Severe bacteremia community-acquired methicillinresistant Staphylococcus aureus pneumonia in a young adult
2020-10-17DanliCaiXiaqingZhouYesongWangLingcongWang
Dan-li Cai, Xia-qing Zhou, Ye-song Wang, Ling-cong Wang
1 Department of ICU, the First Aff iliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
2 Department of Critical Care Medicine, the Second Aff iliated Hospital of Zhejiang University School of Medicine, Hangzhou,China
Dear editor,
Adults with community-acquired methicillin-resistantStaphylococcus aureus(CA-MRSA) are prone to necrotizing pneumonia, bacteremia, and high mortality. Several studies have reported the disease worldwide, but only a few cases of CA-MRSA pneumonia in children and adolescents have been reported in China.[1]No CA-MRSA was detected in the pathogens of community-acquired respiratory tract infections in Chinese adults from 2009 to 2010. During the influenza epidemic, such as COVID-19, viral infections are followed by repeated bacterial infections, and methicillin-sensitiveStaphylococcus aureus(MSSA) and MRSA pneumonia are known complications.[2]Thus, identifying and treating these infections is a tricky clinical issue. A case of CA-MRSA pneumonia with bloodstream infection and pneumothorax was successfully treated in our hospital in January 2018.Herein, we presented a case report based on previous studies.
CASE
A 20-year-old female patient who studied at a university was hospitalized on January 9, 2018, due to fever for three days, cough, and expectoration combined with chest pain for two days. This young adult was previously healthy, without any viral infection or risk factors, such as asthma. Also, her family history did not have any risk factors or co-morbidity.Three days before admission, the patient had fever without any inducement, and the body temperature was up to 40 °C.Two days before admission to the university clinic, she had a cough, expectoration with white sputum, and chest pain.Cefradine and azithromycin were used for the treatment,but the cough accompanied by chest pain was persistent due to resistance to macrolides and penicillin. Hence, she was admitted to the fever department of our hospital. Blood routine plus C-reactive protein showed normal white blood cell count (WBC, [3.5-9.5]×109/L), increased neutrophil(NE, 40%-75%), reduced lymphocyte (LY, 20%-50%) and eosinophil (EO, 0.4%-8.0%), normal hemoglobin (HGB,130-175 g/L), low platelet (PLT, [125-350]×109/L), and high C-reactive protein (CRP, 1-8 mg/L). Myocardial enzymes showed aspartate aminotransferase (AST) 28 U/L,lactate dehydrogenase (LDH) 278 U/L, creatine kinase (CK)154 U/L, and creatinine kinase isoenzyme-MB (CK-MB) 38 U/L. The level of troponin was 0.05 g/L. Electrocardiogram(ECG) revealed sinus tachycardia and a moderate deviation of the right axis. Chest computed tomography (CT) showed interspersed inflammation in both lungs (Figure 1). The patient was sent to the Hospital Infection Department for further diagnosis and treatment. Due to flu-like symptoms and the prevalence of influenza, physicians suspected viral infection. After hospitalization for one hour, venous blood samples were sent for culture, and she was prescribed ceftizoxime 1.5 g q12h combined with azithromycin 0.5 g qd as the anti-infection treatment and Tamiflu 150 mg bid tablets as an antiviral and symptomatic supportive treatment.Since the disease, she was in an alert state, oriented, spirited with decent sleep but not sufficient appetite. No difference was noted in urination, defecation, or weight.

Figure 1. Chest CT showed interspersed inf lammation in both lungs on January 9, 2018.

Figure 2. On January 19, 2018, CT reexamination showed interspersed inf lammation in both lungs together with consolidation of the part of the lung.
After hospitalization, the patient had severe chest distress, dyspnea, and type I respiratory failure at dawn on January 10, 2018, and then she was transferred to the intensive care unit (ICU) for treatment. The subsequent physical examination revealed the following: temperature 37.7 °C, pulse 131 beats/minute, respiratory rate 33 breaths/minute, and blood pressure 122/74 mmHg. The patient had chest distress but no chest pain and remained alert and oriented. The skin and sclera showed no yellow staining, rash or other skin abnormalities; however, pharyngeal hyperemia and swelling of tonsil for type I without suppuration were observed. After using a nasal tube for oxygen inhalation with Fisher Paykel and oxygen concentration at 80%, the oxygen saturation levels reached 90%-93%. The bilateral respiratory tone was slightly coarse and had audible dry rales. The heart rate showed regular beats with no murmurs. The blood gas analysis was performed in the ICU: pH 7.32, the pressure of arterial carbon dioxide (PaCO2) 34 mmHg, the pressure of arterial oxygen (PaO2) 66 mmHg. Diagnosis in ICU confirmed the following: community-acquired pneumonia(bilateral, patients with severe infection), type I respiratory failure, and multiple organs dysfunction syndrome(MODS) (respiration, circulation, and coagulation). After transferred to the ICU, emergency endotracheal intubation was given, and ventilator assistance was provided (Auto/Control model, fraction of inspired oxygen [FiO2] 100%,pressure control [PC] 16 cmH2O, positive end-expiratory pressure [PEEP] 10 cmH2O, 1 cmH2O=0.098 kPa), and meropenem was prescribed as an intravenous drip 1 g q8h combined with moxifloxacin intravenous drip 0.4 g qd as empirical coverage and other therapies for symptomatic supportive treatment. On January 10, 2018, blood routine examination revealed WBC 9.0×109/L, the proportion of neutrophil granulocytes 86.7%, PLT 121×109/L, and CRP 165 mg/L. Antigens, H1N1, B stream virus, adenovirus, and respiratory syncytial virus were negative. Gram-positive cocci and Gram-negative bacilli were cultured in sputum smear, and Mycoplasma pneumonia RNA was negative.Blood coagulation showed that prothrombin time (PT)was prolonged, and D-dimer was increased. The level of procalcitonin (PCT) was 17.52 ng/L. Chest radiograph reexamination revealed that the left pneumothorax was 20%. Consequently, closed thoracic drainage was used for treatment in the left lung. Acute Physiology and Chronic Health Evaluation II (APACHE II) was 18, Sequential Organ Failure Assessment (SOFA) 5, and mortality 42.9%.On January 12, 2018, the body temperature reached 39.9 °C,and oxygenation index was <100. Bronchoscopy displayed pale, bloody sputum in the trachea and main bronchus, and hence, pituitrin and carbazochrome sodium sulfonate were prescribed for hemostasis. On January 13, 2018, the results of blood and sputum culture (blood samples on January 9,and sputum specimens on January 10) showed thatS. aureuswas resistant to clindamycin, erythromycin, and oxacillin,and sensitive to levofloxacin and linezolid (the minimum inhibitory concentration [MIC]=2), vancomycin (MIC=1),and teicoplanin were similar. According to drug sensitivity results and clinical characteristics, we diagnosed severe MRSA with bloodstream infection and used linezolid (0.6 g q12h by intravenous drip) combined with daptomycin(50 mg qd intravenous drip) and meropenem (1 g q8h intravenous drip) to resist infection. On January 16, 2018,the oxygenation index was again <100. Bronchoscopy was performed, yellow mucus was noticed in the pulmonary bronchus of the left upper, right upper, left lower, and congestion edema, and then sputum suction was performed.On January 19, CT scan showed interspersed inf lammation in both lungs with the consolidation of a part of the lung,and a slight effusion was detected in the thoracic cavity(Figures 2, 3). The peak in the temperature declined, and the oxygenation index was improved. Finally, ventilator weaning and extubation were used on January 24, 2018, and daptomycin (a course of 14 days) was stopped on January 26, 2018. Chest CT (Figure 4) displayed interspersed inf lammation in both lungs, with the consolidation of a part of the lung showing significant absorption more than that detected on the previous CT (January 19, 2018). Hence, the patient was transferred to the Respiratory Department for further treatment on January 29, 2018, and discharged with improved health condition on February 8, 2018.
DISCUSSION
CA-MRSA pneumonia often occurs in healthy children and young people. Although the incidence of pneumonia in adults remains low, it is characterized by rapid progression and high mortality.[3]Such patients often have necrotizing pneumonia and complications of bloodstream infections.This mortality rate could be 75%. In 1999, Centers for Disease Control and Prevention (CDC) reported four deaths due to CA-MRSA infection in children who did not have the risk factors of CA-MRSA infection, and thus CA-MRSA has attracted widespread attention.[4]It was rather common in the USA, Canada, Australia, and Taiwan Province in China.[5]CA-MRSA commonly exists in the skin and soft tissue infections, followed in lower respiratory tract infections. CA-MRSA pneumonia is common in previously healthy people with some risks, which include fatigue, cold,or secondary to the skin and soft tissue infection; also, it has an acute onset of illness.[6]Positive blood cultures are common in severe pneumonia patients. Endocarditis can be excluded by echocardiography in this case. Clinical manifestations of this disease included chills, fever, cough,and sputum, and some patients had chest pain and blood in sputum. The strain often carries Panton-Valentine leukocidin(PVL), making the infection severe, and promotes rapid progression of pulmonary infection exhibiting characteristics such as necrosis, cavitation, and abscess.[7]Patients with severe infection might have septic shock,[8]while patients with bacterial pneumonia had higher levels of PCT than those with pneumonia caused by viruses and tuberculosis.PCT level was positively correlated with the severity of pneumonia. In severe community-acquired pneumonia, the PCT level was positively correlated with the positive rate of sputum bacterial culture and the severity of the disease.This patient was a healthy adult with no skin and soft tissue infections and fatigue. She presented high PCT, blood culture and sputum culture wasS. aureuspositive, and the condition was critical.

Figure 3. On January 19, CT reexamination showed interspersed inf lammation in both lungs together with consolidation of part of the lung with little effusion in the thoracic cavity.
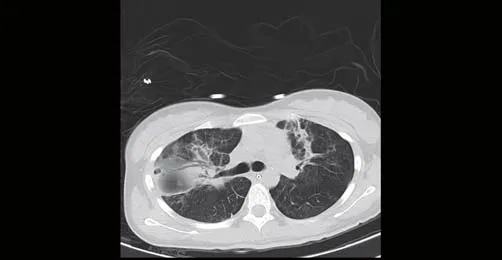
Figure 4. Chest CT (January 29, 2018) reexamination results demonstrated interspersed inflammation in both lungs together with consolidated part of the lung showing signif icant absorption more than that on the previous CT (January 19, 2018).
Diagnosis of CA-MRSA infection was based on the definition by the CDC in the USA. However, the CDC has provided recommendations regarding clinical epidemiology,with limitations. At present, the diagnosis of CA-MRSA pneumonia is mainly based on clinical manifestations,drug sensitivity tests, and genetic typing.[9]Currently,molecular epidemiology, staphylococcal chromosomal cassette mec (SCCmec), and virulence factor detection are used internationally to distinguish between the genetic background and transmission of MRSA bacteria. Multilocus sequence typing (MLST) is currently the best method for molecular epidemiology. The strains of this patient were tested by the MLST method, and the results showed that sequence type (ST) was classified as 59, which is an epidemiological pedigree of CA-MRSA isolates. Taiwanese CA-MRSA isolates that belong to ST59 can be grouped into two clones: a virulent Taiwan clone and a commensal Asian-Pacif ic clone. The Taiwan clone carrying the PVL genes and the SCCmec type VT is isolated from critically ill patients.The Asian-Pacific clone is PVL-negative, carries SCCmec type IV, and is a frequent colonizer of healthy children.CA-MRSA clones from Taiwan clone have enhanced virulence in both human and animal models of infection.The evolutionary habit of PVL, the higher expression of the α-toxin, and the possible loss of most of the β-hemolysinconverting prophage may have a higher pathogenic potential than Asian-Pacific clone.[10]We also tested the resistant gene of this strain. The results showed that it carried genes resistant to antibiotics, aph(3’)-III and ant(6)-Ia, with homology 100. Lipid resistance genes included macrocyclic ermB and ion-lactamide resistance genes (blaZ and mecAI).Among these, aph(3’)-III, ant(6)-Ia, and ermB are present in the same region, and it is possible that there may be no other resistant genes on one drug resistance gene island. We also found that the strain carried multiple common virulence genes, which included aur, scn, and leukocidin (lukF-PV,lukS-PV). PVL is one of the virulence factors produced byS. aureusand is co-coded by lukS-PV and lukF-PV genes.PVL acts as a molecular biological marker of CA-MRSA,attacks neutrophils, and acts on the cell membrane of WBCs,leading to increased cell permeability, loss of potassium ions,and eventually cell death. Also because of the destruction of WBCs, the ability to process and present antigens was decreased, the defense barrier and immune response were damaged, and the effective specific immunity could not be established, which is critical in the skin and soft tissue infections caused byS. aureusand necrotic pneumonia. In this case, f lu-like symptoms and decreased WBCs during the early stage of the disease were observed and ascribed to PVL toxicity.
The ideal treatment of CA-MRSA pneumonia is yet to be explored. For a long time, vancomycin has been the f irst choice for treating MRSA. With continuous clinical usage,the bacterial resistance to vancomycin has grown, the MIC drift of glycopeptides has declined, and the heterogeneity of the molecule has increasedS. aureus(hVISA or hGISA)infection. This, in turn, resulted in decreased clinical eff icacy.Because of the high concentration of linezolid in alveolar epithelial lining f luid and the inhibitory effect on PVL, this patient was treated with linezolid. Consecutively, the use of only linezolid for patients with CA-MRSA complicated with bloodstream infection caused complications such as abscess and pleural effusion, and the treatment time was long with frequent sequelae.[11]For treating MRSA bloodstream infections, the American Society of Infectious Diseases for MRSA Guidelines recommended daptomycin for the treatment of bloodstream infections with evidence of AI.Daptomycin is a new class of cyclic lipopeptide antibiotics with novel antibacterial mechanism and should not be used in pneumonia (failed non-inferiority) as a pulmonary surfactant that inhibits antibiotic effect and also increases the risk of eosinophilic pneumonia.[12]Furthermore, daptomycin has rapid concentration-dependent bactericidal effects on Gram-positive bacteria as assessed byin vitroassays with rare drug resistance and cross-resistance with other kinds of antibiotics.[13]For patients in this column, linezolid combined with daptomycin was used for short treatment time and had few complications. Thus, this strategy could provide a novel approach for treating CA-MRSA combined with bloodstream infections in other patients in the future.
ACKNOWLEDGMENT
The successful rescue of this case is owed to the guidance of Ren-hua Sun, Director of ICU of Zhejiang People’s Hospital, and the nurses and doctors of ICU at the Xiasha Hospital of Zhejiang Traditional Chinese Medicine Hospital.
Funding:This study was supported by the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents(2014-108) and the Medical and Health Research Program of Zhejiang Province (2017KY385).
Ethical approval:Not needed.
Conf licts of interest:All authors declare that they have no conf licts of interest, f inancial, or otherwise.
Contributors:DLC and XQZ contributed equally to this study.LCW and YSW, study design; XQZ, data collection; DLC, writing.
杂志排行
World journal of emergency medicine的其它文章
- Improving antibiotic prescribing in the emergency department for uncomplicated community-acquired pneumonia
- Outcome prediction value of National Early Warning Score in septic patients with community-acquired pneumonia in emergency department: A single-center retrospective cohort study
- Effects of f luid balance on prognosis of acute respiratory distress syndrome patients secondary to sepsis
- Effects of sepsis on hippocampal volume and memory function
- Death and do-not-resuscitate order in the emergency department: A single-center three-year retrospective study in the Chinese mainland
- The general public’s ability to operate automated external def ibrillator: A controlled simulation study
