Nitroso Glutathione Reductase GSNOR1 for Positive Regulation of Plant Resistance against Phytophthora
2020-10-28LIUXingshaoSONGSiyuGAOXianxianSHANWeixingandQIANGXiaoyu
LIU Xingshao, SONG Siyu, GAO Xianxian, SHAN Weixing and QIANG Xiaoyu
(1.College of Agronomy, Northwest A&F University, Yangling Shaanxi 712100, China;(2.College of Plant Protection, Northwest A&F University, Yangling Shaanxi 712100, China)
Abstract Oomycetes, especially Phytophthora, cause devastating crop diseases such as potato late blight caused by P.infestans.To conquer the disease , it is of great significance to explore the plant resistance mechanism and identify novel factors for resistance breeding.Nitroso glutathione reductase 1(GSNOR1)is a highly conserved reductase in plant nitric oxide signaling, which involves in regulating R-gene mediated resistance and non-host resistance.However, its potential function and mechanism in resistance against Phytophthora remain unclear.In this study, the resistance phenotypic analysis on Arabidopsis T-DNA insertion mutant atgsnor1-3 was used to determine the positive regulation of AtGSNOR1 in resistance to P.parasitica.Furthermore, by exploiting tobacco rattle virus(TRV)for virus-induced gene silencing(VIGS),the Nicotiana benthamiana plants with reduced GSNOR1 homolog levels was used, we demonstrated that silencing of GSNOR1 homolog in N.benthamiana enhances plant susceptibility, accompanied with the suppression of reactive oxygen species(ROS)production, induction of PR genes and MAPK signaling upon infection by P.parasitica.This study revealed the highly conserved function and mechanism of GSNOR1 in plant resistance against Phytophthora, which facilitated further exploration and potential application of GSNOR1 inbreeding of potatolate blight disease resistance.
Key words GSNOR; Phytophthora parasitica; Nicotiana benthamiana; Arabidopsis; Potato late blight
Phytophthora, a genus of oomycetes, consists of more than 120 species which cause destructive diseases on agricultural crops, forests and natural ecosystems.For instance, the potato late blight caused byP.infestansand tobacco black shank caused byP.parasiticathreaten the sustainable crop production worldwide[1-2].Since the virulence variation ofPhytophthorais rapid, the loss of genotype-specific plant resistance is a key problem in crop production.Take the potato late blight as a typical example, the disease control is highly dependent on pesticides, the outcome of which is the environmental and ecological pollution.Therefore, to identify novel factors those confer durable resistance and decipher their resistance mechanisms are of great importance particularly in accelerating resistance breeding inSolanaceousplants and contributing to sustainable agriculture.P.parasitica, a model oomycete, is a typical hemibiotrophic pathogen, which has a wide range of hosts[2].The interacting model systems ofP.parasiticawithArabidopsisas well asN.benthamianaprovide a powerful tool to establish these mechanistic studies[3].
In natural environment, to avoid pathogenic infection, plants have evolved two levels of innate immune responses against pathogens[4].The first level is triggered by pathogens-associated molecular pattern(PAMP), which is termed as PAMP-triggered immunity(PTI).The immune response is activated through recognition of PAMPs by plant plasma membrane-localized pattern recognition receptors(PRRs), which typically produce reactive oxygen and nitrogen intermediates(ROIs and RNIs, respectively)and induction of mitogen-activated protein kinase(MAPK)signaling cascade[5].The second is triggered by pathogen effectors, which is termed as effector-triggered immunity(ETI).The immune response is activated through recognition of effectors by nucleotide binding-leucine rich repeat(NB-LRR)proteins encoded by plant resistance genes(Rgenes)[6].
In the course of evolutionary changes during plant-pathogen interactions, pathogens have developed various invasion strategies, to which the host plant responded with rapid and marked changes in cellular redox status[7].These changes are mostly triggered by the accumulation of ROIs and RNIs, which are significant features of the plant defense response.Although the enzymes required for apoplastic ROI synthesis are well established[8], the source of RNIs remains elusive[9-10].Increasing evidence suggests that the redox active, small molecules integrate various plant defense mechanisms.For instance, the signaling function of nitric oxide(NO)is shown during both highly conserved PTI and highly specific ETI, which typically accompanied by the hypersensitive response(HR)at the site of infection[11].
As an endogenous signaling molecule, NO is such kind of free radical gas that can diffuse rapidly through biological membranes[12].To constitute a relatively stable store of NO bioactivity, S-nitroso glutathione(GSNO)is formed by the covalent attachment of NO to the cysthiol within the antioxidant tripeptide, glutathione(GSH)[13-14].The addition of an NO moiety to a protein cysteine(Cys)thiol to form an S-nitrosothiol(SNO)has emerged as a prototypic redox-based, post-translational modification, which is termed as S-nitrosylation[15-17].Cellular GSNO homeostasis is controlled by the enzyme GSNO reductase(GSNOR).This enzyme functions highly conserved among animals, plants and bacteria[18].A.thalianaS-nitroso glutathione reductase 1(AtGSNOR1), was identified to modulate the extent of cellular SNO formation following nitrosative stress,and alterations in total SNO levels strongly affect signaling transduction and modulate multiple modes of plant immunity[17,19-21].
The phenolic metabolite salicylic acid(SA)is a crucial immune activator in plants.Emerging evidence suggests that the absence of GSNOR1 activity increases cellular GSNO levels and leads to significantly reduced SA accumulation and weak activation of SA-dependent defense genes[19].Arabidopsisplants with compromised GSNOR1 function were shown to be disabled inRgene-mediated protection mediated by either the Toll interleukin(TOLL)or coiled-coiled class of NB-LRR proteins, which posse different signaling requirements[22].Moreover,atgsnor1-3plants are disabled in basal resistance against the bacterial pathogenPseudomonassyringaepv.tomato(Pst)DC3000 and the oomycete,Hyaloperonosporaarabidopsidis.Non-host resistance(NHR)can protect plants against the majority of potential pathogens.However, the underlying molecular mechanisms of NHR are less well understood.Notably,atgsnor1-3plants enable the growth of the wheat powdery mildew pathogen,Blumeriagraminisf.sp.tritici, which is a major wheat pathogen but is not adapted for growth onArabidopsis.Besides,atgsnor1-3plants are also host forPseudomonassyringaepv.phaseolicolaandPseudomonasfluorescens; while wild-typeArabidopsisplants do not support the growth of these non-adapted bacterial pathogens.Thus, GSNOR1 also regulates the development of non-host resistance(NHR)against both fungaland bacterial pathogens[19].Collectively, these data imply that GSNOR1 is involved in modulating multiple modes of plant disease resistance.
As a crucial and highly conserved reductase in plant NO signaling, the potential function of GSNOR in plant resistance againstPhytophthoraremains unexplored.In this study, with the benefit of interacting model systems ofP.parasiticawithArabidopsisas well asN.benthamiana, we performed set of analyses on the resistance function ofAtGSNOR1and theGSNOR1homolog inN.benthamianaagainstP.parasitica.We demonstrate that plantGSNOR1might have conserved function in plant resistance againstPhytophthorathrough positively regulating ROS production, induction ofPRgenes, andMAPKcascade signaling at different stages of attempted infection.This study reveals the conserved mechanism ofGSNOR1in plant resistance toPhytophthora, which facilitate further exploration and potential application ofGSNOR1on potato late blight disease resistance breeding.
1 Materials and Methods
1.1 Materials
ArabidopsisT-DNA insertion mutants in Col-0 background,atgsnor1-3(GABI_315D11)was obtained from theArabidopsisBiological Resource Center(ABRC).N.benthamianawas obtained from State Key Laboratory of Crop Stress Biology for Arid Areas of Northwest A&F University.
LB culture medium(500 mL): Trptone 5 g, yeast extract 2.5 g, NaCl 5 g, Agar 4.0 g(Liquid medium without Agar), add to 500 mL with ddH2O.
5% CA culture medium(500 mL): 5% carrot juice, β-sitosterol 0.02 g, CaCO30.3 g, Agar 4.0 g(Liquid medium without Agar), add to 500 mL with ddH2O.
Fast pfu DNA polymerase, EasyTaqDNA Polymerase, T4DNA Ligase, restriction endonuclease(promega):EcoRⅠ,XhoⅠ, Antibiotics: Kanamycin, Gentamicin, Rifampin.
1.2 Methods
1.2.1 Plant Materials and Growth Condition For the root inoculation assay,A.thalianaseeds were surface-sterilized before being plantedon 1/2 MS medium at 4 ℃ for 3 days and then transferred the seeds to 23 ℃ for about 10 days before infection assays.For other experiments,A.thalianaandN.benthamianaseeds were surface-sterilized and transferred to soil for about one month.A.thalianaandN.benthamianawere grown in the same conditions as previously described[23].
1.2.2 Plasmid Constructs To create TRV-based virus induced gene silencing(VIGS)constructs, the full length cDNA sequences ofAtGSNOR1(AT5G43940)were firstly acquired from TAIR homepage(TheArabidopsisInformation Resource, https://www.arabidopsis.org/).Thereafter, through BLAST tool from Solanaceae Genomics Network(https://solgenomics.net/tools/blast/),the full length cDNA sequences ofGSNOR1homolog inN.benthamianawere obtained.The sequence ofGSNOR1homolog was cloned fromN.benthamianacDNA and inserted into tobacco rattle virus(TRV)TRV2 vector withEcoRⅠ andXhoⅠ sites[24-25].The coding sequence ofNbGSNOR1was cloned fromN.benthamianacDNA using gene-specific primers(Table 1).
1.2.3P.parasiticaInoculation Assay The culture and zoospore production ofP.parasiticastrainPp016were conducted as previously described[3].For the leaf inoculation assay, detached leaves were inoculated on the abaxial leaf surface with a 10 μL droplet containing—200P.parasiticazoospores μL-1.Hyphae in epidermal cells were observed at 48 hpi with fluorescence microscope andP.parasiticainfected leaf discs were measured at 72 hpi.For the root inoculation assay, 5-day-oldA.thalianaseedlings grown in 1/2MS medium were inoculated withP.parasitica, and resistance phenotype was observed at 10 dpi.Col-0 was used as wild-type control plant in the infection assays.For the inoculation assay onN.benthamianadetached leaves, approximately 8 mm diameter of mycelium plugs were cut from theP.parasiticastrainPp016cultures and inoculated near the infiltrated sites.Lesion diameters were measured at 2 dpi and then stained with trypan blue.
1.2.4 TRV-based VIGS inN.benthamianaAgrobacteriumtumefaciensstrain GV3101 harbouring pTRV2::NbGSNOR, pTRV2::GFPor pTRV2::PDSconstruct was mixed with strains carrying pTRV1 vector in a 1∶1 ratio to achieve final concentration of OD600=0.25 for each component.The largest leaves of 3-week-old plants were chosen for infiltration and the silenced plants were used to inoculate withP.parasitica3 weeks later as previouslydescribed[26].Three independent experiments were performed.
1.2.5 Gene Expression Analysis Plant total RNA was extracted using TRIzol(Invitrogen)reagent.For qRT-PCR analysis, cDNA was synthesized from 1 μg of total RNA using PrimeScriptTMRT reagent Kit(TaKaRa).20 ng of cDNA was used as template for the amplification of candidate genes using SYBR premix Kit(Roche)according to the manufacturers’ instructions.Quantitative analysis for the relative expression level of the tested genes was performed using the SYBR Premix Kit(Roche, Basel, Switzerland)on Q7 Real Time Cycler(Thermo Fisher Scientific).The Ct values of tested genes were normalized toNbACTINinN.benthamiana.The primers used are listed in Table 1.Expression fold changes were calculated by the 2-△△Ctmethod.
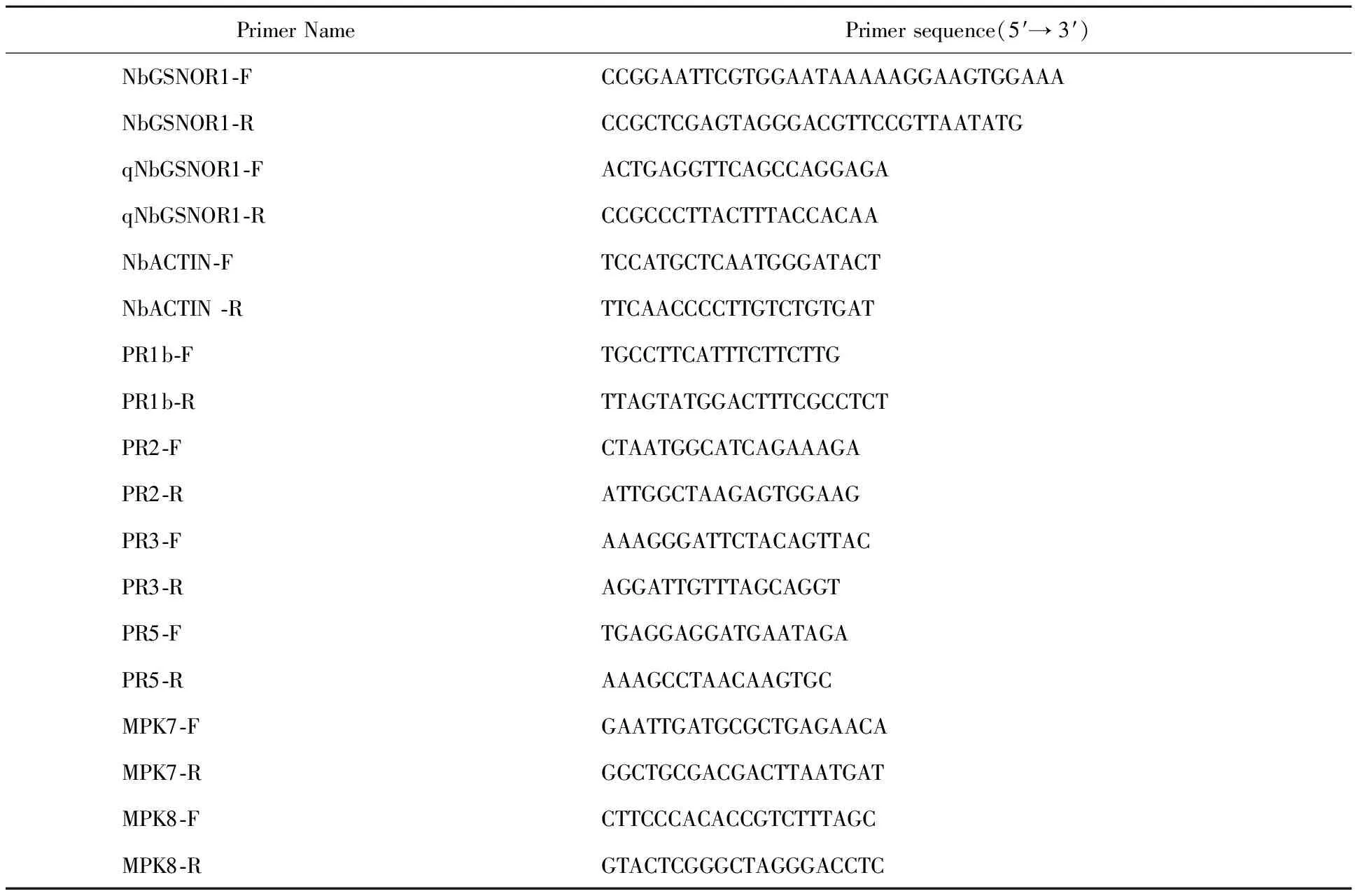
Table 1 Primer sequences used in this study
1.2.6 Trypan Blue Staining Assay DetachedN.benthamianaleaves were stained with lactophenol-trypan blue(10 mL lactic acid, 10 mL glycerol, 10 g phenol, and 10 mg trypan blue, dissolved in 10 mL of distilled water).After boiling for 2 min in the staining solution and de-staining in 2.5 g/mL chloral hydrate, the leaves were mounted in 70% glycerol for microscopic observation.
1.2.7 DAB Staining Assay DetachedN.benthamianaleaves were blocked in DAB solution(1 g/mL DAB, modulate pH to 3.0 with 0.2 mol/L HCL)supplemented with 0.05% Tween 20 and 10 mmol/L Na2HPO4for 12 h.The leaves were decolorized with decolorizing solution(Ethylalcohol absolute∶Acetic Acid∶Glycerol = 3∶1 ∶1)and boiled in distilled H2O for 15 minutes.Thereafter, the leaves were photographed.
1.2.8 Protein Immunoblot Assays Total protein was extracted with GTEN lysis buffer(10% glycerol, 25 mmol/L Tris pH 7.5, 1 mmol/L EDTA, 150 mmol/L NaCl)supplemented with 2% PVPP, 10 mmol/L DTT, 1×protease inhibitor cocktail(Sigma)and 0.1% Tween 20.Proteins were separated by SDS-PAGE and transferred from the gel to a PVDF membrane(Roche)in transfer buffer(25 mmol/L Tris, 200 mmol/L glycine, and 20% methanol).The membrane was then blocked in TBST buffer(Tris-buffered saline with 0.05% Tween 20[pH 7.2])containing 10% non-fat dry milk under gentle shaking.The blocked membrane was incubated with anti-MPK6(Agrisera, Sweden; AS12-2633)dissolved in TBSTM(TBST with 5% non-fat dry milk)at a ratio of 1∶5 000 and incubated at 4 ℃ with shaking at 50 rpm overnight, followed by three washes(10 min each)with TBST.Next, the membrane was incubated with a secondary antibody HRP goat anti-rabbit IgG(H + L)antibody(#AS014, ABclonal), which was also dissolved in TBST at a ratio of 1∶2 000, at room temperature for 1.5 h with shaking.Thereafter the membrane was washed three times(10 min each)with TBST and one time with TBS, then incubated with ECL(#CW0049S, ComWin)before photographing using a molecular imager(ChemiDoc XRS+, Bio-Rad).
2 Results
2.1 Atgsnor1 mutants exhibit both leaf and root susceptibility to P.parasitica
To explore the potential function ofGSNOR1in plant resistance toPhytophthora, we first examined resistance ofatgsnor1-3mutant againstP.parasitica.The detached leaves of six-week-oldatgsnor1-3mutants as well as WT Col-0 plants were inoculated byP.parasiticazoospores.At 72 hpi, leaves of WT Col-0 plants displayed severe water-soaked lesions, indicative of susceptibility againstP.parasiticainfection(Fig.1-A).In comparison, the water-soaked lesions inatgsnor1-3mutant were expanded almost all over the whole leaf, indicating thatatgsnor1-3mutant is more susceptible toP.parasitica(Fig.1-A).Furthermore, visible GFP expressing hyphae colonized WT Col-0 leaves, following infection with stable GFP-expressingP.parasiticatransformant.Conversely,atgsnor1-3mutant plant leaves showed heavier colonization(Fig.1-B).Consistently, the disease index statistic for infected leaves indicated thatatgsnor1-3mutant plants were obviously more susceptible than WT Col-0 plants toP.parasiticainfection(Fig.1-C).
To examine whetherGSNORfunctions in root resistance, 2-week-old seedlings were dip inoculated withP.parasiticazoospores and incubated for 10 days.Most WT Col-0 seedlings were infected and colonized withP.parasiticaat 10 dpi, whereas much heavier infection and root wilt symptom were observed inatgsnor 1-3mutant seedlings(Fig.1-D).Taken together, these results implied thatAtGSNOR1plays an important role in plant resistance toPhytophthora.
A.Phenotype of detached leaves of wild type Col-0 and atgsnor1-3 mutant after 3 days inoculation with P.parasitic; B.Hypha colonization of GFP expressing P.parasitica in detached leaves after 3 days inoculation with P.parasitic, Scale bar, 500 μm; C.Disease severity index(DSI)from level 1 to level 4 was recorded at 48 hpi(Level 1:disease symptom is less than 1/3 of whole leaf area; Level 2: disease symptom is between 1/3 and 1/2 of whole leaf area; Level 3: disease symptom is between 1/2 and 2/3 of whole leaf area; Level 4: disease symptom is more than 2/3 of whole leaf area).D.The root phenotype of wild type Col-0 and atgsnor1-3 mutant after 10 days inoculation with P.parasitica.
2.2 Silencing of GSNOR1 homolog in N.benthamiana enhances plant susceptibility to P.parasitica
To further investigate the resistance function ofGSNOR1homolog in solanaceous plant toPhytophthora, we set out to obtainN.benthamianaplants with reducedGSNOR1levels by exploiting tobacco rattle virus(TRV)for virus-induced gene silencing(VIGS).For identifying target sequences for VIGS, we first acquired the full length cDNA sequences ofAtGSNOR1(AT5G43940)from TAIR homepage(TheArabidopsisInformation Resource, https://www.arabidopsis.org/).Thereafter, through BLAST tool fromSolanaceaeGenomics Network(https://solgenomics.net/tools/blast/), the full length cDNA sequences ofGSNOR1homolog inN.benthamianawere obtained and further sequence alignment showed its high sequence similarity toAtGSNOR1, which is 90.69%(Fig.2-A).Based on this, we generated a VIGS construct that targetsNbGSNOR1.Quantitative reverse transcription(Q-RT)-PCR analyses showed strongly reduced levels ofNbGSNOR1mRNA, reaching on average only 12% of the normal level inN.benthamiana(Fig.2-B).Simultaneously, silencing of the endogenousphytoenedesaturase(PDS)gene, which causes photo bleaching, was used as a control for VIGS efficiency(Fig.2-C).
To study the role ofNbGSNOR1in defense againstPhytophthora, we performed infection assays on leaves detached fromGSNOR1-silencedN.benthamianaplants withP.parasitica.The results showed that the water-soaked lesions onGSNOR1-silenced plants(TRV:GSNOR1)are larger when compared with the lesions on control plants(TRV:GFP)at 2 dpi(Fig.2-D and 2-E).This indicates that in both plant species,GSNOR1is required to counteract the pathogen.

A.N.benthamiana GSNOR1 homologous sequences were obtained by BLAST sequence alignment; B.Relative quantification(RQ)of GSNOR1 expression at 21 dpi with TRV constructs in the qRT-PCR assay.NbACTIN expression was used for normalization.GSNOR1 expression in the TRV:GFP-treated plant was set at 100%.The experiment was repeated three times.Error bars indicate the SE from three biological replicates; C.silencing of the endogenous phytoenedesaturase(PDS)gene, which causes photo bleaching; D.Leaves of NbGSNOR1 silenced plants and control plants were inoculated by P.parasitica, and were strained by trypan blue at 2 dpi.The stained leaves were photographed; E.The lesion diameter statistical analysis of N.benthamiana leaves after 2 days inoculation with P.parasitic.
2.3 Silencing of NbGSNOR1 suppresses plant reactive oxygen species burst upon infection by P.parasitica
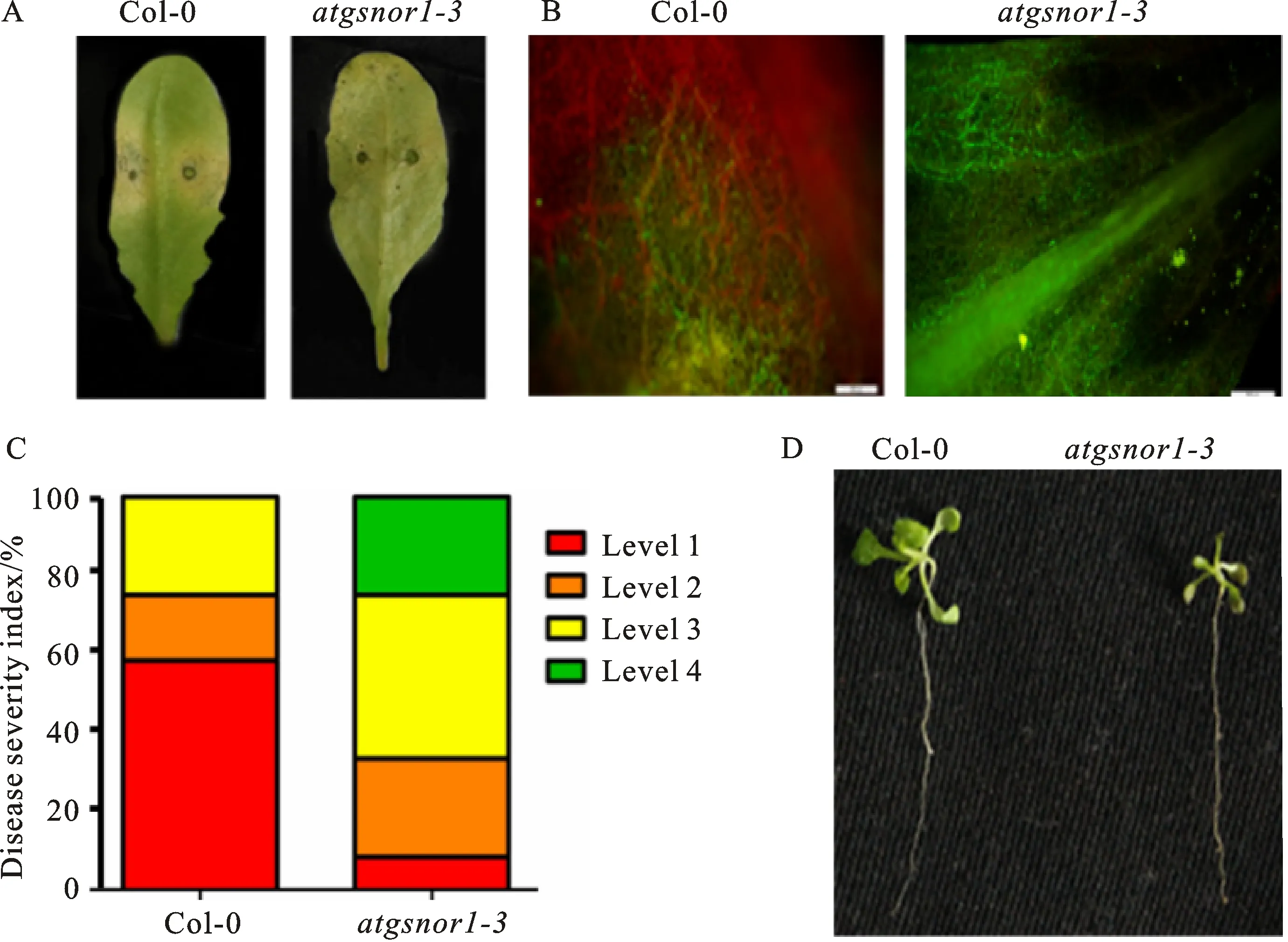
Production of reactive oxygen species(ROS)is critical for successful activation of immune responses against pathogen infection[23].In leaves, the active epitopes of bacterial flagellin(flg22)triggers the oxidative burst and induces defense gene transcription[27-28].To further elucidate whetherGSNOR1modulates ROS burst in response toP.parasitica, we analyzed the production of H2O2, a ROS that can be visualized with 3, 3′-diaminobenzidine-tetrahydrochloride(DAB)staining, in bothGSNOR1-silenced plants and control plants upon infection byP.parasitica.No obvious difference in DAB staining was observed in bothGSNOR1-silenced plants and control plants at 0 hpi(Fig.3).As a control assay, clear staining was visible in control plants(TRV:GFP)treated with flg22, whereas much weaker staining exhibited inGSNOR1-silenced plants treated with flg22(Fig.3).Similarly, the DAB staining was weaker at 3 hpi, but became gradually stronger from 6 hpi to 12 hpi in control plants, indicating an increase of H2O2production in response to the pathogen.By contrast, the DAB staining was hardly detectable at 3 hpi, and much weaker staining at 6 hpi and 12 hpi was observed inGSNOR1-silenced plants(Fig.3), demonstrating that production of H2O2was clearly reduced.These results suggest that the ROS burst appears to be attenuated inGSNOR1-silenced plants upon infection byP.parasitica.
Leaves of NbGSNOR1 silenced plants and control plants were inoculated by P.parasitica, and were strained by DAB at 0 h, 3 h, 6 h and 12 h post inoculation.The DAB staining of 10 mmol/L flg22 injected N.benthamiana leaves were used as positive controls.The stained leaves were photographed
2.4 Silencing of NbGSNOR1 suppresses induction of MPK genes and attenuates accumulation of MPK6 upon infection by P.parasitica
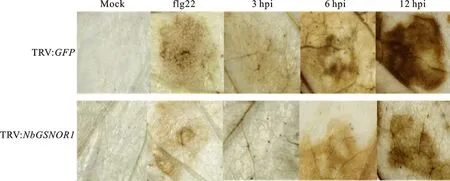
The activation of MAPK cascades is one of the marker events for PTI and plays a crucial role in defense response to pathogens in plants[29-30].InA.thaliana,MPK3,MPK4andMPK6are activated by a broad range of biotic stresses[31-33].Furthermore,MPK7andMPK8are reported to mediate ROS signaling and regulateMPK6in response to jasmonic acid, which is an important phytohormone for activating plant defense response against necrotrophic pathogens[32,34-35].These prompted us to examine whetherGSNOR1might involve in the regulation of MAPK signaling in response to the pathogen.The detached leaves of bothGSNOR1-silencedN.benthamianaplants and control plants inoculated withP.parasiticawere harvested at 3, 6 and 12 hpi.The qRT-PCR analyses showed strongly reduced levels ofNbGSNOR1mRNA inGSNOR1-silencedN.benthamianaplants during the infection byP.parasiticaat indicated time points(Fig.4-A).Based on this, we first monitored the dynamic transcript levels ofMPK7andMPK8.As expect, bothMPK7andMPK8were highly up-regulated in control plants during the infection, although theMPK8showed significant up-regulation from 6 hpi(Fig.4-C and 4-D).By contrast, inGSNOR1-silencedN.benthamianaplant, the induction ofMPK7was significantly reduced during the infection and the induction ofMPK8was only slightly reduced at 6 hpi(Fig.4-C and 4-D).Notably, at very early infection stage(3 hpi),MPK8showed almost no induction in control plants, whereas the expression ofMPK8appeared down-regulated compared to that exhibited at 0 hpi inGSNOR1-silencedN.benthamianaplants(Fig.4-D).Although the up-regulation ofMPK8at 12 hpi was detected inGSNOR1-silencedN.benthamianaplants(Fig.4-D), it might be due to the attenuated level ofGSNOR1silencing at indicated time point(Fig.4-A).
A.Relative quantification(RQ)of GSNOR1 expression of leaves of NbGSNOR1 silenced plants which were inoculated by P.parasiticaat 0 h, 3 h, 6 h and 12 h post inoculation.In the qRT-PCR assay,NbACTIN expression was used for normalization. GSNOR1 expression in the TRV:GFP-treated plant was set at 100%.The experiment was repeated three times.Error bars indicate the SE from three biological replicates; B.Protein expression of MPK6.Total proteins were extracted from detached leaves of NbGSNOR1 silenced plants inoculated by P.parasitica at 6 h, 12 h and 48 h post inoculation.The accumulation of MPK6 was detected by immunoblotting using anti-MPK6 antibody.Ponceau staining of the membrane was used to show equal loading; C, D The dynamic expression of MPK7 and MPK8 were evaluated by qRT-PCR.Leaves of NbGSNOR1-silenced plants and control plants were inoculated by P.parasitica.Total RNA was extracted from inoculated leaves at 0h, 3h, 6h and 12h post inoculation.The ratio of candidate gene expression to plant housekeeping gene NbACTIN was calculated by the △△Ct method.Three independent experiments showed similar result.Error bars indicate the SE from three biological replicates
To complement this, we further examined the level of MPK6 protein accumulation in bothGSNOR1-silencedN.benthamianaplants and control plants upon infection by the pathogen.The detached plant leaves were inoculated withP.parasiticaand harvested at 6, 12 and 48 hpi.Interestingly, we did not detect a clear accumulation of MPK6 protein at early biotrophic infection stage until 48 hpi, which has already developed into necrotrophic infection in eitherGSNOR1-silencedN.benthamianaplants or control plants(Fig.4-B).Notably, at this infection stage, the accumulation of MPK6 was strongly increased in control plants.In comparison, approximately 75% weaker accumulation level of MPK6 was detected inGSNOR1-silencedN.benthamianaplants(Fig.4-B).Taken together, these results suggest that silencing ofNbGSNOR1might attenuate induction ofMPK7andMPK8during early biotrophic infection and suppress the accumulation of MPK6 protein during necrotrophic infection byP.parasitica.
2.5 Silencing of NbGSNOR1 affects induction of PR genes upon early infection by P.parasitica
The activation ofPathogenesis-Related(PR)genes is a typical marker event in plant immunity and plays a crucial role in defense response to pathogens in plants[29-30].Previous research has shown thatPhytophthorainfection induces the expression ofPR1a[36-37].These prompted us to further examine, to what extentGSNOR1might affect the expression ofPRgenes in response toP.parasitica.The dynamic transcript levels ofPR1b,PR2,PR3andPR5were further monitored in leaves of bothGSNOR1-silencedN.benthamianaplants and control plants during early infection.The results showed thatPR1b,PR2andPR3were highly induced from 3 to 12 hpi, andPR5exhibited clear up-regulation at 12 hpi in control plants(Fig.5).By contrast, inGSNOR1-silencedN.benthamianaplants,PR1b,PR2andPR3showed resembled expression level at very early infection stage(3 hpi)to that at 0 hpi, whereas expression ofPR5appeared down-regulated at 3 hpi compared to that exhibited at 0 hpi(Fig.5).These results implied that silencing ofNbGSNOR1might attenuate induction ofPR1b,PR2,PR3andPR5at very early infection phase.

The dynamic expression of PR1b, PR2, PR3 and PR5 were evaluated by qRT-PCR.Leaves of NbGSNOR1 silenced plants and control plants were inoculated by P.parasitica.Total RNA was extracted from inoculated leaves at 0 h, 3 h,6 h and 12 h post inoculation.The ratio of candidate gene expression to plant housekeeping gene NbACTIN was calculated by the △△Ct method.Three independent experiments showed similar result.Error bars indicate the SE from three biological replicates
3 Discussion
The mechanistic insight into the function of highly conserved reductase GSNOR in plant immunity remains elusive, especially within the plant-Phytophthorainteracting systems.In this study, we demonstrate that plantGSNOR1plays a crucial role in the positive regulation of resistance againstPhytophthora.BothAtgsnor1mutant plants andGSNOR1-silencedN.benthamianaplants show significantly enhanced susceptibility toP.parasitica(Fig.1 and Fig.2).This is consistent with previous studies, showing thatAtgsnor1mutant plants are compromised in basal resistance to bacterial pathogenPseudomonassyringaepv.tomato(Pst)DC3000 and non-host resistance againstBlumeriagraminisf.sp.tritici[19].These results indicate that GSNOR1 might be an important and highly conserved candidate, which can be applied to improve broad spectrum resistance in crop plants.
GSNOR1 is the key reductase to participate in regulating protein S-nitrosylation[17], which has been shown to modulate the activity of a number of key regulators integral to plant immune function including SA binding protein 3(SABP3)[38], non-expresser of PR1(NPR1)[39], and the basic leucine zipper(bZip)protein TGA1[40].Furthermore, the NADPH oxidase, RBOHD, an essential enzyme for ROS production in plant defense[41-42], has also been shown to be S-nitrosylated on Cys890[43].Based on these, we speculate thatGSNOR1indirectly functions in modulating plant signaling transduction and multiple modes of plant immunity.This might explain its conserved biological function in plant resistance to a broad spectrum of pathogens.
MostPhytophthoraspecies, includingP.parasitica, are hemibiotrophic pathogens, thus these pathogens first establish a biotrophic interaction with host plant and later switch to a destructive necrotrophic lifestyle[44].As one of the earliest plant immune responses to pathogens, rapid production of ROS is important for resistance against the biotrophic pathogens because it helps drive cell death development[45].The emerging data suggest that the levels and timing of ROS production are important determinants of cell death in incompatibleP.parasitica-N.benthamianainteractions and correlate with compatibleP.parasiticaproliferation in susceptible plants[46].Furthermore,Arabidopsisroot resistance againstP.parasiticarequires an NADPH oxidase mediated oxidative burst[47].These findings indicate that ROS is important for plant resistance toPhytophthorapathogens.Our data suggest that H2O2accumulation is triggered rapidly either by flg22 treatment orP.parasiticainfection in control plants(Fig.3).In comparison, the production of H2O2is significantly reduced inGSNOR1-silenced plants during the early infection phase(Fig.3).These results indicate that the timing and level of the oxidative burst are of importance for plant resistance againstPhytophthoraand further thatGSNOR1plays a key role in regulating these processes.
Plants have developed different defense systems against infections by biotrophic and necrotrophic pathogens.Generally, SA signaling is essential to resist infection from biotrophic pathogens, whereas jasmonic acid/ethylene(JA/ET)signaling is necessary for inhibiting infections from necrotrophic pathogens[48-49].Interestingly, several reports have shown that both SA and JA/ET signaling are required to defend infection byPhytophthorapathogens in bothArabidopsisandN.benthamiana[36-37, 50].The induction ofPRgenes is marker event for SA signaling.Particularly,PR1has been shown to play an important role in plant defense againstP.capsici[50].In addition,PR1also participates inArabidopsisroot resistance againstP.parasitica[36]and plays a crucial role in plant resistance to biotrophic pathogens mediated by the novel susceptibility factorRTP1[23].Our kinetic expression analyses on set of PR genes inN.benthamianaduring early infection byP.parasiticashowed high induction ofPR1b,PR2,PR3andPR5in control plants.However, during the very early infection(3 hpi), we did not detect the induction ofPR1b,PR2andPR3but the down-regulation ofPR5inGSNOR1-silenced plants(Fig.5-A).Our findings imply thatGSNOR1might involve in regulating the induction ofPRgenes during early biotrophic infection byPhytophthora, which suggests thatGSNOR1may affect SA signaling for its function in plant resistance againstPhytophthora.
In plants, MAPK cascades play essential roles in the transduction of environmental signals, including response to a range of stresses caused by ROS and pathogen infections[51].Increasing evidence has demonstrated that MAPK cascades have emerged as battlegrounds of plant-pathogen interactions.Activation of MAPKs is one of the earliest signaling events after plant sensing of PAMPs and pathogen effectors[30].Importantly, hormones such as jasmonic acid(JA)and salicylic acid(SA)are known to influence signaling through MAPK cascades.For instance, JA involves in regulating the activation of MPK6[34].Interestingly, out studies showed the protein accumulation of MPK6 inGSNOR1-silenced plants was not detected until 48 hpi, representing the necrotrophic infection phase(Fig.4-B).Moreover, theGSNOR1-silenced plants exhibited strong suppression of MPK6 accumulation compared to control plants(Fig.4-C).These results imply that MPK6 might function during the necrotrophic infection byPhytophthoraandGSNOR1may also affect JA signaling for its function in plant resistance againstPhytophthora.Furthermore,MPK7could be activated by ROS triggered by plant stress stimuli[52-53].InArabidopsis, through interacting withMPK8, LSF2(LIKE SEX FOUR2)functions in the regulation of plant oxidative stress, resulting in modulating ROS homeostasis[54].The results showed that the induction ofMPK7andMPK8was significantly suppressed inGSNOR1-silenced plants during earlyP.parasiticainfection(Fig.4-B).These data indicate thatGSNOR1may involve in activating MAPK cascade signaling for its resistance function againstPhytophthora.
In conclusion,this study demonstrated that through positively regulating ROS production, induction ofPRgenes,MPK7andMPK8at the early biotrophic infection stag, and the accumulation of MPK6 protein at the later necrotrophic infection,GSNOR1plays an important role in the positive regulation of plant resistance againstPhytophthora.
