白芍总苷对葡聚糖硫酸钠诱导的IBD模型小鼠炎症因子水平的影响
2020-10-09葛冰景曹红燕戴彦成阙任烨傅志泉
葛冰景 曹红燕 戴彦成 阙任烨 傅志泉
〔摘要〕 目的 探討白芍总苷对葡聚糖硫酸钠(dextran sulfate sodium, DSS)诱导的小鼠炎症性肠病(inflammatory bowel disease, IBD)模型的作用及相关机制研究。方法 将50只SPF级C57BL/6小鼠随机分成正常组(N组)、模型组(M组)、柳氮磺吡啶组(L组)、低剂量白芍总苷组(BL组)、高剂量白芍总苷组(BH组),每组10只。M组及治疗组小鼠自由饮用30 g/L DSS,同时予以相应药物灌胃1次;N组自由饮水、进食,连续7 d后处死。期间每天记录小鼠体质量及粪便情况,进行结肠炎疾病活动指数(disease activity index, DAI)评分,利用结肠组织HE染色进行组织病理学评价,利用ELISA法检测血清中IL-8、IL-10、IL-23、IL-36、CCL20、CCR6的表达。结果 与M组比较,用药组DAI评分均显著降低(P<0.05或P<0.01),各用药组之间差异无统计学意义(P>0.05);与M组比较,用药组均可下调IL-8、IL-23、IL-36、CCL20和CCR6的表达,上调IL-10的表达,并以L组、BH组最为显著(P<0.05或P<0.01);病理组织学结果显示,L组、BH组结肠组织的炎症反应得到了显著改善。结论 白芍总苷可以有效调节炎症因子表达,减少结肠组织的炎症损伤,并改善小鼠的腹泻及便血症状。
〔关键词〕 白芍总苷;炎症性肠病;葡聚糖硫酸钠;炎症因子;疾病活动指数
〔中图分类号〕R285.5 〔文献标志码〕A 〔文章编号〕doi:10.3969/j.issn.1674-070X.2020.08.010
〔Abstract〕 Objective To explore the effect and the underlying mechanism of total glucosides of paeony on inflammatory factors in dextran sulfate sodium (DSS) induced-mice model of inflammatory bowel disease (IBD). Methods A total of 50 SPF-class C57BL/6 mice were randomly divided into a normal group (N group), a model group (M group), a sulfasalazine group (L group), a low-dose of total glucosides of paeony group (BL group) and high-dose of total glucosides of paeony group (BH group), with 10 mice in each group. The mice in M group and treatment groups were free to drink 30 g/L DSS, and were given the corresponding medicine by gavage once, while the mice in the N group were free to drink water and eat. All the mice were executed 7 days later. During this period, the status of weight and feces of the mice were recorded, and colitis disease activity index (DAI) score were assessed. In addition, HE staining of colon tissue were performed to evaluate the histopathological injury, and the expression of serum IL-8, IL-10, IL-23, IL-36, CCL20 and CCR6 were determined by using ELISA. Results Compared with the M group, DAI scores of mice in the treatment group were significantly decreased (P<0.05 or P<0.01), and the differences in each treatment group was not statistically significant (P>0.05). Compared with the M group, the expression of IL-8, IL-23, IL-36, CCl20, and CCR6 were decreased in the treatment group, while the expression of IL-10 was increased, in which the L group and the BH group were most significant (P<0.05 or P<0.01). The histopathological results showed that the inflammatory response of colon tissue in the L group and the BH group was significantly improved. Conclusion Total glucosides of paeony could effectively regulate the expression of inflammatory factors, reduce the inflammatory injury of colon tissues, and improve the diarrhea and hematochezia symptoms in mice.
〔Keywords〕 total glucosides of paeony; inflammatory bowel disease; dextran sulfate sodium; inflammatory factor; disease activity index
炎癥性肠病(inflammatory bowel disease, IBD)是一组以腹痛、腹泻、黏液脓血便为主要表现的慢性非特异性肠道炎症性疾病,包括克罗恩病(Crohn's disease, CD)和溃疡性结肠炎(ulcerative colitis, UC)。其病因和发病机制均未明确,目前认为该疾病与遗传、免疫、精神应激等因素相关[1]。由于该病迁延难愈,又易转化为结肠癌,因此被世界卫生组织定义为世界难治病之一,发病率为5/10万~12/10万[2]。我国对此病尚无完整的统计,但就临床所见病例而言并非罕见,且有增多趋势[3]。
根据IBD临床表现的腹泻、腹痛、里急后重、黏液或脓血便等症状,中医学将其归属于“肠癖”“泄泻”“痢疾”“肠风”“脏毒”等范畴[4],认为其病因与湿热之邪、饮食所伤、情志郁结及禀赋不足等有关,肝郁脾虚为其本,湿热蕴结为其标,并常见本虚标实、寒热错杂之证。因此,健脾疏肝、清湿热、化瘀血之治法已成为目前中医药治疗IBD的重要策略。白芍味酸甘,性微寒,可养血敛阴,又能柔肝,炒制后酸甘微寒,焦助肝阴,补肝之体,缓中止痛作用增强。研究表明,白芍的主要成分为白芍总苷、氧化芍药苷、苯甲酰芍药苷、芍药内酯苷等,具有抗炎、抗溃疡、抗氧化、调节免疫等作用[5]。白芍总苷能抑制副交感神经的兴奋性而具有解痉作用,在动物模型中对胃肠道电运动有明显的抑制作用;通过延长豚鼠结肠平滑肌横肌收缩时间而促进结肠运动[6]。故本研究采用葡聚糖硫酸钠(dextran sulfate sodium, DSS)诱导小鼠IBD模型,观察白芍总苷对小鼠一般情况、结肠病理及炎症相关指标的影响,探讨其防治IBD的作用机制。
1 材料与方法
1.1 实验动物
C57BL/6雄性小鼠50只,SPF级,6~8周龄,体质量(20±2) g,由上海中医药大学实验动物中心提供。动物许可证号:SYXK(沪2014-008)。所有小鼠于动物中心饲养、造模和观察,自由饮食饮水。
1.2 药物和主要试剂
白芍总苷(宁波立华制药有限公司,批号:190305);柳氮磺吡啶肠溶片(上海福达制药有限公司,批号:2215272);DSS,分子量为36 000~50 000 DA(MP Biomedicals公司,批号:Q4299);粪隐血测试盒(南京建成生物工程研究院,批号:20180521);IL-8 ELISA试剂盒(批号:MEI023)、IL-10 ELISA试剂盒(批号:MEI025)、IL-23 ELISA试剂盒(批号:MEI036)、IL-36 ELISA试剂盒(批号:MEI048)、趋化因子CCL20 ELISA试剂盒(批号:MEI055)、趋化因子CCR6 ELISA试剂盒(批号:MEI045)均由上海博谷生物科技有限公司提供。
1.3 方法
1.3.1 分组及造模 将50只SPF级C57BL/6小鼠按照体质量分层随机的方法分成:正常组(N组)、模型组(M组)、柳氮磺吡啶组(L组)、白芍总苷低剂量组(BL组)、白芍总苷高剂量组(BH组),每组10只。适应性饲养1周后,N组自由饮食饮水,其余组小鼠自由饮用3% DSS溶液,连续7 d,建立小鼠炎症性肠病的急性模型。
1.3.2 给药方式 根据动物实验等效剂量系数折算法,将人每天9.1倍剂量折算成小鼠等效剂量。白芍总苷人常用剂量为0.6 g,1天2~3次口服。将柳氮磺吡啶(300 mg/kg)、白芍总苷低剂量(180 mg/kg)和白芍总苷高剂量(250 mg/kg)用0.3%羧甲基纤维素钠稀释成混悬液。自造模起,各用药组每日定时根据小鼠体质量以0.02 mL/g混悬液灌胃1次,对照组给予0.9%氯化钠灌胃1次,均连续灌胃7 d。
1.3.3 样品采集与处理 禁食12 h后,用3%戊巴比妥钠,按照2 mL/kg体质量的剂量腹腔注射麻醉,由下腔静脉收集血液,将小鼠结肠、直肠组织用PBS溶液冲洗干净。选取肛门上1 cm组织,置于10%福尔马林中,剩余肠组织装入冻存管,-80 ℃保存。肠组织于福尔马林中固定24 h后予以脱水、包埋,并制成切片,以苏木精-伊红染色后于显微镜下观察。血液予以4 ℃下静置3 h后,在低温离心机4 ℃环境下3 000 r/min离心15 min,分离血清,分装后于-70 ℃保存待用。
1.4 观测指标
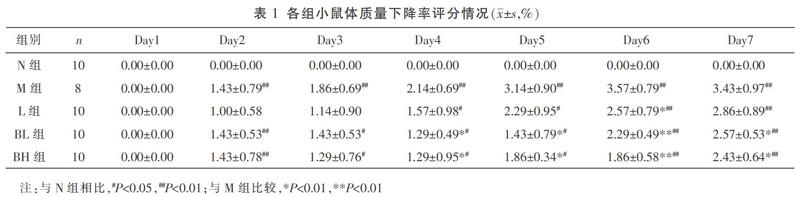
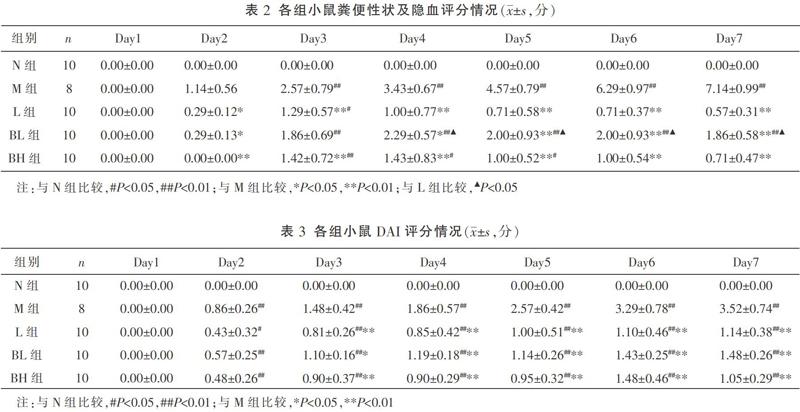
1.4.1 结肠炎疾病活动指数(disease activity index, DAI)评分 每日灌胃前称小鼠体质量,计算小鼠体质量下降率=(当天体质量/实验前体质量)×100%,观察粪便性状,测定粪便隐血情况,并进行相应评分。DAI评分标准具体如下:(1)体质量评分:无变化或增加,0分;减轻1%~5%,1分;减轻5%~10%,2分;减轻10%~15%,3分;减轻大于15%,4分[7-8]。(2)粪便性状评分:正常,0分;软便、球状便,1分;膏状或半球状便,肛门无附着,2分;膏状便,肛门有附着,3分;稀便,4分。(3)粪便隐血评分:阴性,0分;弱阳性,1分;阳性,2分;强阳性,3分;肉眼血便,4分。
DAI评分=(体质量评分+粪便性状评分+粪便隐血评分)/3
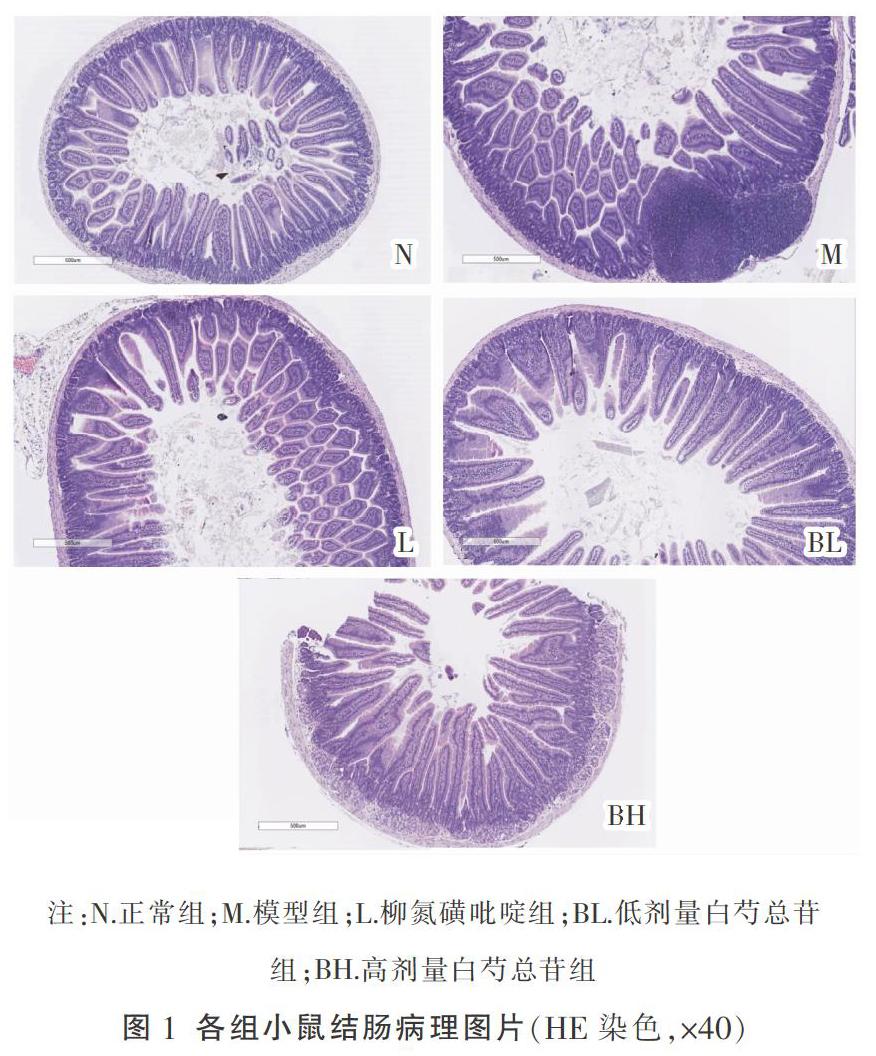
1.4.2 直肠病理组织学评价 切片以苏木精-伊红染色后于显微镜下观察,病理组织学评判标准如下:(1)炎症程度:无、轻、中、重;(2)病变累及深度:无病变、黏膜层、黏膜下层、透壁;(3)隐窝破坏情况:无破坏、基底1/3破坏、基底2/3破坏、基底破坏仅上皮完整;(4)病变累及范围:1%~25%,26%~50%,51%~75%,76%~100%。
在本实验中,L组与BH组显著降低了体质量下降率,随着治疗周期的延长更为显著,对便血、腹泻的改善两者最终无差异。同时BH组能有效减少杯状细胞和淋巴细胞浸润。ELISA的结果证实炎症因子的表达受到了有效抑制。由此可见,白芍总苷在治疗DSS诱导的IBD小鼠模型切实有效。当肠道发生急性黏膜损伤时,白芍总苷显著抑制了IL-8、CCL20和CCR6的表达,降低了对炎症因子的趋化作用,使IL-23表达减少,IL-36的生成亦减少,最终抑制了Th1-Th17型免疫反应,保护了肠道黏膜上皮的完整性。
综上所述,白芍总苷可显著抑制炎症因子的表达,减少炎性细胞的浸润,调节免疫功能,从而改善IBD小鼠腹泻及便血症状,同时改善结肠组织的炎性损伤。
参考文献
[1] 傅志泉,李 珍,赵思宇,等.精神情志对炎症性肠病影响的作用机制及中医药干预对策的研究现状[J].世界中医药,2017,12(8):1979-1984.
[2] HAN Y, MA T M, LU M L, et al. Role of moxibustion in inflammatory responses during treatment of rat ulcerative colitis[J]. World Journal of Gastroenterology, 2014, 20(32): 11297-11304.
[3] FRATILA O C, CRACIUN C. Ultrastructural evidence of mucosal healing after infliximab in patients with ulcerative colitis[J]. Journal of Gastrointestinal and Liver Diseases: JGLD, 2010, 19(2): 147-153.
[4] 吴淑芬,桂茜茹,张小萍.溃疡性结肠炎中医药治疗认识[J].江西中医药,2013,44(3):14-16.
[5] 王朝虹,闵知大.芍药化学成分及药理研究[J].时珍国医国药,1999,10(7):3-5.
[6] 向军英,欧阳钦,胡仁伟,等.白芍总苷对小鼠潰疡性结肠炎的作用[J].中华临床营养杂志,2010,18(4):230-234.
[7] 王旭丹,袁学勤,邱泽计,等.四神丸改善TNBS及DSS诱导小鼠实验性结肠炎的研究[J].北京中医药大学学报,2014,37(11):781-785,4.
[8] 王 新.小檗碱对DSS结肠炎小鼠保护作用的研究[D].广州:广东药科大学,2016.
[9] YAN Y T, KOLACHALA V, DALMASSO G, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis[J]. Plos One, 2009, 4(6): e6073.
[10] RANDHAWA P K, SINGH K, SINGH N, et al. A review on chemical-induced inflammatory bowel disease models in rodents[J]. The Korean Journal of Physiology & Pharmacology, 2014, 18(4): 279-288.
[11] ALEX P, ZACHOS N C, NGUYEN T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis[J]. Inflammatory Bowel Diseases, 2009, 15(3): 341-352.
[12] MEDINA-CONTRERAS O, HARUSATO A, NISHIO H, et al. Cutting edge: IL-36 receptor promotes resolution of intestinal damage[J]. Journal of Immunology (Baltimore, Md.: 1950), 2016, 196(1): 34-38.
[13] SCHEIBE K, BACKERT I, WIRTZ S, et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo[J]. Gut, 2017, 66(5): 823-838.
[14] AWASTHI A, RIOL-BLANCO L, JAGER A, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells[J]. Journal of Immunology (Baltimore, Md.: 1950), 2009, 182(10): 5904-5908.
[15] ZENEWICZ L A, FLAVELL R A. Recent advances in IL-22 biology[J]. International Immunology, 2011, 23(3): 159-163.
[16] BECKER C, DORNHOFF H, NEUFERT C, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis[J]. Journal of Immunology (Baltimore, Md.: 1950), 2006, 177(5): 2760-2764.
[17] KHN R, LHLER J, RENNICK D, et al. Interleukin-10-deficient mice develop chronic enterocolitis[J]. Cell, 1993, 75(2): 263-274.
[18] SPENCER S D, DI MARCO F, HOOLEY J, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor[J]. Journal of Experimental Medicine, 1998, 187(4): 571-578.
[19] GEUKING M B, CAHENZLI J, LAWSON M A, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses[J]. Immunity, 2011, 34(5): 794-806.
[20] HUBER S, GAGLIANI N, ESPLUGUES E, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner[J]. Immunity, 2011, 34(4): 554-565.
[21] ZIGMOND E, BERNSHTEIN B, FRIEDLANDER G, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis[J]. Immunity, 2014, 40(5): 720-733.
[22] SHOUVAL D S, BISWAS A, GOETTEL J A, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function[J]. Immunity, 2014, 40(5): 706-719.
[23] GIRARD-MADOUX M J, OBER-BLBAUM J L, COSTES L M, et al. IL-10 control of CD11c + myeloid cells is essential to maintain immune homeostasis in the small and large intestine[J]. Oncotarget, 2016, 7(22): 32015-32030.
[24] GLOCKER EO, KOTLARZ D, BOZTUG K. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor[J]. The New England Journal of Medicine, 2009, 361(21): 2033-2045.
[25] PIGNEUR B, ESCHER J, ELAWAD M, et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: A survey of the Genius Working Group[J]. Inflammatory Bowel Diseases, 2013, 19(13): 2820-2828.
[26] BEGUE B, VERDIER J, RIEUX-LAUCAT F, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease[J]. American Journal of Gastroenterology, 2011, 106(8): 1544-1555.
[27] COOK D N, PROSSER D M, FORSTER R, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue[J]. Immunity, 2000, 12(5): 495-503.
[28] DIEU-NOSJEAN M C, MASSACRIER C, HOMEY B, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors[J]. The Journal of Experimental Medicine, 2000, 192(5): 705-718.
[29] IWASAKI A, KELSALL B L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokinesmacrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine[J]. The Journal of Experimental Medicine, 2000, 191(8): 1381-1394.
[30] DIEU M C, VANBERVLIET B, VICARI A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites[J]. The Journal of Experimental Medicine, 1998, 188(2): 373-386.
[31] LI Q, LAUMONNIER Y, SYROVETS T, et al. Recruitment of CCR6-expressing Th17 cells by CCL20 secreted from plasmin-stimulated macrophages[J]. Acta Biochimica et Biophysica Sinica, 2013, 45(7): 593-600.
[32] COMERFORD I, BUNTING M, FENIX K, et al. An immune paradox: How can the same chemokine axis regulate both immune tolerance and activation?: CCR6/CCL20 chemokine axis balancing immunological tolerance and inflammation in autoimmune disease[J]. BioEssays, 2010, 32(12): 1067-1076.
[33] YAMAZAKI T, YANG X O, CHUNG Y, et al. CCR6 regulates the migration of inflammatory and regulatory T cells[J]. Journal of Immunology, 2008, 181(12): 8391-8401.
[34] KWON J H, KEATES S, BASSANI L, et al. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease[J]. Gut, 2002, 51(6): 818-826.
[35] KASER A, LUDWICZEK O, HOLZMANN S, et al. Increased expression of CCL20 in human inflammatory bowel disease[J]. Journal of Clinical Immunology, 2004, 24(1): 74-85.
[36] SKOVDAHL H K, GRANLUND A V, STVIK A E, et al. Expression of CCL20 and its corresponding receptor CCR6 is enhanced in active inflammatory bowel disease, and TLR3 mediates CCL20 expression in colonic epithelial cells[J]. Plos One, 2015, 10(11): e0141710.
[37] LIU J Z, VAN SOMMEREN S, HUANG H L, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations[J]. Nature Genetics, 2015, 47(9): 979-986.
[38] MACKAY C R. Moving targets: Cell migration inhibitors as new anti-inflammatory therapies[J]. Nature Immunology, 2008, 9(9): 988-998.
[39] LEE H J, CHOI S C, LEE M H, et al. Increased expression of MIP-3alpha/CCL20 in peripheral blood mononuclear cells from patients with ulcerative colitis and its down-regulation by sulfasalazine and glucocorticoid treatment[J]. Inflammatory Bowel Diseases, 2005, 11(12): 1070-1079.
[40] HE C, ZHANG S L, HU C J, et al. Higher levels of CCL20 expression on peripheral blood mononuclear cells of Chinese patients with inflammatory bowel disease[J]. Immunological Investigations, 2010, 39(1): 16-26.
[41] SPAGNUOLO R, DATTILO V, DANTONA L, et al. Deregulation of SGK1 in ulcerative colitis: A paradoxical relationship between immune cells and colonic epithelial cells[J]. Inflammatory Bowel Diseases, 2018, 24(9): 1967-1977.
(本文編辑 匡静之)
