A New Species of Microhyla (Amphibia:Anura:Microhylidae) from Langbian Plateau,Central Vietnam
2020-09-30VanChungHOANGMaiAnhLUONGQuangTruongNGUYENNikolaiORLOVYouhuaCHENBinWANGandJianpingJIANG
Van Chung HOANG ,Mai Anh LUONG ,Quang Truong NGUYEN,5 ,Nikolai L.ORLOV ,Youhua CHEN ,Bin WANG and Jianping JIANG,2*
1 Chinese Academy of Sciences (CAS) Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization and Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province,Chengdu Institute of Biology,CAS,Chengdu 610041,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
3 Vietnam National Museum of Nature,Vietnam Academy of Science and Technology,18 Hoang Quoc Viet Road,Hanoi,Vietnam
4 Institute of Ecology and Biological Resources,Vietnam Academy of Science and Technology,18 Hoang Quoc Viet Road,Hanoi,Vietnam
5 Graduate University of Science and Technology,Vietnam Academy of Science and Technology,18 Hoang Quoc Viet Road,Cau Giay,Hanoi,Vietnam
6 Zoological Institute,Russian Academy of Sciences,Universitetskaya nab 1,St.Petersburg 199034,Russia
Abstract We describe a new species,Microhyla hongiaoensis sp.nov.,from Lam Dong Province,southern Vietnam based on morphological data and molecular evidences.The new species is sister to M.pulchella by molecular phylogenetics and also most closely resembles M.pulchella in morphological characteristics,albeit differs from its congeners by a combination of the following morphological features:(1) size medium (SVL 13.6-14.7 mm in males and 18.3-18.6 mm in females); (2) fingers II-IV with small disks,dorsal surface of disks,without median longitudinal groove; (3) webbing formula I1½ -2II1 -2III1 -2½IV2¼ -1V; (4) toe disks with dorsal median longitudinal groove; (5) dorsal back without two small black spots; (6) one small black spot adjacent behind the eyes; (7) few small black scapular spots in the flanks-belly and inguinal region; (8) palm with two small metatarsal tubercles; (9) tibiotarsal reaching beyond snout.M.hongiaoensis sp.nov.occurs in evergreen montane tropical forests at an elevation of 1500 m a.s.l.
Keywords new species,Microhyla,Bi Doup-Nui Ba,national park,morphology,molecular phylogeny
1.Introduction
The genusMicrohylaTschudi,1838 is an assemblage of generally small,mostly ground-dwelling frogs.The genus currently contains 50 species with a distribution range from the Ryukyu Archipelago (Japan) and eastern China westwards to the northern part of India,Sri Lanka and mainland Southeast Asia,and southwards to Indonesia (Frost,2019).Remarkably,23 new species ofMicrohylahave been described during the last decade(Frost,2019) and recent molecular studies have discovered numerous highly divergent lineages and cryptic species (Matsuiet al.,2013; Hasanet al.,2015; Howladeret al.,2015; Seshadriet al.,2016; Wijayathilakaet al.,2016; Yuanet al.,2016; Khatiwadaet al.,2017; Zhanget al.,2018; Garget al.,2019; Liet al.,2019; Nguyenet al.,2019; Poyarkovet al.,2014,2019).
In Vietnam,17 species ofMicrohylahave been recorded so far (Frost,2019) and the greatest diversity of the genus occurs in the central and southern parts of the Truong Son Range(also known as the Central Highlands or the Tay Nguyen Plateau) (Figure 1).Tay Nguyen Plateau,including Langbian Plateau,harbors the highest diversity of amphibians in Indochina with a high level of local endemism and new species discovery (Bain and Hurley,2011; Geissleret al.,2015; Chenet al.,2018; Nguyenet al.,2019).This region appears to be one of the centers of radiation for the genus (Poyarkovet al.,2014).The following 14 species ofMicrohylahave been recorded from the Tay Nguyen Plateau:M.annamensisSmith,1923;M.aurantiventrisNguyen,Poyarkov,Nguyen,Nguyen,Tran,Gorin,Murphy,and Nguyen,2019;M.berdmorei(Blyth,1856)Bain and Nguyen,2004;M.butleriBoulenger,1900;M.darevskiiPoyarkov,Vassilieva,Orlov,Galoyan,Tran,Le,Kretova,and Geissler,2014;M.fuscaAndersson,1942;M.heymonsiVogt,1913;M.minutaPoyarkov,Vassilieva,Orlov,Galoyan,Tran,Le,Kretova,and Geissler,2014;M.mukhlesuriHasan,Islam,Kuramoto,Kurabayashi,and Sumida,2014;M.nanapollexaBain and Nguyen,2004;M.pineticolaPoyarkov,Vassilieva,Orlov,Galoyan,Tran,Le,Kretova,and Geissler,2014;M.pulchellaPoyarkov,Vassilieva,Orlov,Galoyan,Tran,Le,Kretova,and Geissler,2014;M.pulchra(Hallowell,1861) Bain and Nguyen,2004;M.pulverataBain and Nguyen,2004 (Ingeret al.,1999;Bain and Nguyen 2004; Hoanget al.,2013; Poyarkovet al.,2014;Nguyenet al.,2019).
During our recent field surveys in Langbian Plateau of Lam Dong Province in 2018,we collected a series of adult microhylid frogs that morphologically resembleM.pulchella,a species that was recently described from Bi Doup-Nui Ba National Park in Lam Dong Province (Poyarkovet al.,2014).However,these specimens are smaller in size than any known specimen ofM.pulchellaand have greyish-brown to light-brown dorsum with dark-brown markings extending from their interorbital bar to hindlimb,forming a double-waisted figure usually in a light-brown color.Furthermore,they were reproducing syntonically withM.pulchellathat differed markedly from those of sympatricM.pulchella.Subsequent analyses based on morphological and molecular data showed that these specimens represented an independent evolutionary lineage that could not be assigned to any known species ofMicrohyla.We herein describe the population ofMicrohylafrom Lam Dong province as a new species.
2.Materials and Methods
2.1.SamplingField surveys were conducted in Bi Doup-Nui Ba National Park,Lam Dong Province,Vietnam (Figure 1) in May 2018 by C.V.Hoang,A.M.Luong,Y.T.Nguyen,N.L.Orlov,L.Iogansen (hereafter C.V.Hoanget al.).Geographic coordinates and elevation were obtained using a Garmin GPSMAP 78S (WGS 84 data).After photographing the specimens alive,they were euthanized in a closed vessel with a piece of cotton wool containing ethyl acetate (Simmons,2002),fixed in 80% ethanol for five hours,and then transferred to 70%ethanol for permanent storage.Tissue samples were preserved separately in 70% ethanol prior to fixation.Eleven specimens of the new form (Tables 1,3) were collected and subsequently deposited in the collection of the Vietnam National Museum of Nature (VNMN 07385,07388, 07390,07477),Institute of
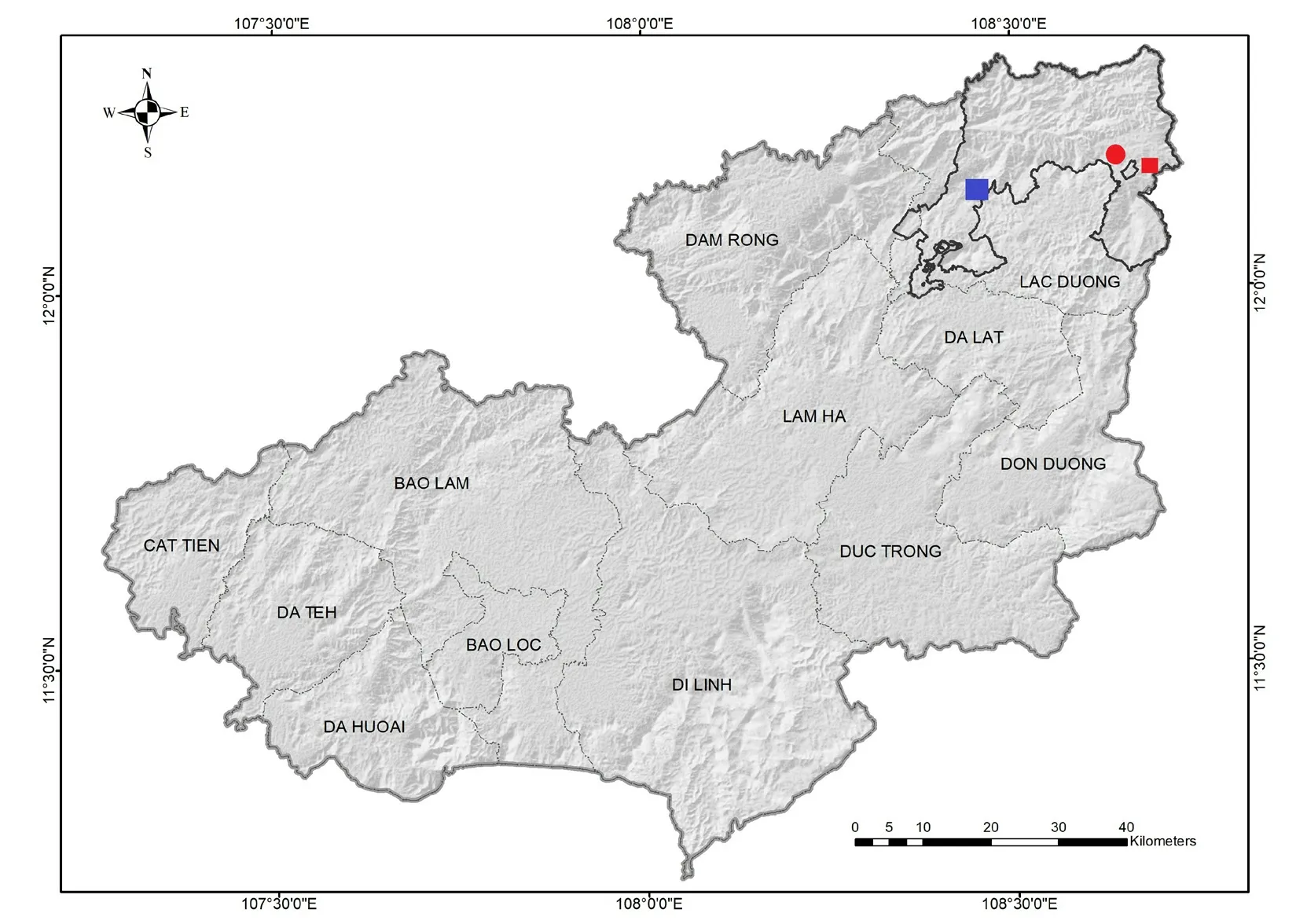
Figure 1 Map showing the type locality (red circle) of Microhyla hongiaoensis sp.nov.in Lam Dong Province,Vietnam (12°11′18.3"N,108°40′29.0"E); blue square is a known distribution point of M. pulchella (12°5′55.81"N,108°21′26.83"E); red square is a known distribution point of M. hongiaoensis sp.nov.and M. pulchella (12°10′27.44"N,108°42′24.64"E).
Ecology and Biological Resources (IEBR 4573-4576),Hanoi,Vietnam.For comparative specimens,21 specimens ofMicrohyla pulchella(Table 3) were collected in Bi Doup-Nui Ba National Park,Lam Dong Province,Vietnam (VNMN 07478,07718,07710,07389,07713-07714,07391,07693,07705-07708,07683,07581,07694,07386,07437,07531,07691,07614,07574).Three specimens of the new form (VNMN 07385,07388,07390) and two specimens ofMicrohyla pulchella(VNMN 07389,07391)were collected in the same breeding pond.Sex was determined by direct observation of calling males or by gonadal dissection after euthanasia.
2.2.Molecular analyses
DNA extraction and sequencing In this study,we amplified a 1936 base pair (bp) length fragment of the 12S rRNA-16S rRNA mitochondrial gene and the complete sequence of tRNAVal that was used recently forMicrohyla(Nguyenet al.,2019).DNA of 21 tissue samples were extracted using TIANamp Genomic DNA kit (TIANGEN BIOTECH,Beijing,China),Tiangen following the manufacturers’ instructions (Table1).Total DNA wasamplified using an Eppendorf PCR machine.PCR total volume was 25 μL,consisting of 12 μL of Mastermix,6 μL of water,1 μL of each primer at a concentration of 10 pmol/μL,and 5 μL of DNA.Primers used in the PCR and sequencing were as follows:12SAL (5’-AAACTGGGATTAGATACCCCA CTAT-3’; forward),16S2000H (5’-GTGATTAYGCTACCTT TGCACGGT-3’; reverse) (Zhanget al.,2008)and LR-N-13398(5’-CGCCTGTTTACCAAAAACAT-3’; forward),LR-J 12887(5’-CCGGTCTGAACTCAGATCACGT -3’; reverse) (Simon,1994).PCR conditions:94°C for 5 minutes of initial denaturation;with 35 cycles of denaturation at 94°C for 30 s,annealing at 56°C for 30 s,and extension at 72°C for 45 s; and the final extension at 72°C for 7 minutes.PCR products were sent to Tsingke Biological Technology company for sequencing (http://www.tsingke.net).The obtained sequences were deposited in GenBank under the accession numbers MN475176-475196(Table 1).
Phylogenetic analysisIn addition to 11 sequences of the new form from Bi Doup-Nui Ba National Park and 10 sequences ofM.pulchellafrom newly collected samples,we used 35 available sequences of 12S rRNA-16S rRNA in GenBank (Garget al.,2019) and a sequence (MN453610) ofM.marmoratafrom Kon Tum Province,Vietnam,for phylogenetic analyses of the genusMicrohyla.Sequences ofKaloula pulchrawere included in the analysis as the outgroup (Van Bocxlaeret al.,2006).Locality information and accession numbers for all sequences included in the analysis can be found in Table 1.
Phylogenetic trees were constructed by using maximum likelihood (ML) and Bayesian inference (BI) analyses.Chromas Pro software (Technelysium Pty Ltd.,Tewantin,Australia)was used to edit the sequences,and then aligned using the ClustalW (Thompsonet al.,1997) option in MEGA 7.0 (Kumaret al.,2016) with default parameters and subsequently optimized manually in BioEdit 7.0.5.2 (Hall,1999).We then checked the initial alignments by eye and adjusted slightly.Prior to ML and Bayesian tests,phylogenetic analyses were performed in MrBayes 3.2 (Ronquistet al.,2012).We chose the optimum substitution models for entire sequences using Kakusan 4(Tanabe,2011) based on the Akaike information criterion(AIC).The best model selected for ML was the general time reversible model (GTR:Tavaré 1986) with a gamma shape parameter (G:0.232 in ML and 0.249 in BI).The BI summarized two independent runs of four Markov Chains for 10,000,000 generations.A tree was sampled every 100 generations and a consensus topology was calculated for 70,000 trees after discarding the first 30,001 trees (burn-in=30 001) (Nguyenet al.,2017).We checked parameter estimations and convergence using Tracer version 1.5 (Rambaut and Drummond,2009).The strength of nodal support in the ML tree was analyzed using non-parametric bootstrapping (MLBS) with 1000 replicates.We regarded tree nodes in the ML tree with bootstrap values of 75% or greater as sufficiently resolved (Hillis and Bull,1993;Huelsenbeck and Hillis,1993),and nodes with a BPP of 95% or greater as significant in the BI analysis (Leaché and Reeder,2002).
2.3.Morphological analysis
Specimens examinedA total of 32 specimens ofMicrohylawas collected from Bi Doup-Nui Ba National Park,Lac Duong District,Lam Dong Province,in May 2018 (Table 3).
MeasurementsMeasurements were taken from preserved specimens by V.C.Hoang with a digital caliper to the nearest 0.1 mm under a stereo microscope (Table 3).The following morphological characteristing were used (see Matsui,2011;Matsuiet al.,2013; Poyarkovet al.,2014):(1) snout-vent length(SVL,from the tip of snout to cloaca); (2) head length (HL,from tip of snout to posterior margin of jaw angle); (3) snout length(SL,from the anterior corner of eye to the tip of snout); (4) eye length (EL,the distance between the anterior and posterior corners of the eye); (5) nostril-eye length (NEL,the distance between the anterior corner of the eye and the nostril); (6) head width (HW,the maximum width of the head on the level of mouth angles in ventral view); (7) internarial distance (IND);(8) interorbital distance (IOD,the shortest distance between the medial edges of eyeballs in dorsal view); (9) upper eyelid width (UEW,the widest distance from the medial edge of eyeball to the lateral edge of the upper eyelid); (10) forelimb length (FLL,length of straightened forelimb to tip of third finger); (11) lower arm and hand length (LAL,distance from elbow to tip of third finger); (12) hand length (HAL,from proximal end of outer palmar [metacarpal] tubercle to tip of third finger); (13) inner palmar tubercle length (IPTL,maximal distance from proximal to distal ends of inner palmar tubercle);(14) outer palmar tubercle length (OPTL,maximal diameter of outer palmar tubercle); (15) hindlimb length (HLL,length of straightened hindlimb from groin to tip of fourth toe); (16)tibia length (TL,the distance between the knee and tibiotarsal articulation); (17) foot length (FL, from distal end of tibia to tip of toe IV); (18) inner metatarsal tubercle length (IMTL,maximal length of inner metatarsal tubercle); (19) first toe length (1TOEL,from distal end of inner metatarsal tubercle to tip of first toe);(20) outer metatarsal tubercle length (OMTL); (21) first finger width (1FW,measured at the distal phalanx); (22-25) finger lengths (1-3FLO,2-4FLI; for outer side (O) of the first,inner side (I) of the fourth,and both sides of the remaining fingers,measured between tip and the junction of the neighbouring finger); (26-28) finger disk diameters (2-4FDW); and (29-33)toe disk diameters (1-5TDW).Terminology for describing eye coloration in life followed Glaw and Vences (1997); webbing formula followed Savage (1975).
Morphological comparisonsMorphological comparisons were based on 50 specimens examined and data from literatures (Tables 3,4): Boulenger (1897,1900); Smith (1923);Parker (1928,1934); Andersson (1942); Bourret (1942); Parker and Osman (1948); Pillai (1977); Inger and Frogner (1979); Inger(1989); Dutta and Ray (2000); Bain and Nguyen (2004); Daset al.(2007); Das and Haas (2010); Feiet al.(2012); Matsui (2011);Matsuiet al.(2013); Hasanet al.(2014); Poyarkovet al.(2014);Howladeret al.(2015); Seshadriet al.(2016); Wijayathilakaet al.(2016); Khatiwadaet al.(2017); Zhanget al.(2018); Nguyenet al.(2019); Garget al.(2019); Poyarkovet al.(2019); and Liet al.(2019).
Principal component analysis (PCA)Measurement data were size-corrected and then were used to compare the morphometric difference between eight males and three females of the new form vs.the male holotype,17 males and four females ofM.pulchellafrom Bi Doup-Nui Ba National Park.All statistical analyses were performed using PAST 2.17b software (Hammeret al.,2001).
3.Results
3.1.Molecular systematics
Sequence variationIn the final alignment of 12S rRNA-16S rRNA,993 sites were conserved and 964 sites exhibited variation,of which 557 were found to be potentially parsimony-informative.The transition-transversion bias (R)was estimated as 1.Nucleotide frequencies were A=32.20%,T=23.15%,C=23.85%,and G=20.81% (data for ingroup only).
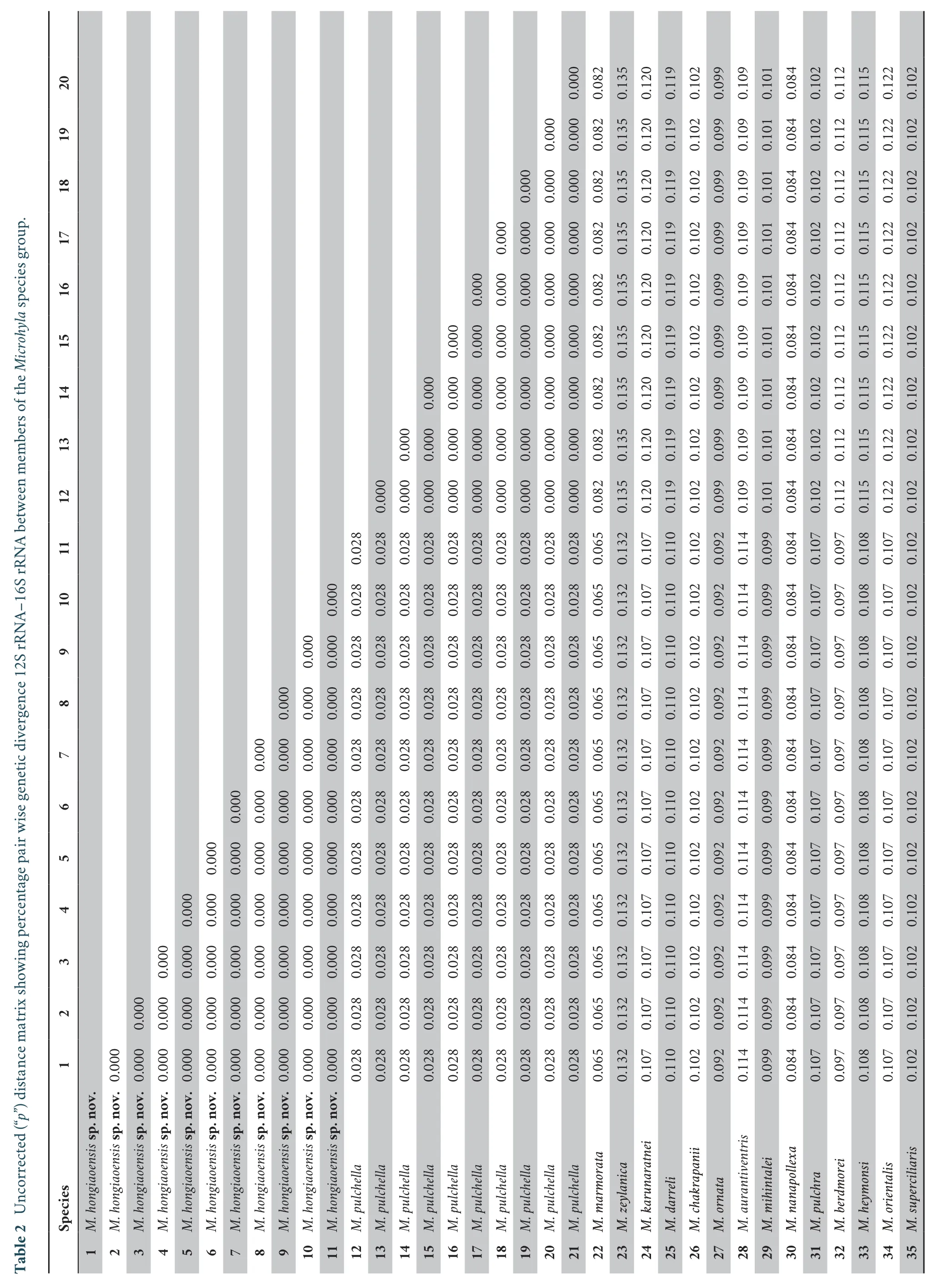
Interspecific uncorrected p-distanceIn theMicrohylaspecies group,the uncorrected p-distance ranged from 1.7% (betweenM.mixturaandM.okinavensis) to 16.4% (betweenM.annectensandM.zeylanica) (Table 2).The genetic divergence of the new form and its congeners ranged from 2.8% (compared withM.pulchella) to 13.2 % (compared withM.zeylanica).These values were higher than those between some other recognized species ofMicrohyla(2.6% betweenM.karunaratneiandM.darreli,M.borneensisandM.malang,M.mukhlesuriandM.fissipes; 1.9%betweenM.okinavensisandM.beilunensis; 1.7% betweenM.okinavensisandM.mixtura).The new form is separated fromM.pulchella,the most closely related species in morphology,by a genetic distance of approximately 2.8% based on 12S-16S rRNA mtDNA fragments (Table 2).It is noted that three specimens of the new form (VNMN 07385,07388,07390) and two specimens ofMicrohyla pulchella(VNMN 07389,07391),collected from the same breeding pond,are also distinguished from each other by a genetic difference of at least 2.8%.
Phylogenetic relationshipsThe BI and ML analyses produced topologies with -lnL=13118.5911 and 13107.9365,respectively.BI and ML analyses obtained similar topologies(Figure 2) that differed only at several poorly supported basal nodes.Our matrilineal genealogy was consistent with those of Matsuiet al.(2011),Pelosoet al.(2016) and Garget al.(2019).The BI genealogy inferred the following set of phylogenetic relationships:Monophyly of Microhylinae was not supported and the relationships among microhylid subfamilies remained unresolved (Nguyenet al.,2019) (Figure 2).In both analyses,the newly collectedMicrohylaform was recovered as sister group toM.pulchella(Poyarkovet al.,2014) from Lam Dong Province,Langbian plateau,Vietnam with high nodal support values(1.0/99.7).
3.2.Morphological analysisThe new form ofMicrohylafrom Bi Doup-Nui Ba National Park,Lam Dong Province,Vietnam,is compared with all known congeners as following.
The new formofMicrohylafrom Lam Dong Province differs from other species ofMicrohylaby having a smaller size,slender habitus; bluntly round snout; skin on dorsum scattered by small tubercles; finger I shorter than one-half the length of finger II;tips of three outer fingers (II-IV) weakly dilated,forming weak disks,largest on finger III,without dorsal median longitudinal grooves; tips of all toes distinctly dilated into wide disks with narrow peripheral grooves; and tibiotarsal articulation reachingwell beyond snout tip (Table 4).

Figure 2 Bayesian inference matrilineal genealogy of Microhyla derived from the analysis of 12S rRNA-16S rRNA mtDNA sequences.Numbers above and under branches are Bayesian posterior probabilities and ML bootstrap values.



The new formofMicrohylafrom Lam Dong Province most closely resemblesM.pulchellain morphology as well as supported by molecular phylogenetic relationships (Figure 2),butthey are differentiated from each other in the following characteristics:The new form has a notably slender body habitus than that ofM.pulchella,which is reflected in a number of diagnostic morphometric characteristic ratios to SVL(Figures 4,7E,Table 6).Males ofM.pulchellahave two small black scapular spots on dorsum,both usually bordered by a thin white line (vs.absent in males of the new form,see Figures 4,7E,7F) (Poyarkovet al.,2014).Dorsum skin of the new form scattered by small tubercles (vs.dorsum skin smooth inM.pulchella).M.pulchellahave few black scapular spots behind the eyes (vs.only one small black spot behind the eye in the new form,see Figures 4,9) (Poyarkovet al.,2014).M.pulchellahas a median longitudinal groove on dorsal surface of finger disc (vs.absent in the new form).M.pulchellaalso differs from the new form in the webbing formula (I1-2II1-2½III1-2½IV2½-1Vvs.I1½-2II1-2III1-2½IV2¼-1V in the new form) (Table 6).
Moreover,statistical results indicated that the new form ofMicrohylafrom Lam Dong Province could be separated fromM.pulchellabased on morphometric data.PCA extracted four principal component axes with eigenvalues greater than 0.001,and of these,the first two component axes accounted for 82.19%of the variation (Table 5).The first two principal component axes could separate the new formfromM.pulchellaby 19 characteristics (Figures 6A,6B),mainly based on limb and head measurements,namely:HL,SL,EL,NEL,HW,IND,UEW,FLL,LAL,HAL,1FL,OPTL,3FDD,HLL,TL,FL,IMTL,1TOEL,3TDD (Table 5).Species with a larger and positive score on PC1 reflected shorter SVL,HL,SL,EL,NEL,HW,IND,UEW,FLL,LAL,HAL,1FL,OPTL,3FDD,HLL,TL,FL,IMTL,1TOEL and 3TDD while a negative score signified smaller IOD.The PC2 with negative scores was associated with species having shorter SL,EL,NEL,IND,1TOEL,3TDW whereas positive scores were associated with species with larger morphological traits such as HL,HW,IOD,UEW,FLL,LAL,HAL,OPTL,1FL,3FDW,HLL,TL and FL (Table 5).
3.3.Description
Microhyla hongiaoensis sp.nov.
(Figures 3A,3B,3C,3D,3E,3F; Tables 3,4)
HolotypeVNMN 07477,adult male,collected in Hon Giao Forest,Bi Doup-Nui Ba National Park,Da Chai Commune,Lac Duong District,Lam Dong Province (12°11′18.3"N,108°40′29.0"E,elevation ca.1548 m a.s.l.,Figure 1); collected by C.V.Hoanget al.on 8 May 2018.

Figure 3 Dorsal and ventral views of the holotype (VNMN07477,male) of Microhyla hongiaoensis sp.nov.:in life (A,B) and in preservative (C,D); underside of right hand (E) and right foot (F) .Photos by Chung V.Hoang.

Table 3 Measurements (in mm) and proportions of the type series of Microhyla hongiaoensis sp.nov.and M.pulchella.
Paratypes(n=10) All specimens collected by C.V.Hoanget al.,the same location data as the holotype:2 adult males (VNMN 07385,07388) and 1 adult gravid female (VNMN 07390),collected on 7 May 2018; 5 adult males (IEBR 4573=VNMN 07405,IEBR 4574=VNMN 07406,IEBR 4575=VNMN 07718,VNMN 07483,VNMN 07617) and 2 adult gravid females(IEBR 4576=VNMN 07478,VNMN 07448),collected on 8 May 2018.
DiagnosisMicrohyla hongiaoensissp.nov.is assigned to the genusMicrohylabased on the molecular phylogenetic data and the following morphological characteristics:relatively small body size; maxillary and vomerine teeth absent; vomer divided into two parts,disappearing at the posterior edge of the choana;tongue round posteriorly; skin smooth or with tubercles;tympanum hidden; palate with 1−2 rows of horizontal skin ridges; fingers without webbing; toes slightly webbed or free of webbing; metacarpal tubercles 2 or 3; and the absence of skin ridge or skin projection between the subarticular tubercles of toes III and IV.



Microhyla hongiaoensissp.nov.is distinguished from its congeners by a combination of the following characteristics:(1) size medium (SVL 13.6-14.7 mm in males and 18.3-18.6 mm in females); (2) fingers II-IV with small disks,dorsal surface of disks,without median longitudinal groove; (3) webbing formula I1½ -2II1 -2III1 -2½IV2¼ -1V; (4) dorsal surface of toe disks with median longitudinal groove; (5) dorsal back without two small black spots; (6) one small black spot present behind the eye; (7) few small black scapular spots in the flank-belly and inguinal region; (8) palm with two small metatarsal tubercles;(9) tibiotarsal reaching beyond snout.
Description of holotypeSmall-sized frog,SVL 14.3 mm;habitus slender,head longer than wide (HL/HW 1.1); snout bluntly round,slightly protruding beyond margin of lower jaw,longer than diameter of eye (SL/EL 1.41); eyes protuberant,pupil round; dorsal surface of head flat,canthus rostralis round,distinct; loreal region oblique,weakly concave; nostril round,lateral,on canthus rostralis,closer to tip of snout than to eye(NEL 1.3 mm); interorbital distance wide (IOD 2.3 mm),much greater than internarial distance (IND 0.9 mm) and upper eyelid width (UEW 0.8 mm); tympanum hidden,supratympanic fold weakly developed,from posterior corner of eye to arm insertion; vomerine teeth absent,tongue round posteriorly and free at the rear half of its length; slit-like openings to a median vocal sac.
Forelimbs relatively short,about three times shorter than hindlimbs (FLL/HLL 0.4); hand shorter than a half of forelimb length (HAL/FLL 0.5); fingers slender,free of webbing,dorsoventrally flattened,fingers without skin fringes; the first finger slightly reduced notably,less than one-half the length of the second finger (1FLO/2FLO 0.2); the second finger slightly longer than fourth (2FLI/4FLI 1.7),and much shorter than the third (2FLI/3FLI 0.6); relative finger lengths:I<IV<II<III.Tip of first finger round,not enlarged; tips of fingers II-IV slightly dilated,forming weak round disks; same width as basal phalanges on the fourth and second fingers,much narrower than basal phalange on the third finger; third finger with basal phalange twice larger than that of the first finger (3FDW/1FW 2.0); narrow peripheral grooves absent on all fingers; grooves on dorsal surface of disks present in fingers II,III,IV,absent in the first finger; relative finger disk widths:IV<II<III; subarticular tubercles on fingers distinct,round,formula:1 :1 :2 :1 (given for fingers I :II :III :IV,respectively); thumb present on anterior part of forearm; nuptial pad absent; inner palmar tubercle absent,outer palmar tubercle round and prominent.
Hindlimbs slender and long (HLL 26.2),tibia longer than half of snout-vent length (TL/SVL 0.6); tibiotarsal articulation reaching beyond snout when limb adpressed along body; foot longer than tibia (FL/TL 1.3); relative toe lengths:I<II<V<III<IV;tarsus smooth,inner tarsal fold absent; tips of all toes slightly dilated,forming truncated disks,much wider than those of fingers (disk diameter of the third toe 0.8 mm; 3FDW/3TDW 0.7),disks of all toes with peripheral grooves,dorsal surface of toe disks II-V with short median longitudinal grooves;relative toe disk widths:I<IV<V<II<III; webbing between toes:preaxial side of toes II-IV,postaxial side of toe IV reaching distal subarticular tubercles,at all toes webbing reaching disk as fringe,webbing formula:I1-2II1-2½III1-2½IV2½-1V;subarticular tubercles on toes distinct,round,formula:1 :1 :2 :3 :2 (for toes I :II :III :IV :V,respectively); two metatarsal tubercles:inner metatarsal tubercle elongated,prominent,outer metatarsal tubercle weak.

Table 5 Variable loadings for principal components with eigenvalue greater than 0.01,from morphometric characters corrected by SVL.All measurements were given in millimeter (mm).
Dorsal skin slightly bumpy with low tubercles,scattered over dorsal surfaces of limbs; eyelid without supraciliary spines;supratympanic fold weakly developed,from posterior corner of eye to arm insertion; lateral side of head and flank smooth;ventral side of body and limbs smooth,vent area smooth.
Coloration of holotype in life.Dorsal surface of head and trunk greyish-brown to light-brown with dark-brown markings,a distinct dark-brown interorbital bar between eyelids,forming a reverse arrow in shape,running posteriorly towards scapular region and not covering dorsal surfaces of upper eyelids; one small black spot adjacent behind the eyes; a dorsal dark-brown marking consisting of two reverse V-shaped figures forming an hourglass-shaped marking:an anterior reverse V-shaped figure runs from scapular area posteriorly and laterally,opening at level of axilla and getting narrower again posteriorly on middle of dorsum; posterior reverse↓-shaped figure starts at middle of dorsum and runs laterally towards groin; few small black scapular spots in the flankbelly and inguinal region,both usually bordered with a thin light-brown line; supratympanic fold light-brown; dorsolateral surfaces of trunk and upper arm brown with gray-pattern a few small black spots; dorsal surfaces of thigh,tibia,and tarsus brown with dark-brown cross-bars on each hindlimb; fingers and toes dorsally brown with dark-brown cross-bars; throat and chest white-grey with intense small dark-grey mottling,belly lighter with indistinct grayish mottling,limbs ventrally white-grey with small dark-grey mottling; hand and foot ventrally greyish-brown; pupil black,fine golden reticulations throughout iris.
Coloration of holotype in preservative.In ethanol,dorsal coloration changed to dark greyish-brown,ventral surface of chest,belly,and limbs greyish-beige; dorsal pattern,dark spots on dorsum and stripes on dorsal surfaces of limbs unchanged,dark brown pattern changed to dark grey; iris completely black.

Figure 4 Paratypes of Microhyla hongiaoensis sp.nov.in life.A,male IEBR4575 and female IEBR4576 in amplexus; B,female VNMN 07390; C,female VNMN 07449; D,male VNMN 07449; E,male VNMN 07388; F,male IEBR4573; G,male IEBR4574; H,male CIB-VNMN 07617.Photos by Chung V.Hoang.

Figure 5 Habitat of Microhyla hongiaoensis sp.nov.in Bi Doup-Nui Ba National Park,Lam Dong Province,Vietnam.Photos by Chung V.Hoang.

Figure 6 Plots of the first principal component (PC1) versus the second (PC2) Microhyla hongiaoensis sp.nov.(blue,blue 24A is the holotype) and topotype of M.pulchella (red,red 1A is the holotype).Males (A) and the females (B).
Variation(Table 3,Figures 4A,4B,4C,4D,4E,4F,4G,4H).Specimens vary in body size and dorsal pattern from dark brown markings or with black scapular spots.All adult males have thumbs on anterior part of forearm but absent in adult females.Three female paratypes (VNMN 7390,7447,CIBVNMN 07448 have a larger body size than those of male paratypes (SVL 23.27 ± 1.64 mm [18.1-25.8 mm;n=3] mm vs.17.40 ± 1.86 mm [14.7-21.6 mm;n=8] in males); the male VNMN 7385 has a dark brown background color with blackish-brown markings.Two male paratypes (IEBR 4573,4574) have fewer dark spots in the axilla area compared to the holotype.The male (CIB-VNMN 07617) has an unsymmetrical hourglassshaped marking.Three females (VNMN 7390,IEBR 4576,CIBVNMN 07483) have a lighter greyish ventral surface compared to the holotype.
Natural historyAll specimens were collected at night,from 19:00 to 23:00 h,on the banks of a small temporary pond that was formed after heavy rain,along the sides of a recently constructed road (Figures 5A,5B).Specimens of the new species were recorded at an elevation of ca.1513 m a.s.l.Male and female frogs were found in amplexus on the water surface of the pond.Males were found calling about 1 m from the pond at night.The 27C road separated the Hon Giao forest into two patches.The new species was found sympatrically with five congeners,includingM.pineticola,M.mukhlesuri,M.annamensis,M.berdmorei,M.pulchella(Figures 7A,7B,7C,7D,7E,7F) andMicryletta inornata,all of which were reproducing simultaneously with the new species and sharing the same breeding site.Other anurans such asFeihyla palpebralis(Smith),Polypedatessp.(theP.leucomystaxspecies complex),Rhacophorus calcaneusSmith,Fejervarya limnocharis(Gravenhorst),andOccidozyga martensii(Peters) also encountered in surroundings.Larval stages and eggs of the new species are unknown.
DistributionThe new species currently known only from the type locality in Bi Doup-Nui Ba National Park,Lac Duong District,Lam Dong Province,Vietnam (Figure 1).
Conservation statusThe new species is likely to be endemicto Langbian Plateau,central Vietnam.However,the extent of its actual distribution range should be confirmed in further studies.Given the available information,we suggest this species be considered as Data Deficient following IUCN’s Red List categories (IUCN 2001).

Table 6 Selected diagnostic characters for the comparisons between the new species and M.pulchella.
EtymologySpecific epithet is in reference to the type locality of the Hon Giao forest.We recommend “Hongiao Narrowmouth Frog” as the common English name and “Nhái b‵âu hòn giao” as the Vietnamese name.
4.Discussion
Our matrilineal genealogy was consistent with those of Matsuiet al.(2011),Pelosoet al.(2016),Nguyenet al.(2019) and Garget al.(2019).The BI genealogy showed that monophyly of Microhylinae was not supported and the relationships among microhylid subfamilies remained unresolved (Nguyenet al.,2019) (Figure 2).In our phylogenetic analyses,the newly collectedMicrohylaspecimens were recovered as sister toM.pulchella(Poyarkovet al.,2014) with high nodal support values(1.0/99.7).Furthermore,the results of morphometric analyses(PCA) indicated thatM.hongiaoensiscould be well separated fromM.pulchella(Poyarkovet al.,2014).Interestingly,both species were found sympatrically at the same habitat and elevation (ca.1500 m.s.l) and they also share the same breeding site with other microhylid species in Hon Giao forest,Bi Doup-Nui Ba NP,Lam Dong Province,Langbian plateau,Vietnam.This gives us a hypothesis that during the formation of the two sister’s species ofMicrohyla,the Liangbian plateau region had been strongly geographically divided for a long time.Later,the geographical barriers between the two species faded away and created common habitat nests for the two sister’s species.However,further studies are required to prove this hypothesis.The herpetofauna of Truong Son Range is well known in terms of species richness of local endemism with many new species have been discovered recently (eg.Hoanget al.,2013;Ingeret al.,1999; Orlovet al.,2005; Rowleyet al.,2010b,2016;Ziegleret al.,2008).The discovery ofM.hongiaoensisbrings the total number of known species in the genusMicrohylato 50 and the species number in Vietnam to 18 (Frostet al.,2019). The Truong Son Range harbors the highest diversity of the genusMicrohylawith 15 recorded species so far.However,habitat loss is one of the greatest threats to amphibians in Southeast Asia,and the amphibians of the region appear to be particularly vulnerable to habitat alterations (Rowleyet al.,2010a,2016).The need for biological exploration in this region is made urgent due to intensifying logging,road construction,increasing agricultural pressure and other human activities (De Koninck 1999; Kuznetsov and Kuznetsova 2011; Laurance 2007; Meijer 1973; Meyfroidt and Lambin 2008).
AcknowledgementsWe would like to thank the directorate of the Bi Doup-Nui Ba NP for support of our work and issuing of relevant permits.Many thanks L.Iogansen(St.Petersburg),T.H.Ninh,T.Y.Nguyen,T.C.Pham,T.D.Nguyen (Hanoi) for their assistance.Thanks to Tuan Anh Tran(Hanoi) for providing the map.This research is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA23080101),NSFC (31471964),The World Academy of Sciences (TWAS),CAS-TWAS president fellowship program,RFBR 19-54-54003 Vietnam,and Ideal Wild.
杂志排行
Asian Herpetological Research的其它文章
- Morphological Variations,Distribution and Population Estimation of Indian Spiny Tailed Lizard (Uromastyx hardwickii Gray,1827) from District Bahawalnagar,Punjab,Pakistan
- Effects of Dietary Protein Variations at Different Life-stages on Vocal Dominance of the African Clawed Frogs
- Tail Display Intensity is Restricted by Food Availability in an Asian Agamid Lizard (Phrynocephalus vlangalii)
- Flexibility as a Strategy for Avoiding Call Overlap in Male Anhui Treefrogs
- No Evidence for the Compensation Hypothesis in the Swelled Vent Frog(Feirana quadranus)
- Age and Body Size of the Shangcheng Stout Salamander Pachyhynobius shangchengensis (Caudata:Hynobiidae) from Southeastern China
