Improving antibiotic prescribing in the emergency department for uncomplicated community-acquired pneumonia
2020-10-17RebekahShawEricaPopovskyAlyssaAboMarniJacobsNicoleHerreraJamesChamberlainAndreaHahn
Rebekah Shaw, Erica Popovsky, Alyssa Abo, Marni Jacobs, Nicole Herrera, James Chamberlain, Andrea Hahn
1 Division of Pediatrics, Children’s National Health System, Washington, DC, USA
2 Division of Emergency Medicine, Children’s National Health System, Washington, DC, USA
3 Department of Pediatrics, George Washington University School of Medicine and Health Sciences, Washington, DC, USA
4 Division of Biostatistics and Epidemiology, Children’s National Health System, Washington, DC, USA
5 Division of Infectious Diseases, Children’s National Health System, Washington, DC, USA
KEYWORDS: Antibiotics; Community-acquired pneumonia; Emergency department; Pediatrics
INTRODUCTION
Community-acquired pneumonia (CAP) is the most common severe bacterial infection in children, with more than 3 million cases each year in the United States,resulting in 7.2% of all pediatric hospitalizations.[1-3]In 2011,the Pediatric Infectious Disease Society (PIDS) and the Infectious Disease Society of America (IDSA) published the f irst evidence-based guidelines for the diagnosis and the treatment of uncomplicated CAP in children. The American Academy of Pediatrics (AAP) subsequently endorsed these guidelines, which recommended the use of aminopenicillins (e.g., amoxicillin and ampicillin) as the f irst-line agents for the treatment of uncomplicated CAP in previously healthy and fully-immunized children.[4,5]
Recent studies have demonstrated poor compliance with these guidelines, with only 10%-50% of patients admitted to the hospital on narrow-spectrum antibiotics such as ampicillin.[1,6-9]These studies[6,8,10-11]also demonstrated no difference in clinical outcomes for patients treated with narrow-spectrum antibiotics compared with broad-spectrum antibiotics. In pediatric inpatient units and outpatient settings, implementation of clinical practice guidelines (CPGs) results in increased adherence to recommended guidelines.[12-15]It is important to study compliance with these guidelines in the emergency department (ED) because broad-spectrum antibiotics are typically continued on the inpatient unit when initiated in the ED.[1]
In December 2015, the Children’s National Health System (CNHS) ED implemented a new CPG for uncomplicated CAP. We performed this study to test:(1) providers’ knowledge of the guidelines for narrowspectrum antibiotics; and (2) the actual practice change in the proportion of patients receiving narrow-spectrum antibiotics for CAP. We hypothesized that providers are aware of the guidelines and that implementation of the CPG would result in increased rates of the use of narrowspectrum antibiotics.
METHODS
Setting
CNHS is a free-standing, tertiary-care, pediatric academic medical center with 323 licensed inpatient beds located in Washington, DC that serves the Washington,DC metropolitan area, northern Virginia, and Maryland.The health system includes a tertiary ED at the main campus and a community ED in Southeast DC. The combined volumes are approximately 120,000 ED visits per year, and there are more than 10,000 admissions to the inpatient teams per year. There are pediatric emergency medicine (PEM) trained attending physicians,pediatric associate physicians (non-pediatric emergency trained general pediatricians), PEM fellows, physician assistants, pediatric residents, family medicine residents,and emergency medicine (EM) residents caring for and prescribing medications for patients in the two EDs. This study was approved by the CNHS Institutional Review Board with a waiver of consent.
Survey data
Study data were collected and managed using REDCap electronic data capture tools hosted at Children’s National Medical Center.[16]Prior to CPG implementation, a case-based survey was distributed to ED providers, including pediatric residents,pediatric associate physicians, PEM fellows, and PEM attendings. The survey was anonymous and consisted of demographics information (training background and years in practice) and two cases of pediatric CAP.The survey required respondents to choose which antibiotic(s) they would prescribe in each case in an otherwise healthy and appropriately immunized ED patient. The f irst case and follow-up question addressed administering intravenous (IV) ampicillin or oral amoxicillin in a preschool-aged child with mild hypoxia and CAP presumed to be bacterial in origin. The second case and follow-up question addressed administering oral amoxicillin or IV ampicillin plus oral azithromycin in a school-aged child with CAP, potentially caused by an atypical pathogen. An additional question after each case evaluated the provider’s knowledge of the 2011 PIDS/IDSA CAP guideline.
Implementation of the CPG
Prior to approval, a focus group with PEM attendings and fellows was held for feedback on the new CPG.The CPG was approved and implemented in December 2015. Knowledge of implementation of the CPG was disseminated via email that identif ied the new CPG and included a summary of the guidelines as well as where to find the complete CPG for use. One email was sent to the ED division, which included attendings, fellows,associates, and physician assistants; and a separate email was sent to the pediatric residents. The CPG consisted of two parts. The first part was the CPG as well as a decision-making assistance algorithm, which was located on the hospital’s internal webpage amongst other CPGs. The second part was an order-set that was located in a folder for ED providers, where other order-sets were located. Accessibility to the CPG and order-set is relatively easy given the ED has multiple CPGs, and this CPG is found amongst the other CPGs.
Data collection
A REDCap database was compiled from the electronic medical records of patients seen in the CNHS ED diagnosed with pneumonia, administered IV antibiotics, and admitted to the hospital between January 1, 2015, and February 28, 2017.[16]We chose this time frame to represent one year prior to implementation through 14 months after the implementation of the CPG.Inclusion criteria for the study were: age three months to 18 years, receiving age-appropriate vaccinations (per parental report), an ICD-9/10 discharge diagnosis code including CAP or pneumonia (ICD-9 codes: 480.0,480.2, 480.8, 480.9, 481.0, 482.1, 482.3, 482.4, 482.8,482.9, 485.0, 486.0, 487.0 and ICD-10 codes: J12.9,J13.0, J15.8, J15.9, J16.8, J18.1, J18.8, J18.9), receiving IV antibiotics in the ED, and chart review confirmed diagnosis of CAP. Patients were excluded if: they had received outpatient oral antibiotics for ≥ 48 hours prior to presentation to the ED as these patients may represent outpatient treatment failure; patients were hospitalized in the last 30 days and thus at the risk of hospital-acquired pneumonia; patients on chart review had a diagnosis or suspected diagnosis of sepsis; patients had complex chronic conditions, including immunodeficiencies,known airway anomalies, or underlying chronic lung disease; patients were ventilator-dependent; and patients had penicillin-family allergies. Appropriate first-line therapy was def ined as empiric receipt of IV ampicillin.
Data analysis
Practitioner survey
Question responses based on demographics were evaluated using Fisher’s exact test. The frequency of aminopenicillin prescribing versus ceftriaxone or other non-aminopenicillin antibiotics based on the f irst question from the provider survey was compared to the actual frequency of IV ampicillin prescribing versus ceftriaxone or other antibiotics pre-CPG implementation using a Chisquare test.
CPG implementation
For actual practice data, the primary outcome measure was the proportion of eligible patients seen in the CNHS ED with CAP receiving appropriate f irst-line IV antibiotic therapy (ampicillin) in the ED among those children who received IV antibiotics. Control charts with 2σlimits, with limits calculated using pre-intervention prescribing patterns as the baseline reference (mean monthly sample size of 10), were constructed to evaluate whether post-intervention prescribing patterns improved post-baseline. Mann-Kendall test was used to evaluate the linear trends in prescribing patterns. Demographics and other clinical parameters between the pre- and post-CPG groups and between the ampicillin and ceftriaxone groups post-CPG were analyzed using Chi-square tests with odds ratio (OR) reported for categorical data andt-test for normally distributed variables; Wilcoxon-Mann-Whitney test was used for those that were not normally distributed. All analyses were done using SAS 9.4; signif icance was def ined asP<0.05.
RESULTS
Survey results
Overall, 82 clinicians responded to the survey (39.0%response rate). The training background of the survey respondents and response rates are shown in Table 1.
Using the first case-based scenario, the percentage of ED prescribers reporting that they would prescribe a narrow-spectrum antibiotic (amoxicillin or ampicillin)for uncomplicated CAP in the ED was compared to those choosing a broad-spectrum agent (e.g., ceftriaxone).A narrow-spectrum antibiotic was chosen by 93.2%(n=41) of trainees versus 80.7% (n=25) of non-trainees(P=0.15). Of PEM-trained clinicians, 82.7% (n=24)chose a narrow-spectrum agent compared to 91.3%(n=42) of non-PEM clinicians (P=0.30).
Direct questioning of respondent’s knowledge of the PIDS/IDSA CAP guideline yielded the following results:86.4% (n=38) of trainees correctly identified a narrowspectrum antibiotic agent as the first-line treatment for uncomplicated CAP versus 93.6% (n=29) of nontrainees (P=0.30), while 86.7% (n=26) of PEM providers correctly chose a narrow-spectrum agent versus 91.1%(n=41) of non-PEM providers (P<0.99). Other provided answers included the selection of a broad-spectrum agent(e.g., ceftriaxone) or identifying that the respondent was not familiar with the guideline.
Using the first case-based scenario in the survey of a preschool-aged patient with uncomplicated CAP, 85.7%(n=66) of all survey respondents reported they would prescribe an aminopenicillin prior to the implementation of the CAP CPG. Other selected antibiotic choices included ceftriaxone (n=9), cefdinir (n=1), and azithromycin (n=1).In contrast with these survey results, we found that in the 11 months prior to the implementation (described below), only 41.2% (n=40) of patients with uncomplicated CAP admitted to the hospital for IV antibiotics were prescribed ampicillin.The other patients were prescribed ceftriaxone (n=54) or ampicillin/sulbactam (n=3). The difference in the rate of the survey reported versus observed prescribing patterns for narrow-spectrum antibiotics in CAP was clinically and statistically signif icant (P<0.01) (Figure 1).
CPG implementation results
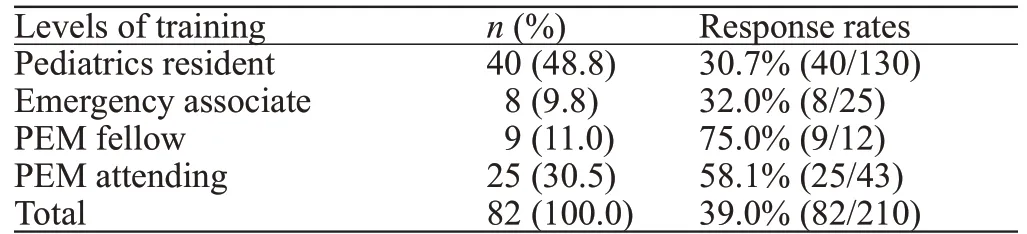
Of 1,537 patients seen in the ED with a diagnosisTable 1. Characteristics of survey respondents and overall response rates
code of CAP, 278 met inclusion criteria: 94 preimplementation (PRE) and 184 post-implementation(POST) (Figure 2). Baseline characteristics of the PRE and POST patient groups are shown in Table 2. The median age was higher in the POST group (36.3 months vs. 21.5 months).
In the PRE period, ceftriaxone was the most commonly prescribed antibiotic (55%), and ampicillin was the second most common (42%). Ampicillin use varied widely, with monthly rates ranging from 17% to 64% (Figure 3).
During the POST period, the proportion of patients who empirically received ampicillin did not differ significantly from the pre-implementation period (42% PRE vs. 46%,POST,P=0.57). Controlling for age did not significantly change the assessment of antibiotic prescription in PRE and POST groups (P=0.62). During the POST period, ampicillin use varied widely, with monthly rates ranging from 10%to 58%, excluding January 2017 as the rate of ampicillin prescription was outside the two standard deviation control limits withn=10 (Figure 3). In an analysis of trends, there was no signif icant increase in ampicillin prescribing (P=0.40,Mann-Kendall test, Figure 4).
Table 3 depicts the differences in demographic and clinical characteristics between those children who received ampicillin (n=84) and ceftriaxone (n=97) in the POST period. No significant differences were noted between the two groups.

Figure 1. Survey frequency of case-based ampicillin versus observed ampicillin use. The P-value determined using Chi-square; other antibiotics included azithromycin (n=1, survey), cefdinir (n=1,survey), and ampicillin/sulbactam (n=3, pre-CPG).
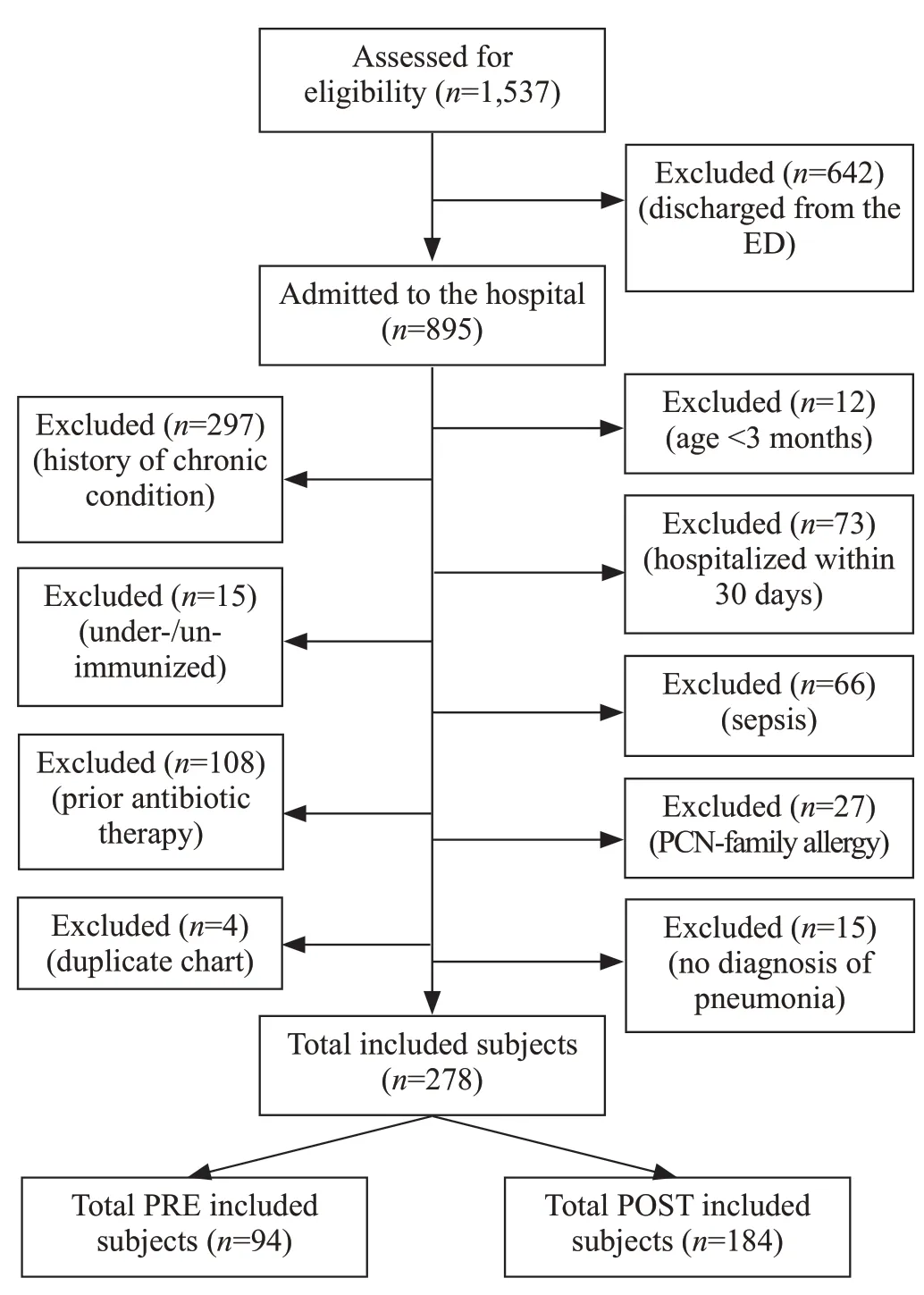
Figure 2. Flow diagram of selection of eligible subjects for study between January 2015 and February 2017.

Figure 3. Rates of ampicillin prescribing by month pre- and post-CPG implementation. UCL: upper control limit; LCL: lower control limit;CPG: clinical practice guideline.

Table 2. Characteristics of enrolled patients pre- and post-CPG implementation, n (%)
Lastly, it should be noted that macrolide prescriptions at our institution were low during the study period.Only four patients were prescribed macrolides in the pre-implementation period, and six patients in the post-implementation period; all prescriptions were in conjunction with ampicillin or ceftriaxone.
DISCUSSION
Overall, our study did not show a difference preand post-CPG implementation in antibiotic prescribing habits for providers in the ED for patients receiving IV antibiotics for uncomplicated CAP. Moreover, there was a significant difference between what providers knew about the guidelines for narrow-spectrum antibiotics and their actual practice. These data together indicate that appropriate knowledge and the institution of CPGs are not sufficient to affect significant change in prescribing patterns in a short period of time.
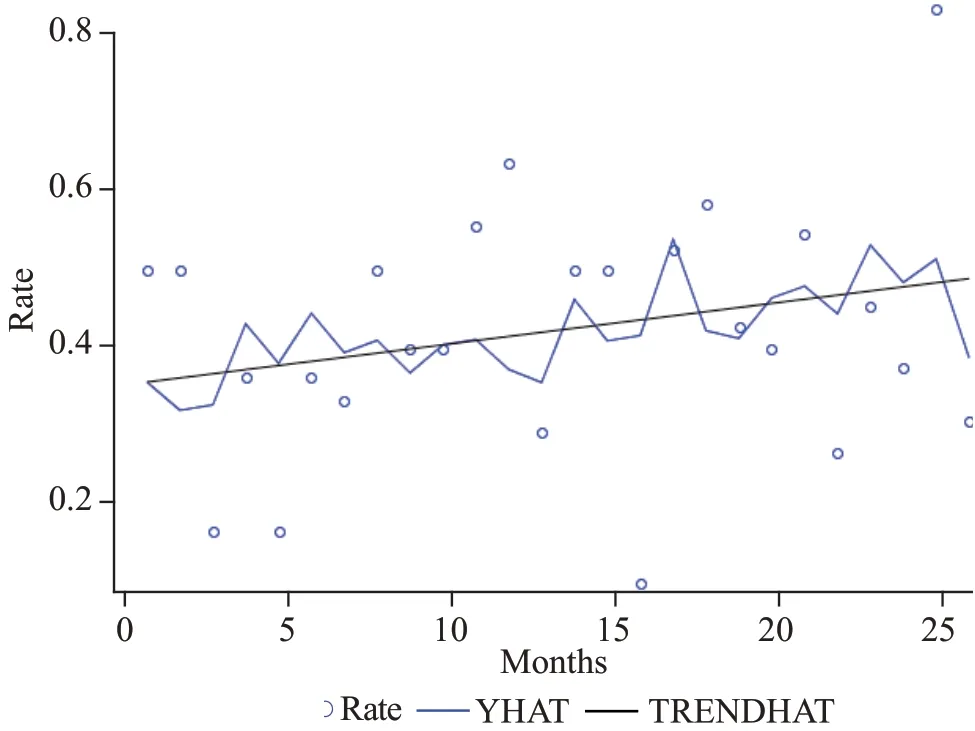
Figure 4. Trend-analysis of IV ampicillin use over time. YHAT:the full model prediction values; TRENDHAT: the autocorrelation corrected regression line of the structural predicted values; the trend was not statistically signif icant (P=0.40, Mann-Kendall test).
Several studies have shown increased adherence to guidelines with the implementation of CPGs plus the addition of other implementation measures in the ED, in the inpatient units, and in the outpatient clinic. A CAP CPG consisting of a one-page decision support algorithm along with three CPG training sessions presented to the physicians and residents working in the ED led to increased adherence to the PIDS/IDSA guideline and increased use of aminopenicillins as the first-line antibiotics for uncomplicated CAP in both the inpatient setting and outpatient setting.[17,18]Similarly, the use of an educational session on current guidelines for the treatment of CAP plus quarterly feedback of prescribing patterns for individual providers led to a decrease in the use of broad-spectrum antibiotics for bacterial respiratory tract infections in the outpatient clinic setting.[13]An additional study demonstrated quality improvement (QI)methodology, including in-person education regarding guideline recommendations as well as pocket-sized reference books with recommended antibiotic choices in bulleted form distributed to providers led to increased adherence to PIDS/IDSA guidelines for uncomplicated CAP in the ED and in the inpatient unit.[12]Finally, a study comparing hospital adoption of guidelines found that changes in practice following new guidelines creation and publication were seen more substantially in those hospitals that implemented guidelines-related dissemination activities, thus pointing to the importance of dissemination strategies for timely uptake of new guideline recommendations.[8]
These results differ from those seen in our study, in which we did not see increased adherence to guidelinesfollowing the implementation of a CPG in the ED. Our study did not include any in-person training sessions regarding the PIDS/IDSA CAP guideline, nor did we provide periodic feedback on guideline adherence.In our study, providers were notified via email of implementation of the CPG and encouraged in this email to reference the practice guideline and an associated order set when caring for patients with uncomplicated CAP in the ED. This difference in the dissemination of the CPGs could be responsible in part for the difference seen in the results.
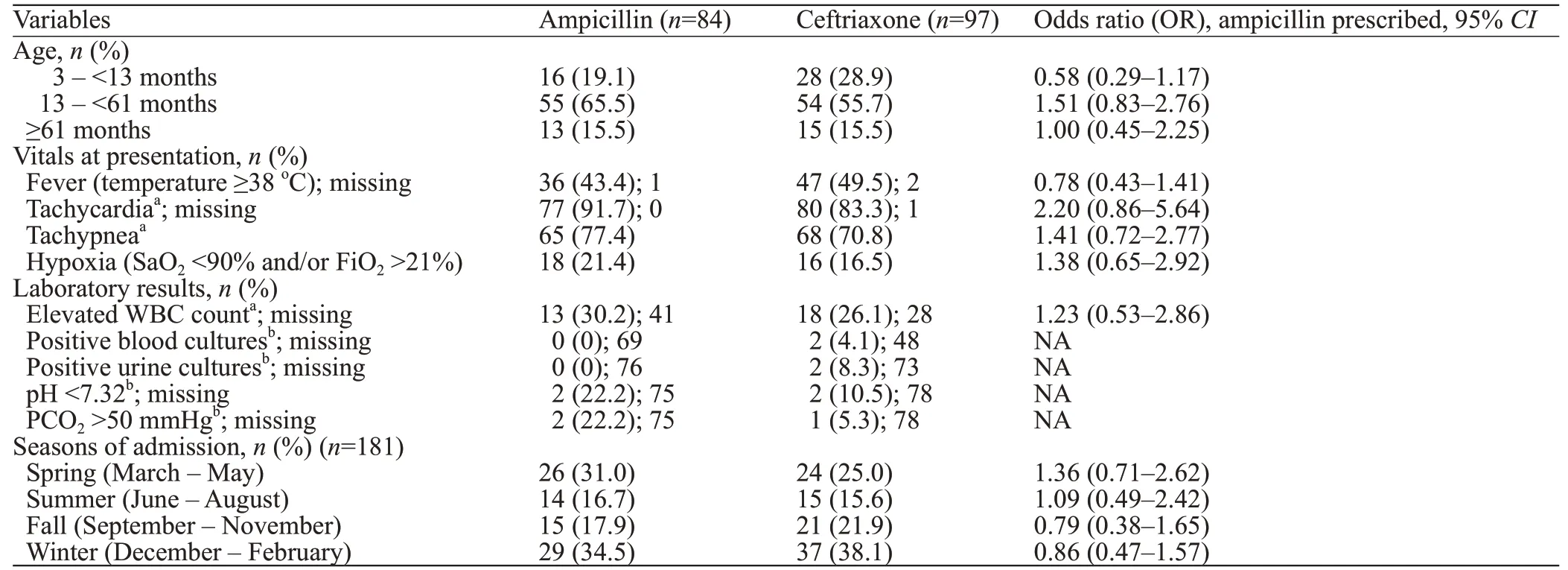
Table 3. Characteristics of patients in POST group prescribed ampicillin versus ceftriaxone
Based on our survey results, providers are aware of the IDSA guidelines and antibiotic recommendations;however, it seems that they have not implemented these guidelines into clinical practice. It is possible that the implementation of a CPG in conjunction with in-person training improves uptake of the CPG. Additionally, the quarterly individualized feedback regarding antibiotic use could improve adherence to guidelines as perhaps providers are unaware of their current practice.[13]The majority of our survey respondents correctly identified the IDSA guideline recommendations and correctly prescribed narrow-spectrum antibiotics in our casebased survey; however, they did not duplicate these results in actual practice. Real-time feedback may be necessary to encourage providers to change clinical practice. Investigation of barriers to guideline-adherent prescribing is an area for further study.
One hypothesis is that patients with more severe illness were preferentially prescribed ceftriaxone;however, subsequent analysis showed that patient characteristics, including vital signs and laboratory values, were similar between those patients who received ampicillin versus ceftriaxone. This suggests another etiology for the discrepancy between survey results and what is seen in practice.
This study has several limitations. First, we did not collect data prospectively, so we could not determine providers’ reasons for prescribing broad-spectrum antibiotic therapy. Second, the study did not involve follow-up reminders after initial implementation in December 2015, and this likely affected uptake of the knowledge of the CPG and associated order set in the 14 months following the implementation. Third, we had a low overall (39.0%) survey response rate, particularly for attendings (30.5%), who likely made the final decision regarding antibiotic therapy, making the inferences from the survey data limited. It is possible that those who did not respond to the survey were those less likely to adopt new recommendations contributing to the difference seen between the survey results and actual practice. Finally,because the survey was anonymous, we did not measure the overlap between survey responders and the clinicians who prescribed antibiotics in actual practice. It is also possible, though unlikely, that survey responders were systematically different from those who prescribed the antibiotics.
CONCLUSIONS
In conclusion, although our study does not show a significant change in prescribing patterns for uncomplicated CAP in the ED, it is reassuring that providers are aware of best practice guidelines as demonstrated by our survey results. Future efforts should include initial and continued education of the CPG,as well as individualized feedback, to best maximize results. Hopefully, through education and awareness of the discrepancy between knowledge of the guidelines and what continues to occur in practice, we will see an increase in narrow-spectrum antibiotics being prescribed for uncomplicated CAP in the ED setting.
ACKNOWLEDGEMENT
Dr. Erica Popovsky is currently completing her pediatric emergency medicine fellowship at Boston Children’s Hospital. Dr. Rebekah Shaw is currently a pediatric hospitalist at C. S. Mott Children’s Hospital at the University of Michigan.
Funding:Drs. Rebekah Shaw and Erica Popovsky received protected time for research during their pediatric residency through the Research, Education, Advocacy, and Child Health Care(REACH) program at Children’s National Health System. During the study period, Dr. Andrea Hahn was also funded in part by the National Institute of Health (NIH) National Heart, Lung, and Blood Institute (K12 HL119994). Biostatical support was provided by the Clinical and Translational Science Institute at Children’s National Health System (UL1TR000075) through the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the off icial views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Ethical approval:This study was approved by the CNHS Institutional Review Board with a waiver of consent.
Conf licts of interest:No benef its in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors:RS wrote the f irst draft. All authors contributed to the design, interpretation of the study, and further drafts.
杂志排行
World journal of emergency medicine的其它文章
- Outcome prediction value of National Early Warning Score in septic patients with community-acquired pneumonia in emergency department: A single-center retrospective cohort study
- Effects of f luid balance on prognosis of acute respiratory distress syndrome patients secondary to sepsis
- Effects of sepsis on hippocampal volume and memory function
- Death and do-not-resuscitate order in the emergency department: A single-center three-year retrospective study in the Chinese mainland
- The general public’s ability to operate automated external def ibrillator: A controlled simulation study
- Aldehyde dehydrogenase 2 preserves mitochondrial morphology and attenuates hypoxia/reoxygenationinduced cardiomyocyte injury
