Rey, F., 1996. Digestive en- zymes in marine species. II. Amylase activities in gut from sea-bream (), turbot () and red-fish ().,113 (4): 827-834, DOI: 10.1016/0305-0491(95)02101-9.
Murashita, K., Fukada, H., Rønnestad, I., Kurokawa, T., and Ma-sumoto, T., 2008. Nutrient control of release of pancreatic en- zymes in yellowtail (): Involvement of CCK and PY in the regulatory loop.,150 (4): 438-443, DOI: 10.1016/j.cbpa.2008.05.003.
Nikolopoulou, D., Moutou, K., Fountoulaki, E., Venou, B., Ada- midou, S., and Alexis, M., 2011. Patterns of gastric evacu- ation, digesta characteristics and pH changes along the gas- trointestinal tract of gilthead sea bream (L.) and European sea bass (L.).,158 (4): 406-414, DOI: 10.1016/j.cbpa.2010.11.021.
Noda, M., and Murakami, K., 1981. Studies on proteinases from the digestive organs of sardine. II. Purification and charac- terization of two acid proteinases from the stomach.–,658 (1): 27-34, DOI: 10.1016/0005-2744(81)90246-1.
NRC, 2011.. National Academic Press, Washington D.C., 376pp.
Pinto, W., Engrola, S., Santos, A., Bandarra, N. M., Dias, J., and Conceição, L. E., 2016. Can Senegalese sole post-larvae ef- fectively grow on low dietary DHA and lipid levels during weaning?,463: 234-240, DOI: 10.1016/j.aquaculture.2016.05.027.
Podoler, H., and Applebaum, S., 1971. The α-amylase of the beetleproperties.,121 (2): 321-325, DOI: 10.1042/bj1210321.
Psochiou, E., Mamuris, Z., Panagiotaki, P., Kouretas, D., and Moutou, K. A., 2007. The response of digestive proteases to abrupt salinity decrease in the euryhaline sparidL.,147 (2): 156-163, DOI: 10.1016/j.cbpb.2006.12.021.
Rungruangsak-Torrissen, K., Moss, R., Andresen, L., Berg, A., and Waagbø, R., 2006. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (L.).,32 (1): 7, DOI: 10.1007/s10695-005-0630-5.
Santigosa, E., García-Meilán, I., Valentín, J. M., Navarro, I., Pérez-Sánchez, J., and Gallardo, M. Á., 2011. Plant oils’ in- clusion in high fish meal-substituted diets: Effect on diges-tion and nutrient absorption in gilthead sea bream (L.).,42 (7): 962-974, DOI: 10.1111/j.1365-2109.2010.02679.x.
Santigosa, E., Sánchez, J., Médale, F., Kaushik, S., Pérez-Sán-chez, J., and Gallardo, M., 2008. Modifications of digestive enzymes in trout () and sea bream () in response to dietary fish meal replacement by plant protein sources.,282 (1-4): 68-74, DOI: 10.1016/j.aquaculture.2008.06.007.
Sheridan, M. A., Allen, W. V., and Kerstetter, T. H., 1985. Changes in the fatty acid composition of steelhead trout,Richardson, associated with parr-smolt transformation.,80 (4): 671-676, DOI: 10.1016/0305-0491(85)-90444-4.
Silva-Brito, F., Timoteo, F., Esteves, A., Peixoto, M. J., Ozorio, R., and Magnoni, L., 2019. Impact of the replacement of dietary fish oil by animal fats and environmental salinity on the me- tabolic response of European seabass ().,233: 46-59, DOI: 10.1016/j.cbpb.2019.04.004.
Sivaramakrishnan, T., Sahu, N. P., Jain, K. K., Muralidhar, A. P., Saravanan, K., Ferosekhan, S., Praveenraj, J., and Artheeswa- ran, N., 2017. Optimum dietary lipid requirement ofjuveniles in relation to growth, fatty acid profile, body indices and digestive enzyme activity.,25 (2): 941-954, DOI: 10.1007/s10499-016-0090-1.
Squires, E. J., Haard, N., and Feltham, L., 1986a. Gastric pro-teases of the Greenland cod. I. Isolation and kine- tic properties.,64 (3): 205-214, DOI: 10.1139/o86-030.
Squires, E. J., Haard, N., and Feltham, L., 1986b. Gastric pro- teases of the Greenland cod. II. Structural pro- perties.,64 (3): 215-222, DOI: 10.1139/o86-031.
Sutthinon, P., Thongprajukaew, K., Saekhow, S., and Ketmanee, R., 2015. Juvenile hybrid grouper (×) are euryhaline and can grow in a wide range of salinities.,23 (2): 671-682, DOI: 10.1007/s10499-014-9845-8.
Thanuthong, T., Francis, D. S., Manickam, E., Senadheera, S. D., Cameron-Smith, D., and Turchini, G. M., 2011. Fish oil re-placement in rainbow trout diets and total dietary PUFA con- tent: II) Effects on fatty acid metabolism andfatty acid bioconversion., 322: 99-108, DOI: 10.1016/j.aquaculture.2011.09.026.
Tocher, D. R., Bell, J. G., Dick, J. R., Henderson, R. J., McGhee, F., Michell, D., and Morris, P. C., 2000. Polyunsaturated fatty acid metabolism in Atlantic salmon () undergoing parr-smolt transformation and the effects of dietary linseed and rapeseed oils.,23 (1): 59-73, DOI: 10.1023/a:1007807201093.
Tsuzuki, M. Y., Sugai, J. K., Maciel, J. C., Francisco, C. J., and Cerqueira, V. R., 2007. Survival, growth and digestive enzyme activity of juveniles of the fat snook () reared at different salinities.,271 (1-4): 319-325, DOI: 10.1016/j.aquaculture.2007.05.002.
Ugwumba, A., 1993. Carbohydrases in the digestive tract of the African bony-tongue(Pisces: Osteoglossi- dae).,257 (2): 95-100, DOI: 10.1007/BF00005949.
Usher, M., Talbot, C., and Eddy, F., 1988. Drinking in Atlantic sal- mon smolts transferred to seawater and the relationship be- tween drinking and feeding.,73 (1-4): 237-246, DOI: 10.1016/0044-8486(88)90058-0.
Usher, M., Talbot, C., and Eddy, F., 1990. Effects of transfer to seawater on digestion and gut function in Atlantic salmon smolts (L.).,90 (1): 85-96, DOI: 10.1016/0044-8486(90)90285-U.
Van Anholt, R. D., Spanings, F. A. T., Nixon, O., Bonga, S. E. W., and Koven, W. M., 2012. The effects of arachidonic acid on the endocrine and osmoregulatory response of tilapia () acclimated to seawater and subjected to confinement stress.,38 (3): 703-713, DOI: 10.1007/s10695-011-9552-6.
Wijekoon, M. P. A., Parrish, C. C., and Mansour, A., 2014. Ef- fect of dietary substitution of fish oil with flaxseed or sun- flower oil on muscle fatty acid composition in juvenile steel- head trout () reared at varying tempera- tures.,433: 74-81, DOI: 10.1016/j.aquaculture.2014.05.028.
Woo, N. Y., and Kelly, S. P., 1995. Effects of salinity and nutri- tional status on growth and metabolism ofin a closed seawater system.,135 (1-3): 229-238, DOI: 10.1016/0044-8486(95)01003-3.
Xie, D. Z., Xu, S. D., Wu, Q. Y., Chen, F., Wang, S. Q., You, C. H., and Li, Y. Y., 2018. Changes of visceral properties and digestive enzymes in the herbivorous marine teleostfed on different diets.,37 (2): 85-93, DOI: 10.1007/s13131-018-1165-9.
You, C., Chen, B., Wang, M., Wang, S., Zhang, M., Sun, Z., Ju- ventus, A. J., Ma, H., and Li, Y., 2019. Effects of dietary lipid sources on the intestinal microbiome and health of golden pompano ().,89: 187-197, DOI: 10.1016/j.fsi.2019.03.060.
. Tel: 0086-532-82031590
E-mail: zhouyg@ouc.edu.cn
January 24, 2020;
May 8, 2020;
September 1, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Numerical Simulation and Risk Analysis of Coastal Inundation in Land Reclamation Areas: A Case Study of the Pearl River Estuary
- Variation of Yellow River Runoff and Its Influence on Salinity in Laizhou Bay
- Cold Water in the Lee of the Batanes Islands in the Luzon Strait
- Preliminary Design of a Submerged Support Structure for Floating Wind Turbines
- Inversion of Oceanic Parameters Represented by CTD Utilizing Seismic Multi-Attributes Based on Convolutional Neural Network
- Trace-Norm Regularized Multi-Task Learning for Sea State Bias Estimation
