Effects of Dietary Protein Variations at Different Life-stages on Vocal Dominance of the African Clawed Frogs
2020-09-30YanCAOJiangyanSHENXiaocuiWANGSongTANPingLIJianghongRANYezhongTANGandJingfengCHEN
Yan CAO ,Jiangyan SHEN ,Xiaocui WANG ,Song TAN ,Ping LI ,Jianghong RAN ,Yezhong TANG and Jingfeng CHEN
1 Thematic Area of Biodiversity and Ecosystem Services,Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
2 Key Laboratory of Bio-Resources and Eco-Environment of Ministry of Education,College of Life Sciences,Sichuan University,Chengdu 610041,Sichuan,China
3 University of the Chinese Academy of Sciences,Beijing 100049,China
Abstract How nutritional conditions during early development affect an organism’s phenotype at adulthood is still poorly understood despite a plethora of research on developmental plasticity.The “environmental matching”hypothesis predicts that individuals will have high fitness providing that their adult environment “matches” what they experienced during development.In contrast,the“silver spoon” hypothesis predicts that individuals who obtain better developmental resources will be generally superior.Here we tested these two hypotheses and examined the underlying hormonal mechanisms by manipulating the early dietary protein content of African clawed frogs (Xenopus laevis) for a year with a 2×2 factorial experimental design.We found that only a lowprotein food during development enhanced the vocal competition ability of male X.laevis,and that vocal dominance was associated with higher cortisol levels but not related with testosterone content.These results were not congruent with the “environmental matching”hypothesis or with the “silver spoon” hypothesis,suggesting the behavioral plasticity during development is more complex than our expectation in amphibians.
Keywords aggression,developmental effects,environmentalmatching,silver spoon,social status,vocal competition,Xenopus laevis
1.Introduction
Evolutionary ecologists have long been fascinated by how the interactions between developmental and adult environments shape adult phenotype and performance (Taborsky,2006;Wells,2006; Monaghan,2008; Lindstromet al.,2009; Kellyet al.,2019).Currently two competing hypotheses have been widely investigated.The “silver spoon” hypothesis predicts that the fitness of a phenotype produced as a consequence of inferior nutrition is simply impaired and thereby consistently lower than the fitness of non-impaired individuals (Lindstrom,1999;Monaghan,2008).The inferior fitness could be derived from developmental constraints imposed by resource limitation(Monaghan,2008).Alternatively,the “environmental matching”hypothesis predicts that the developmental circumstances experienced by juvenile trigger the development of alternative phenotype such that fitness is maintained,providing that the adult environment matches that of the developmental stages(West-Eberhard,2003; Gluckmanet al.,2005; Monaghan,2008).However,data supporting the two hypotheses are mixed.Evidence consistent with the “environmental-matching”hypothesis is mainly from studies on humans (Wells,2007).In contrast,investigations in other organisms mostly support the ‘silver spoon’ hypothesis (e.g.butterflyBicyclus anynana(Saastamoinenet al.,2010),cockroachNauphoeta cinereal(Barrettet al.,2009),burying beetlesNicrophorus vespilloides(Hopwoodet al.,2014),ladybird beetlesHarmonia axyridis(Dmitriew and Rowe,2011),cichlid fishSimochromis pleurospilus(Taborsky,2006),oystercatcherHaematopus ostralegus (Van de Polet al.,2006)); or neither (e.g.house cricketsAcheta domesticus(Royauteet al.,2019),southern corroboree frogsPseudophryne corrobore(McInerneyet al.,2016; Sillaet al.,2016),Mallard ducksAnas platyrhynchos(Butler and McGraw,2012)).Regardless of this mixed support,the majority of studies into interaction of developmental and adult nutritional conditions have focused on life-history traits,but few on behavioral phenotypes (Sillaet al.,2016).
The indicator model of sexual selection predicts that sexually selected traits serve as honest indicators of condition to potential mates and rival male competitors (Johnstone,1995).Abundant studies have proved that consuming greater amounts of food or more nutrient-dense food is often associated with increased ornamentation or sexual signaling (Dodmanet al.,1996; Simonet al.,2004; Kayet al.,2010; Pillayet al.,2016; Han and Dingemanse,2017).Comparatively fewer studies have examine the influence of nutrition on aggressive behaviors related to male-male competition (Wilsonet al.,2010),and even less considering the interactive effect of nutritional environments on the contest outcome at different life-stages.The only study was conducted in burying beetles (Hopwoodet al.,2014) and found no evidence for “environmental-matching” or simple “silver spoon” effects.However,the generality of this pattern is still unknown.
Hormonally driven changes in physiology can have a profound influence on aggressive behavior particularly during the breeding season where surges in androgens can enhance the motivation to engage in agonistic encounters when males are contesting access to females (Briffa and Sneddon,2007).A variety of hormones are released from the endocrine system that can have direct and indirect effects on aggression in both the developing and adult individuals (Huntingfordet al.,1995).These hormones can be categorized into three groups:the androgens; the stress hormones and the neurohormones such as biogenic amines (Creel,2005).However,we know little how these hormones mediate the association between nutritional conditions and aggression at different life-stages.
Vocal communication is a hallmark of reproduction in anurans.Early nutrition (low protein) diet has been shown to have important implications for the developmental plasticity of vocalizations (Nowickiet al.,2002).Thus,it is expected the low dietary protein may modulate the vocal system in anurans during developmental period.The African clawed frog (Xenopus laevis) is native to sub-Saharan Africa (Tinsley and Kobel,1996).Previous works found that pairing two sexually mature males resulted in complete or partial suppression of advertisement calling in one and this type of vocal dominance can be achieved even in the absence of physical contact and is not determined by body size (Tobiaset al.,1998; Tobiaset al.,2004; Tobiaset al.,2014).Moreover,a reproductive advantage of the vocal dominance has been commonly recognized in this species(Tobiaset al.,2010; Xuet al.,2012; Rhodeset al.,2014).Therefore,these behavioral characteristics ofX.laevisare quite convenient to test the nutritional hypotheses.
In the current study,we conducted food manipulation experiment with a 2×2 factorial design and determined the effects of diet protein on vocal contest ability as well as rate of growth and hormone levels onXenopus.The ‘silver spoon’hypothesis predict that 1) if individuals that have experienced high protein diet during development and/or adulthood significantly do better than those that experienced low dietary conditions during development and/or adulthood for any given environment experienced in adulthood,and 2) the ‘environmental-matching’ hypothesis predict that if individuals show a better vocal competition performance under the same dietary condition in developmental and adult stages.Moreover,we also predict that vocal competition ability would positively co-vary with testosterone and corticosterone content,considering the interaction between hypothalamuspituitary-gonadal and hypothalamus-pituitary-adrenal axes plays a pivotal role in calling behavior and energy mobilization(Gluckmanet al.,2005; Mooreet al.,2005; Joshiet al.,2019).
2.Material and methods
2.1.Study animalThe entire experiment was conducted from July 1,2014 to September 1,2016.AdultX.laevispurchased from Nasco (Fort Atkinson,WI,USA) and bred in our laboratory.Adult males and adult females received two injections of 0.2-0.3 ml (100 IU/0.1 ml) of human chorionic gonadotropin and paired in separated plastic containers (L×W×H,61cm×43cm×37cm,water depth 20cm).384 tadpoles in total from paired toads were reared at (19±1)°C and a 12L:12D light regime.Before metamorphosis,tadpoles were fed milk powder (Yangping Dairy Company,China).When these tadpoles entered stage 51 (Dekkeret al.,1994),boiled and chop pork liver were added gradually.Three months after metamorphosis all froglets were fed with the gold fish pellet (Gold-inch fish feed Co.,Ltd,Shenzhen).The food was supplied 3 h before the end of the daily light period.Water was changed on a weekly basis to maintain water quality.All animal procedures in this study were carried out in accordance with and approved protocols the Animal Care and Use Committee at the Chengdu Institute of Biology,Chinese Academy of Sciences (Permit number:20140306).
2.2.Diet protein treatmentOne year after metamorphosis,theses froglets were evenly divided into 12 containers (L×W×H,61cm×43cm×37cm,water depth 20 cm).Each box contained 32 individuals.To create low and high protein diet,half of group were fed with either high protein pellet (H group) or with low protein pellet (L group) (day 0).The main difference between the two diets was their protein content:the low diet comprised roughly 15% protein by dry weight,while the high diet comprised 45% (Table 1).The setting of dietary protein gradient was based on the field studies (Tinsley and Kobel,1996; Veneskyet al.,2012; Martinset al.,2013).After 122 days food treatment(DEV period),H and L groups were again randomly allocated into either low or high protein group for the next eight months(ADL stage),thereby creating four different rearing diet groups (HH,HL,LH,and LL) with each group reared in three containers (Figure 1).
Most individuals were sexually mature (e.g.nuptial pad appeared and advertisement calls emitted) at day 277 (five months after re-allocation).Then males and females were separated and then raised in different tanks (same size as above).Each tank contained 13-15 individuals.Meanwhile,all the frogs were labeled with PIT-tag (Biomark,USA) to individually track their body conditions and facilitate the following behavioral tests.
2.3.Behavioral trialsBehavioral test was carried out at 1900-2400 hour from day 321 to day 341.To examine the effects of diet treatment on the contest ability,one male was randomly chosen from each diet group for each trial and composed a quad (mean % difference between members within quads ± SE=(7.8±0.6)% for SVL and (21±2.0)% for mass).Within one quad,each frog contested every other member such that each frog engaged in three contests which form six contrasts (HH versus HL; HH versus LH; HH versus LL; HL versus LH; HL versus LL;LH versus LL).That means each subject was used for three times in total for the experiment.To avoid stressing the subjects,break of 30 minutes was set between the consecutive test trials.Thus,84 individuals were used in a total of 126 contests (21 quad*6 contest/quad=126).Two contests were discarded because both contestants failed to release advertisement calls and thus no clear winner could be identified,leaving a total of 124 contests for statistical analysis.Competitive tests were conducted in paired males in an aquarium arena (150cm×50cm×60cm),which was divided equally into 3 sections (left,middle,and right) along the long dimension with 2 plastic mesh barriers restricting the paired individuals at the end sections.These barriers did not impede sound transmission.One lab-40 hydrophone (Labcore System,Washington,DC) was installed under the water for each holding aquarium to record spontaneous calls before pairing.Two lab-40 hydrophones were installed at the left and right end sections of the competitive aquarium to record calls produced by each male during contests.The hydrophones were connected to a digital recorder (Marantz PMD 660,16bit,44.1 kHz).The temperature and photoperiod conditions were setup as the same as those for home tank.Advertisement call effort(in seconds) were measured with Audition 3.0 software.The call effort was defined as the total time spent in calling during a 45-min session.The winner of the pair competition was defined as the individuals that made more calls than its rival (Tobiaset al.,2004).

Figure 1 The timeline of the whole experiment.
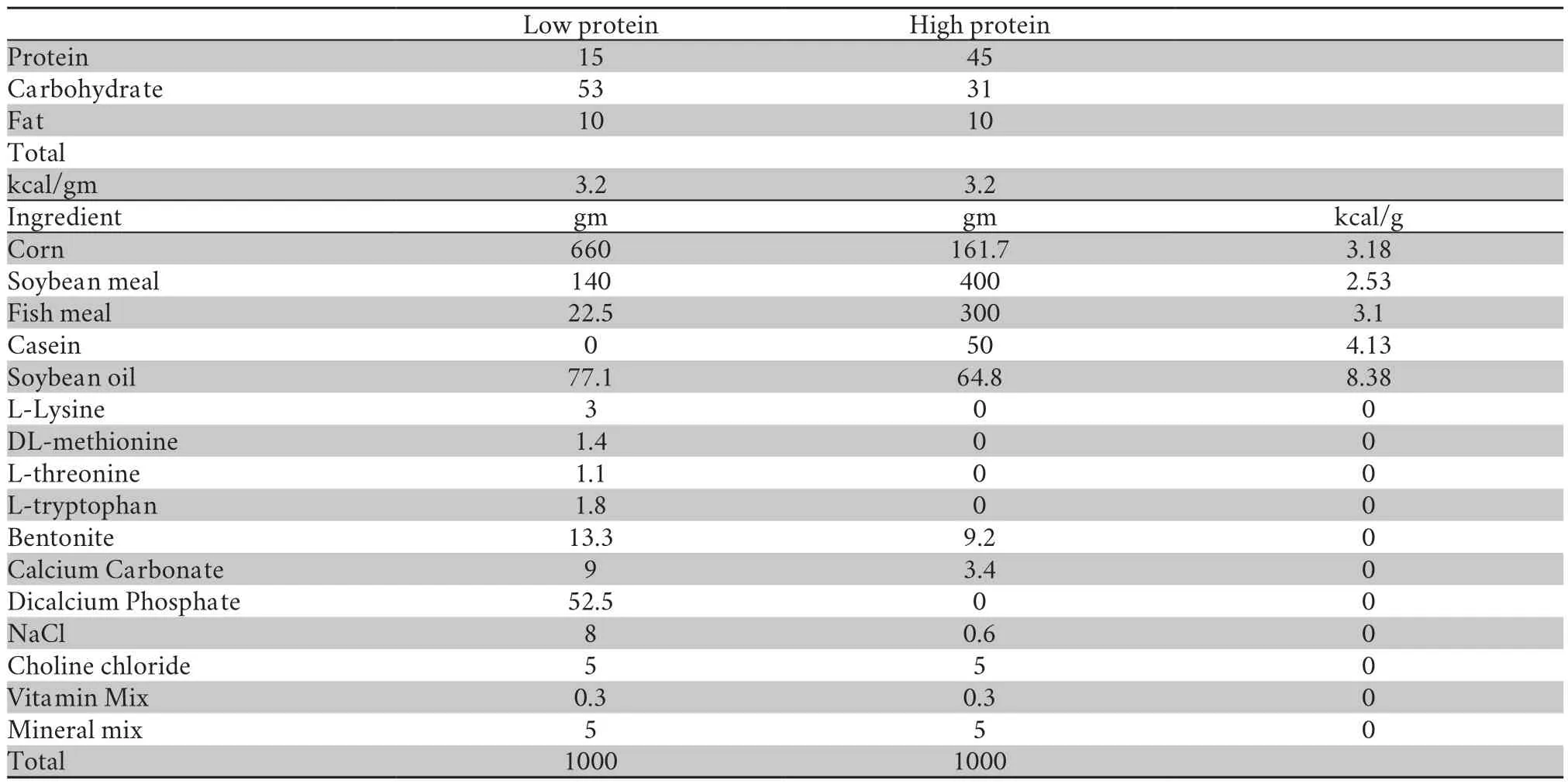
Table 1 Nutrition proportion and ingredient of pellets feeding African clawed frogs.
2.4.Hormone radioimmunoassayAt day 385,all 84 subjects involved in behavioral tests were scarified and their sera were collected.The Sera testosterone and cortisol were measured with Iodine [125I]-Rabbit-Testosterone Radioimmunoassay Kit and Iodine [125I]-Rabbit-Cortisol Radioimmunoassay Kit(Beijing North Institute of Biological Technology,China).The fetal bovine serum was used as the intraplate control.The intra-assay coefficient of variation (CV) was<10% and interassay CV was<15%.The lower and upper effective limits of the testosterone assay kit was between 0.1 ng/mL and 20 ng/mL,and those of cortisol between10 ng/mL and 500 ng/mL,respectively.
2.5.Statistical AnalysesAnalyses were performed in the IBM SPSS Statistics 21 and R package.Cortisol and testosterone concentrations were transformed to yield normality distribution with Cox-Box transformation.T-test was used to examine the differences in body mass at day 0,day 61,day 91,day 122 after food treatments.Effects of diet treatments during DEV,and ADL,and their interactions (DEV×ADL) on the body mass at day153,day 211 and day 243,and cortisol and testosterone contents were analyzed with ANOVA.Repeatedmeasures ANOVAs were used to test male body mass at adulthood (4 time points:day 277,day 305,day336,day 372,the subjects individually labeled during this period) as a function of treatment time,treatment during DEV or ADL,and interaction between the treatment during DEV and ADL.We ran general linear model to analyze the effects of diet treatment during DEV or ADL,and their interaction on the body mass gain (day 277-305,day 305-336,day 336-372) with the initial body mass of each period as covariates.
We examined whether the diet treatment may affect fighting ability using the structured Bradley-Terry model for pair comparisons (Firth,2005).The structured Bradley-Terry model is a form of generalized linear model that can ascribe fighting abilities (derived from the binary contest outcomes) to a series of predictors and estimate the probability of individual i beating individual j (Firth,2005).As each individual is staged with several opponents,the model used at the present study includes an additional term,compatibility with the potential effects of prior experience,where the extra predictor Ziksummarizes the contest history of individual i at the time of contest k.Thus,the full model can be expressed as:
Logit [probability(ibeatsjin contest k)]=λi-λj+δ(Zik-Zjk)
Where λiand λjrepresent the abilities of the two individuals.
We included an experience effect for (up to) the previous two contests rather than only the most recent contest,as (Stuart-Foxet al.,2006) showed that the previous two contest outcomes had the strongest influence on subsequent fighting ability in lizards.We set a score ranging between 0 and 1 for ‘prior wins’(0=no wins,0.5=the penultimate win,1=the most recent win).We also gave each contestant a score for ‘prior losses’ (0=no losses,-0.5=the penultimate losses,-1=the most recent losses).The reason for different score which was assigned to different encounter is based on the fact the advantage of a previous win or disadvantage of a previous loss decreases exponentially over time (Stuart-Foxet al.,2006).We set contest outcome as the dependent variable,and the following predictors of fighting ability as independent variables:‘prior wins’,‘prior losses’,diet treatments (DEV,ADL,and DEV×ADL).To choose the most parsimonious model,we employed a standard stepwise procedure to examine reduction of model Akaike Information Criterion as well as the significance of variables.
3.Results
3.1.Somatic growthPrior to diet treatment (day 0),the body mass of H group and L group were similar (Table2,Table S1).As a consequence of the diet protein treatment,froglets in the H group grew faster than their siblings during the following 2 months.At day 122,the body mass of H group was 25% higher than that of L group (Table 2,Table S1).At day 153,HH and HL frogs were significantly larger than LH and LL group; at day 211 and day 243,both treatment during DEV and ADL but not their interaction markedly affected the body mass,which increased in frogs fed with higher protein diet during either DEV or ADL treatment period.After the males and females were separated at day 277,no significant effect of treatment during DEV and ADL on male or female body mass was found(Table2,Table S1).In addition,there were no significant effects of the dietary treatment and initial body mass on the body mass gain in males,while the body mass gain was dependent largely on the initial mass and dietary treatment in females (Table 3,Table S2).
3.2.Hormone levelsDietary treatment during DEV but not ADL and their interaction significantly affected cortisol concentration ofXenopus(DEV:F(1,80)=5.813,P=0.018; ADL:F(1,80)=0.797,P=0.374; interaction:F(1,80)=2.863,P=0.095; Figure 2).Comparing with LL and LH groups,HH and HL groups had higher cortisol levels in plasma (Table 4,Figure 2).However,diet experience had no influence on the plasma testosterone content (Table 4).
3.3.Vocal competition abilityBradley-Terry model showed that dietary treatment during DEV but not ADL or theirinteraction or previous contest experiences significantly affected vocal contest outcome (Table 5).For males that differ in dietary supplement during juvenile,and all else being equal,the probability that male A (growing under low protein condition)defeats male B (growing under high protein condition) is estimate to be exp (0.6921×2)/ [1+exp (0.6921×2)]=80%.
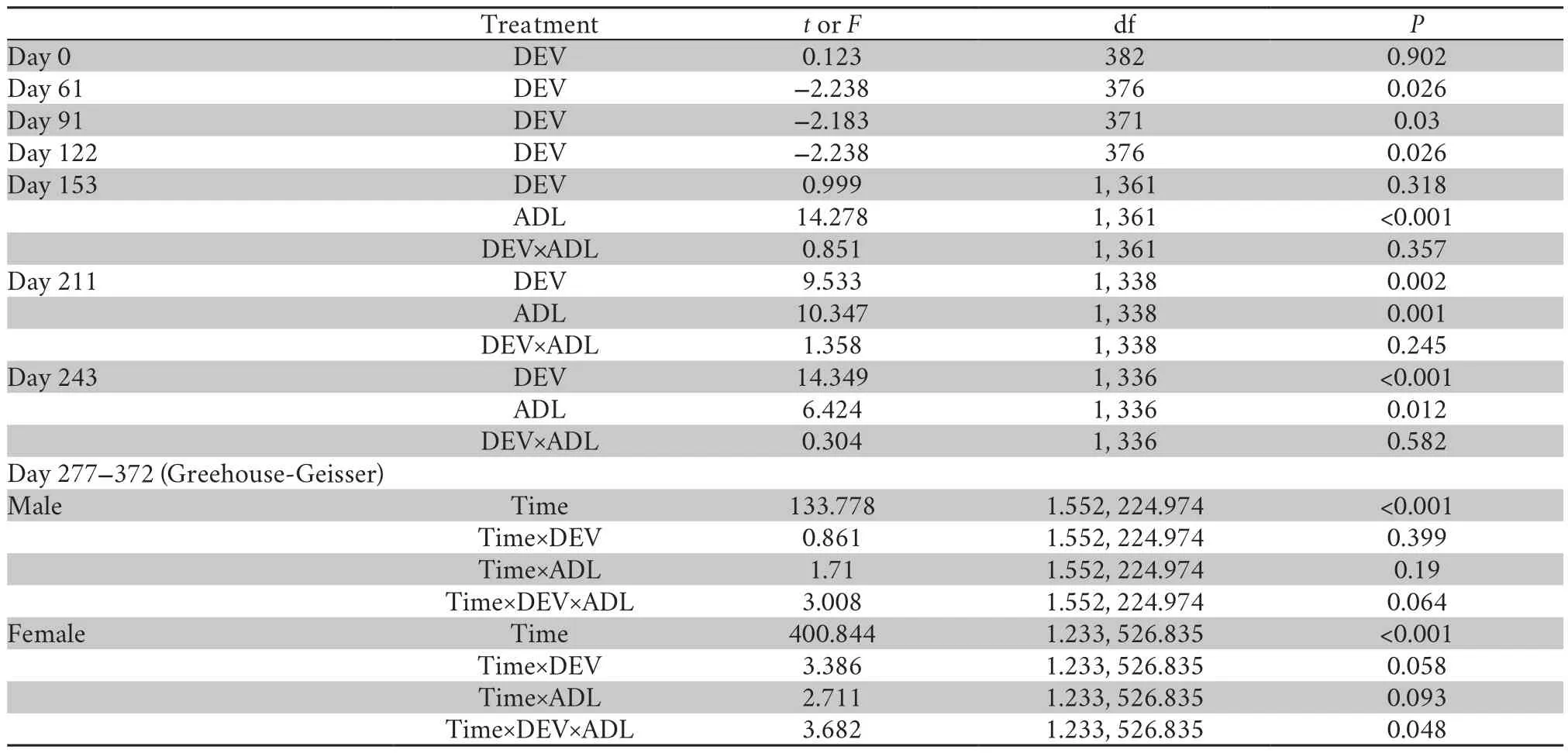
Table 2 The effects of dietary protein on body mass in African clawed frogs during the periods of juvenile (DEV) and adult (ADL).
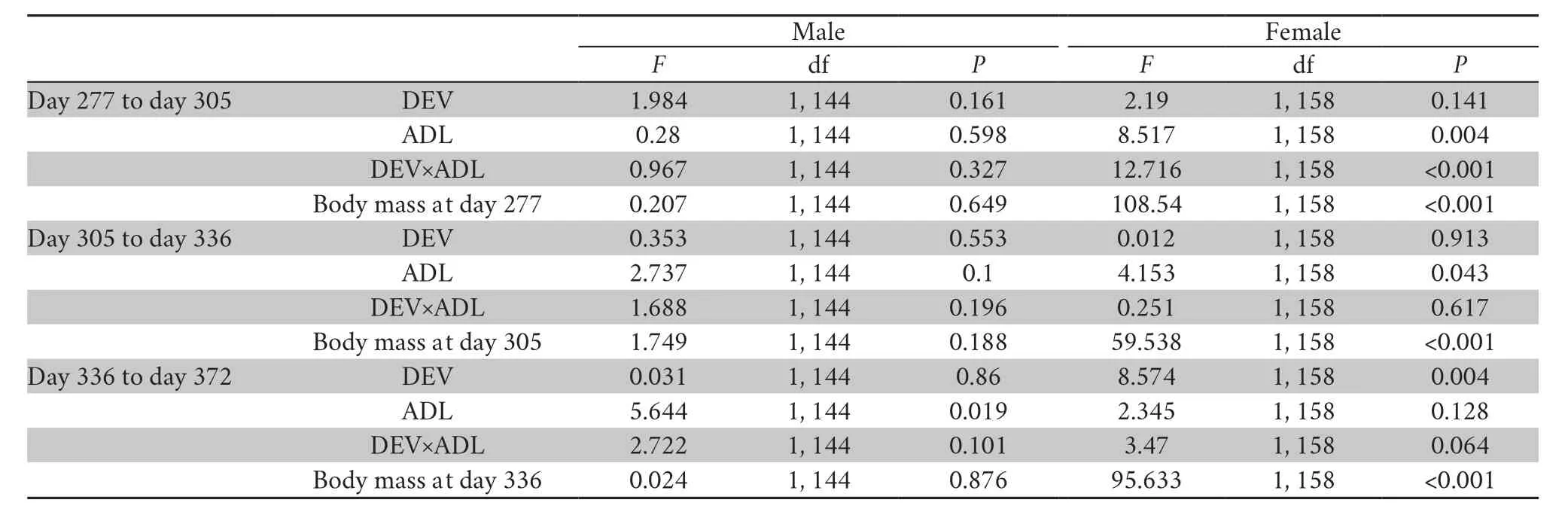
Table 3 mixed model analyses of the effects of diet treatment on body mass gain of male African clawed frogs under the four-diet treatment(Analyses controlled for body mass at the start of each period).

Table 4 The effects of dietary treatment on cortisol and testosterone concentrations in African clawed frogs.
4.Discussions
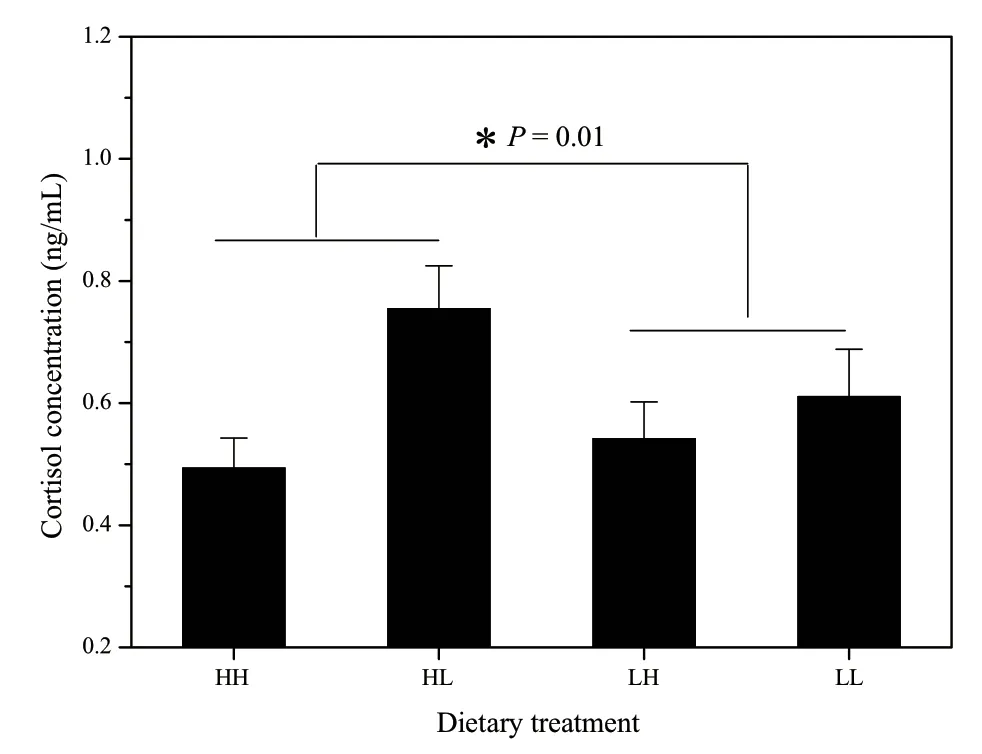
Figure 2 The effects of dietary protein during development(DEV) and during adulthood (ADL) on the serum cortisol content.Asterisks indicate significant diet treatment differences between H-and L-group during development.
The diet manipulations resulted successful in significant differences in the growth trajectories ofXenopus(Figure S1).The juvenile frogs fed with a low-protein diet showed early growth retardation and were smaller in mass at day 61.However,at day 277 there was no group difference in male mass,suggesting the pattern of compensation differed according to the dietary schedule.The modifications of social interactions induced by alterations of group size and sexual separation at day 243 could be responsible for the vanished difference in male mass observed at day 277 and afterwards.However,the modulated social pressure is unlikely a major factor in mass regulation in the present study,because the group difference of female mass was maintained until the end of the experiment.
Based on the “silver spoon” hypothesis,it is expected that the frogs fed with high protein food during development should defeat those fed with low protein food with high probability,while the ‘environmental-matching’ hypothesis posits that this‘silver spoon’ effects depends on a continuation of favorable conditions in to the adult stage.However,our results found that the individuals raised with low protein diet had superior vocal competition ability than those with high protein diet at earlylife stage,which are not in consistence with the “environmentalmatching” hypothesis,and stand in direct opposition to the“silver-spoon” hypothesis.
The elevated vocal competitive ability on low-protein supplement at early-life stagecould be attributed to the effects of changing levels of nutrition on developmental process.Protein limitation can change the relative proportion of growth in different organs (Desaiet al.,1995),thus leading to differences in relative organ size,even despite that overall body size was full compensated at adulthood.In turn,this could create the observed variation in energy metabolism and behaviorally competitive ability,since variation in the relative organ size of the most metabolically active organs (e.g.liver,digestive tract) has been shown to be responsible for some of the interindividual variation in metabolic rate and aggressive activity(Konarzewski and Diamond,1995; Speakman and McQueenie,1996; Mathot and Dingemanse,2015).However,we did not find obvious differences in relative organ size of this species(unpublished data) and thus does not support the explanation of metabolic organ development.
It is also possible that malnutrition impairs neurocognitive functioning by reducing neurons,alternating neurotransmitter functioning,and increasing neurotoxicity,and such neurocognitive impairments predispose to aggressive behavior(Liu and Raine,2006).Little is known of this effect in anurans and we have not examined the function and structure of these frogs’ neurons.Alternatively,a more probable explanation lies in the effect of dietary protein on endocrine system since a variety of endocrine hormones can have a profound effect upon aggressive behavior particularly during reproductive season (Briffa and Sneddon,2007).In term of stress hormone,dominance is often associated with low glucocorticoids in nonmammalian vertebrates,while the converse is true in mammalian vertebrates and avian cooperative breeders (Creel,2005; Briffa and Sneddon,2007).For instance,in pairwise fight experiments between two breeding lines of Rainbow trout,Oncorhynchus mykiss,in which one line shows the lower or while the other displays the higher cortisol releases in response to a stressor,low stress responsive fishes are always dominant over high stress responders (Pottinger and Carrick,2001).In the present study,we found that serum cortisol content of males living on a low-protein food were relatively lower than the males on a high-protein food,suggesting the cortisol secretion could mediate the aggressive activity according to the levels of early nutritional supplement.
Dietary-induced differences in vocal dominance can profoundly affect reproductive successof maleX.laevis,since the superior individuals may dominate the competitors by vocal suppression and have mating priority with the sexually receptive females in the natural breeding conditions,characterizing with high population density and low visibility (Tobiaset al.,1998; Tobiaset al.,2004; Tobiaset al.,2010).However,maintaining the dominant status may also accompany with fitness costs.For instance,high social status is associated with immunosuppressive,which increased the parasite risk and oxidative damage during the mating season(Archieet al.,2012; Beaulieuet al.,2014; Habiget al.,2018).Our data showed that individuals fed with low protein diet during development grew longer forelimb which is mainly related with forage capability (unpublished data),suggesting an energy cost for holding the vocal dominance.
In conclusion,our findings revealed that only low protein acclimation during development may partially increase vocal competition ability ofXenopusat adulthood,neither supporting‘environmental-matching’ nor ‘silver-spoon’ hypothesis.Moreover,the enhanced effects on vocal contest ability may relate with the declined cortisol content in this frog species subjected to low protein food during development.This behavioral modulation could be part of adaptive strategies ofXenopuswhen they experience nutritional stress due to extremes of temperature and drought.
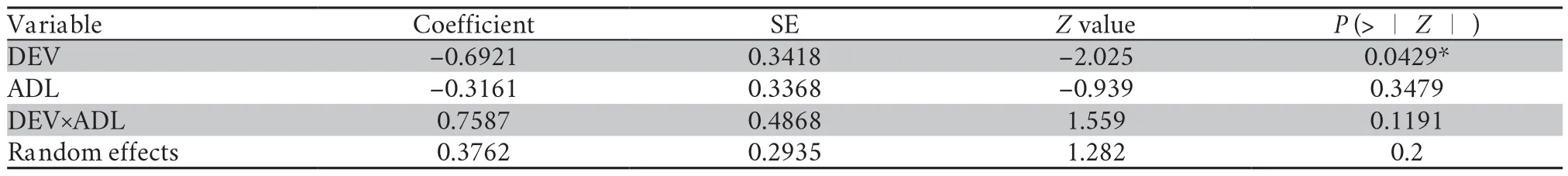
Table 5 Bradly-Terry model showing the group difference in vocal competition and the best predictors of vocal competition ability in African clawed frogs.
AcknowledgementsWe thank all the members of the Behavioral Neuroscience Group of Chengdu Institute of Biology,Chinese Academy of Sciences for their discussion and help with this study.We also thank Professor Jinzhong FU in Department of Integrative Biology,University of Guelph for language revision.The study was conceived and designed by JFC and YC.Experiments were performed by YC,JYS,XCW,ST,PL,JFC; results were analyzed and interpreted by JFC,YZT and YC.The paper was written by JFC and YC.This work was financially supported by grants for the National Natural Science Foundation of China (31370431) to JFC,the Sichuan Provincial Science and Technology Department(2018JY0617) to JFC,the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment,China(2019HJ2096001006) to JFC.
Appendix
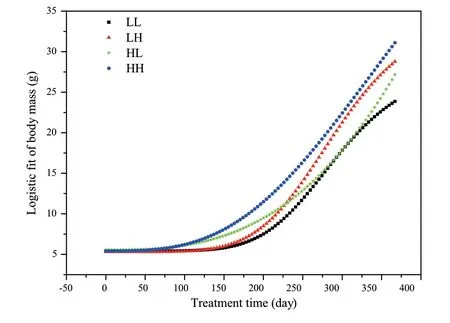
Figure S1 The growth curve of African clawed frogs under different food treatment.

Table S1 The body mass of African clawed frogs during the whole experiment.

Table S2 The body mass gain of African clawed frogs from day 277 to day 372.
杂志排行
Asian Herpetological Research的其它文章
- A New Species of Microhyla (Amphibia:Anura:Microhylidae) from Langbian Plateau,Central Vietnam
- A New Species of Hemiphyllodactylus Bleeker,1860 (Reptilia:Squamata) from Hunan Province,China
- Geographic Variation in the Skull Morphometry of Four Populations of Batrachuperus karlschmidti (Urodela:Hynobiidae)
- The Impact of Stream Landscape on Genetic Structure and Dispersal Patterns in Stream Salamander (Pachyhynobius shangchengensis)
- Age and Body Size of the Shangcheng Stout Salamander Pachyhynobius shangchengensis (Caudata:Hynobiidae) from Southeastern China
- No Evidence for the Compensation Hypothesis in the Swelled Vent Frog(Feirana quadranus)
