Biochemical Factors Affecting the Quality of Products and the Technology of Processing Deep-Sea Fish,the Giant Grenadier Albatrossia pectoralis
2020-09-29PIVNENKOTKARPENKOYuKRASHCHENKOVPOZDNYAKOVAYuandESIPENKOR
PIVNENKOT. N., KARPENKOYu. V., KRASHCHENKOV. V.,POZDNYAKOVAYu. M., andESIPENKOR. V.
Biochemical Factors Affecting the Quality of Products and the Technology of Processing Deep-Sea Fish,the Giant Grenadier
PIVNENKOT. N.*, KARPENKOYu. V., KRASHCHENKOV. V.,POZDNYAKOVAYu. M., andESIPENKOR. V.
,690087,
The composition of muscle tissues of a deep-sea species, the giant grenadier (), and a mesopelagic species, the Alaska pollock(), are compared. Grenadier is proved to have a higher moisture content (91.7%) and lower protein (7.4%) and lipid (0.3%) contents. The factors responsible for the softening and moisture separation during processing of grenadier are identified. Contents of some fractions of non-protein nitrogenous components, including trimethylamine oxide (TMAO) and free amino acids, are clarified. The proportion of the main myofibrillar proteins myosin and actin, which is considered as an indicator of efficiency of structure formation in fish muscle tissue during processing, is almost 20% lower in giant grenadier than in pollock. The effects of endogenous protease, Са2+-ATPase and transglutaminase on the processes of autolysis, denaturation, and possibility of myofibrillar proteins cross-linking were also studied. The proposed technology of getting a nutritionally balanced, gel-like, ready-to-use foodstuff from grenadier fillet includes its enrichment with pollock muscle tissue and supplementing with a binary structure-forming agent containing collagen and chitosan. Nutritional value of the products is assessed by the method of biological assay and by determining the denaturation changes of myofibrillar proteins. The modes of fine mincing and thermal processing, as well as the shelf-life of the finished product are justified. The developed technology of producing a ready-to-eat food from giant grenadier will allow a more efficient use of this underutilized resource.
giant grenadier; Alaska pollock; myosin; actin; proteases; ATPase; transglutaminase; softening; structure formation; ready-to-eat foodstuffs
1 Introduction
One of the most common deep-sea fish species in the northern Pacific Ocean (the Sea of Okhotsk and the Bering Sea) is the giant grenadier, whose commercial harvest is steadily increasing worldwide. The depth of its habitat ranges from 1200 to 3500m. The bio- mass formed by this species is 166050t (Tuponogov., 2008; Tuponogov and Novikov, 2016). According to the data provided by Russian fishing companies, the giant grenadier stocks allow commercial catches up to 30–40t per day using longlines operated from medium-tonnage vessels, while traditional target species of fisheries make up only 4–6t per day (http://www.vostok1.com/produk- tsiya/makrurus/). Currently, the interest of fishing companies in this object is associated with its relatively low cost and the high production of liver and eggs having attractive organoleptic properties and containing valuable nutritional components. Nevertheless, processing the gre-nadier muscle tissue in both industrial and household conditions poses a significant challenge due to its high water content and low moisture holding ability. Therefore, when dealing with the giant grenadier, the major obstacle which fishing companies face is not only the organization of fishing at great depths (NPFMC, 2011; Devinea., 2012), but the complexity of further processing of this fish and the related marketing problems. Inhabiting in deep-sea waters has resulted in significant rearrangements in the structure of its muscle tissues, such as the high moisture content and low protein and lipid contents. The resulting changes affected not only the quantitative redistribution of the main classes of biological molecules, but also the composition of both myofibrillar proteins and enzyme systems (Crapo., 1999b; Morita, 2010; Karau- lova and Yakush, 2017). Traditionally methods for processing raw materials from deep-sea organisms do not allow deep processing to obtain products with satisfactory quality. A study of a number of biochemical factors affecting the quality of pre-cooked and ready-to-eat food items and a comparison of the results with those known for traditional target species of fisheries will make it possible to justify technological approaches to process such a non-standard raw material.
The high moisture content of grenadier muscle tissue suggests two possible approaches for further processing. The first method is dehydrating the fish by salt curing and pressing. This method can cause a loss of initial weight and is unprofitable. The second method can bind the water by creating a disperse system based on endogenous factors and/or introducing a structure-forming agent which can form a continuous spatial framework throughout the volume of the product and hold the water (Crapo., 1999b; Borderías and Sánchez‐Alonso, 2011). We believe that the second approach is more productive. In this case, developing a technology of production of ready-to-eat foodstuffs with balanced biological value from a low-use raw material will give the opportunity to extend the range of fish-derived products with high nutritional value and attractive to consumer.
The present study was designed to identify factors responsible for softening and dehydration of muscle tissue of giant grenadier () and to develop a technology of producing of ready-to-eat foodstuffs with balanced nutritional value.
2 Materials and Methods
2.1 Sample Collection
Giant grenadier,, was caught in the Sea of Okhotsk in 2017 and brought to the laboratory of Innovative Biotechnologies. For analysis, we used speci- men that had been stored frozen for 3 months. The body weight of the whole fish varied from 0.9 to 2.4kg. The body length was from 67 to 93cm. The average yield of fillet was 47.5%. Alaska pollock (), was employed to compare the muscle tissue and the fish was stored under the same conditions.
2.2 Determination of Nitrogenous Extractive Compounds
Protein content was analyzed with a Kjeldahl assay (Nx6.25) on a Kjeltec Auto 10 SO Analyzer (Sweden) according to the Official Methods of Analysis of AOAC International (20th edition, 2016). To determine non-protein nitrogen, the protein fraction of the muscle tissue extract was pre-precipitated using a 15% solution of trichloracetic acid (TCA) at a ratio of 1:4 (v/v), and the supernatant was mineralized in concentrated H2SO4by the Kjeldahl’s assay. Protein concentration in the solutions was determined by the Biuret test.
To determine the amount of trimethylaminoxide (TMAO), a spectrophotometric method was used (Wekell and Barnett, 1991).Samples were homogenized in 9 volumes of ice-cold TCA (5%) on a tissue homogenizer and left on ice for 10min to precipitate protein. After centrifuged at 12000rmin−1for 5min, a reaction of reducing TMAO to TMA was carried out by adding an equimolar mixture of FeSO4and EDTA-Na2in 0.1molL−1acetate buffer (0.8molL−1, pH 4.5). Then absorbance was measured at 410nm and compared to a standard curve.
Amino acid compositionwas determined under standard conditions on a Biochrom-30 amino acid analyzer (Great Britain) using an Ultropac [Li+] 8µm column. To analyze the free amino acids, the homogenized muscle tissue was extracted using 75% ethanol for 5–7d, filtered and evaporated. The rest of the liquid was transferred to a separatory funnel and supplemented with 10mL of ethyl ether. The lower layer was collected and evaporated on a rotary evaporator at 40℃±2℃. To analyze the total aminoacids, tissues were hydrolyzed with 6molL−1HCl at 110℃for 24h.
2.3 Extraction of Myofibrillar Proteins
Sarcoplasmic proteins were isolated from muscle tissue by extraction by a solution containing 5mmolL−1EDTA and 1mmolL−1dithiothreitol at 4–6℃. After 12h, the ex- tracts were centrifuged at 4000–6000rmin−1. The resulting precipitate represented the muscle protein concentrate (MPC). MPC was homogenized in a low-ionic-strength solution (0.1molL−1KCl, 1mmolL−1EDTA, pH 7.0) and supplemented with an equivalent volume of solution containing 1.1molL−1KCl, 0.1molL−1Na2CO3, and 0.1molL−1NaHCO3. Extraction was performed at 3–5℃ for 15– 30min. The precipitate was separated by centrifugation at 6000rmin−1. To isolate Actomyosin, the obtained extract was supplemented with a 15-fold volume of 5mmolL−1EDTA at рН 6.8–7.0 and kept at 4℃ for 10–12h. Actomyosin was precipitated with (NH4)2SO4to a 35% saturation degree. The precipitate was washed washed and then dialyzed with 1mmolL−1EDTA solution. To extract myosin, MPC was homogenized for 2min in a 5mmolL−1phosphate buffer with pH 7, containing 0.1molL−1KCI, 10mmolL−1MgCI2, and 1mmolL−1EDTA. The extract was supplemented with a saturated solution of (NH4)2SO4. The precipitate, obtained in the saturation degree of 35%– 50%, was separated by filtration. Then it was dialyzed with water and then with a 5-fold volume of 5mmolL−1phosphate buffer pH 7, containing 0.6molL−1KCl, 10mmolL−1Na4P2O7, and 1mmolL−1MgCl2(Karaulova., 2003). Actin was extracted with 0.6molL−1KI and then precipitated with (NH4)2SO4at a saturation degree of 15%–25% (Ishikawa, 1983).
2.4 Determination of Enzymes Activities
Proteolytic activity was determinated with casein (рН 8.0) and hemoglobin (pH 2.5; 4.5; 6.5) as the substrates by the method of Kunitz (1965). It is based on measuring the amount of soluble low-molecular-weight proteins form- ed during enzymatic hydrolysis of the substrates. A 2-mL portion of 2% solutions of the appropriated substrate was mixed with 2g muscle tissue homogenate and incubated at 37℃ for 60min. The reaction was terminated by adding of an equal volume of 5% TCA. After filtration, optical density of supernatant was measured at 280nm. A unit of activity was assumed to be the amount of the preparation (g) which causes the absorption to increase by 1 optical unit per minute (Gokcek., 2016).
To determine Ca2+-ATPase activity by the method of Fiske-Subbarow (1925), 1mL of the aqueous extract of muscles was added to a solution containing 0.6mL Tris- maleate buffer (pH 7.0) and 1mL 0.1molL−1CaCl2. Extracts of muscles were obtained by homogenization of 1g of muscles in 80mL of distilled water with a subsequent centrifugation. The volume of the reaction mixture was brought to 9.5mL with distilled water. Then 0.5mL of 20mmolL−1ATP solution was added to the mixture which was then incubated at 25℃ for 10min. The reaction was terminated by adding 5mL of 15% TCA. To determine the amount of formed inorganic phosphate (Pi) a color reaction with the molybdenum reagent was carried out after filtration. Optical density was measured at 680nm. The amount of free Pi (µmolL−1) per min perg of tissue was assumed to be a unit of activity (Upreti, 1984).
The activity of transglutaminase (TGase) was determined by the hydroxamate method (Folk and Cole, 1966) with modifications proposed by Cochón. (2010). The reaction solution contains 63mmolL−1CBZ-l-glutaminyl- glycine, 200mmolL−1Tris-acetate buffer (pH 6.0), 5mmolL−1CaCl2, 10mmolL−1GSH (glutathione), and 0.1 molL−1hydroxylamine. For the analysis, 0.75mL reaction solution was added to a 0.25mL muscle tissue extract containing 100mgmL−1protein. After 30min of incubation at 37℃, it was supplemented with 0.75mL of a mixture of 2.5molL−1HCl, 15% ТСА, and 5% FeCl3(1:1:1). Optical density was measured at 525nm. The final product of the reaction was γ-glutmayl-L-monohydroxamate. The calibration curve of optical densityconcentration was plotted using standard solutions. A unit of activity was assumed to be the amount of 1μmolγ-glutmayl- L-monohydroxamate produced per minute.
2.5 Determination of Water, Water-Holding Capacity, and Viscosity
The amount of water in the muscle was measured on a Kett F-1A infrared moisture balance (Kett Electric Laboratory, Japan). The amount of expressible moisture and water-holding capacity (WHC) were determined by the pressure method. Pressing loss was calculated as a percentage of weight loss before and after compression (1kg, 10min) of fish fillet and expressed as:

The dynamic viscosity () was determined on a Rheo- lograph Sol-535 (Toyo Seki Ltd.) and calculated using the following formula:
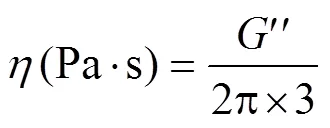
where 3 is knife oscillation frequency (Hz);is the loss modulus (Pa).
For measurements, we subjected the test sample to deformation in an oscillatory, harmonious mode with deformation and stress alternating sinusoidally. In this case, the amplitude (deformation magnitude) should not alter the structure of the sample. The loss modulus () was recorded as a degree of sample resistance to deformation.
2.6 Determination of Protein Denaturation Degree
The denaturation of proteins during thermal processing was determined as variation in the ability of myofibrillar proteins to dissolve in standard saline solutions. The prepared products were subjected to thermal processing until the temperature at the center of a sample increased from 40℃ to 90℃ with a step of 10℃±2℃. The denaturation degree (%) was expressed as the ratio of the difference in the weight fraction of saline-soluble proteins before and after the thermal processing to its initial value.
2.7 Determination of Relative Biological Value
To determine the relative biological value (RBV) of the products ciliatewas cultivatedin 0.1% solution of peptone water with the addition of the studied components (Wheatley., 1994). The dynamics of growth and development of ciliates were observed during 4 days. Number of grown cells was observed counted in a Goryaev’s chamber under the microscope after fixing them with formalin. RBV was calculated as the ratio of the number of ciliates growing in the medium containing the studied products to the control sample (casein).
2.8 Statistical Analysis
All experiments and measurements were carried out at least three times. Statistical processing of experimental data was performed using the Statistica 6.0 and Microsoft Excel,software package. One-way analysis of variance (ANOVA) was applied. Arithmetic mean (), standard deviation (),confidence interval (), and size of analyzed subgroup () were estimated. Differences were con- sidered significant at<0.05. The confidence level was 95%.
3 Results and Discussion
3.1 Composition and Properties of Fish Muscle Tissue Proteins
Deep-sea waters inhabitance undoubtedly influences the composition and functions of organs and tissues of giant grenadier. This, in turn, affects the functional and technological properties of its tissues used for manufacturing foodstuffs. Identification of the factors responsible for the differences in properties of muscle tissues between mesopelagic (such as Alaska pollock) and deep-sea fishes will make it possible to give recommendations for the use of deep-sea fish in the food industry.
Table 1 provides comparative data on the general che- mical composition of muscle tissue of the fish species with specified contents of certain fractions of nitrogenous components. In this table, the composition of the certain protein fractions and the proportion of the main myofibrillar proteins are presented in detail. For the two species considered, all indicators presented in this table significantly differ (<0.05)with the exception of ash. The high moisture content of giant grenadier muscle tissue corresponds to the low protein and lipid contents. After filleting, meat acquires a watery texture. Despite generally re- duced content of nitrogenous substances, including free amino acids, urea, nucleotides, trimethylammonium and nitrogenous bases, the content of trimethylamine-oxide (TMAO) is obviously high. According to the published data (Yansey, 2007; Bockus and Seibel, 2018), TMAO per- forms the following biochemical functions: regulation of osmotic pressure in fish tissues; stabilization of proteins during denaturation when exposed to various chemical compounds and physical factors such as freezing and high pressure. The latter function is obviously important for supporting physiological functions of deep-sea fish, and for preserving the quality of raw materials during storage. It is worth of mentioning that in this fish which has a high content of TMAO its conversion into trimethylamine (TMA) is very low (Treberg and Driedzic, 2002; Bockus and Seibel, 2018). This process occurs under the action of an enzyme TMAO-reductase. This reaction is of great sig- nificance as a factor contributing to spoilage of raw fish, including cool storage. In other fish species, TMA is re- sponsible for the appearance of the spoilage odor and has a very low sensitivity threshold. In giant grenadier, de- spite the presence of large quantities of TMA, this process does not occur and frozen fish retains its quality with the pleasant shrimp taste and smell after a thermal process- ing.

Table 1 The biochemical compositions in the muscle tissues of giant grenadier and Alaska pollock (% of total weight)
Note: Different superscript letters in the same column represent significant difference between the two fishes with the same treatments (<0.05) (=6).
The fractional composition of proteins differed insignificantly between the fish species compared. At the same time, there is a decrease in the ratio of sarcoplasmic and myofibrillar proteins in the pollock muscles compared to the grenadier muscles, which are 1:2 and 1:3, respectively.Sarcoplasmic proteins are believed not to have any substantial effect on the texture of the product. The functional properties of minced muscle tissue are predicted depending on the content of myofibrillar proteins. The presence of the sufficient proportion of lipids is also assumed to facilitate gelation, moisture-binding and emulsifying capacities of minced fish material. However, this parameter also differs slightly between the fish species compared.
Table 1 also shows concentrations of different protein fractions and certain proteins of muscle tissue from the studied fishes isolated by staged extraction. The main myo- fibrillar proteins are myosin and actin. The average myosin/actin ratio for muscle tissue varies from 2 to 4, and in most cases it is 3.5–4.0 (Brown, 1986; Foegeding., 1991; Tolano-Villaverde., 2016; Listrat., 2016). This ratio is used to characterize the animal locomotion activity and, in food technology, the gelation ability of muscle tissues processed. However, myosin is responsible for the gelation function as it contributes to water holding, gel hardness, cohesion, and elasticity, in addition to other properties (Dong and Holley, 2011; Listrat., 2016). This functional property depends on the characteristics of the protein including the number of sulfhydryl groups, types of amino acids, hydrophobicity, charge,., as well as on the external factors including temperature, protein concentration, concentration of salt, and pH value. Other myofibrillar proteins, such as actin or regulatory and cytoskeletal proteins, do not form gels (Tolano-Villaverde., 2016).
It can be seen that grenadier is characterized by a lower value of quantitative ratio of myosin to actin compared to that in pollock and some other mesopelagic fishes (Ka- raulova., 2003; Karaulova and Yakush, 2017). A low myosin content especially the actin content, generally can-not provide the effective binding and retaining water mole- cules and gelation during processing of grenadier.
The differences in the structure of myotomes are considered by some researchers as important (Crapo., 1999a).The size of myotomes in grenadier muscles is almost three times bigger than that in Alaska pollock, and is less densely packed. Thus, the larger muscle fibers provide a greater intercellular space for binding moisture and lead to a lower tissue density. As the structure of muscle tissue is destroyed during the technological processing, the bound water is released into the free state, which causes changes in the viscoelastic properties of the product. The moisture- binding properties of minced meat from the studied fish species and its viscosity is presented in Table 2.

Table 2 Water-binding properties (%) and viscosity of minced fish muscle tissue
Note: Different superscript letters in the same column represent significant difference between two fishes with the same treatment (<0.05) (=6).
Values of total and expressible amounts of moisture in the grenadier fillet samples were significantly higher than those in pollock fillets; accordingly, the WHC and, especially, the viscosity of the grenadier muscle tissue were significantly lower. Expressible moisture is not associated with proteins, but, nevertheless, it plastifies the product, creating a favorable consistency and facilitating food digestion. However, excess moisture can be separated during processing or defrosting. Great losses are observed during thermal processing of this raw material. To prevent such losses, special technological approaches need to be developed.
3.2 Content of Free Amino Acids in Fish Muscle Tissue
Maintaining the osmotic balance is known to be very important for marine organisms. This function is perform- ed by low-molecular-weight nitrogenous compounds in- cluding free amino acids (FAA) together with inorganic ions. Concentration of FAA is particularly high in tissues of marine invertebrates (Ayushin., 1999; Kube., 2006). A comparison of the compositions and quantities of FAA and histidine-containing dipeptides between the tissues of pollock and grenadier showed a significantly lower level of these components in grenadier (Table 3). Tissues in these species are poor with FAA, while the highest amount is represented by histidine. In pollock tis- sues, the dominant FAA is taurine (approximately 25% of total FAA), which is the characteristic of many marine organisms. However, in grenadier its proportion does not exceed 4%. It can be assumed that in grenadier tissues the regulation of osmotic pressure involves other substances.
3.3 Enzyme Activity in Fish Muscle
Another factor that can influence the rheological properties of fish muscle tissue is enzyme system. The enzymes maintaining the functional activity of fish muscle tissue and having significant effects at various stages of harvesting and processing of marine organisms are worth with further investigation. Proteinase, ATPase and TGase are such enzymes. Activities of these enzymes are presented in Table 4.
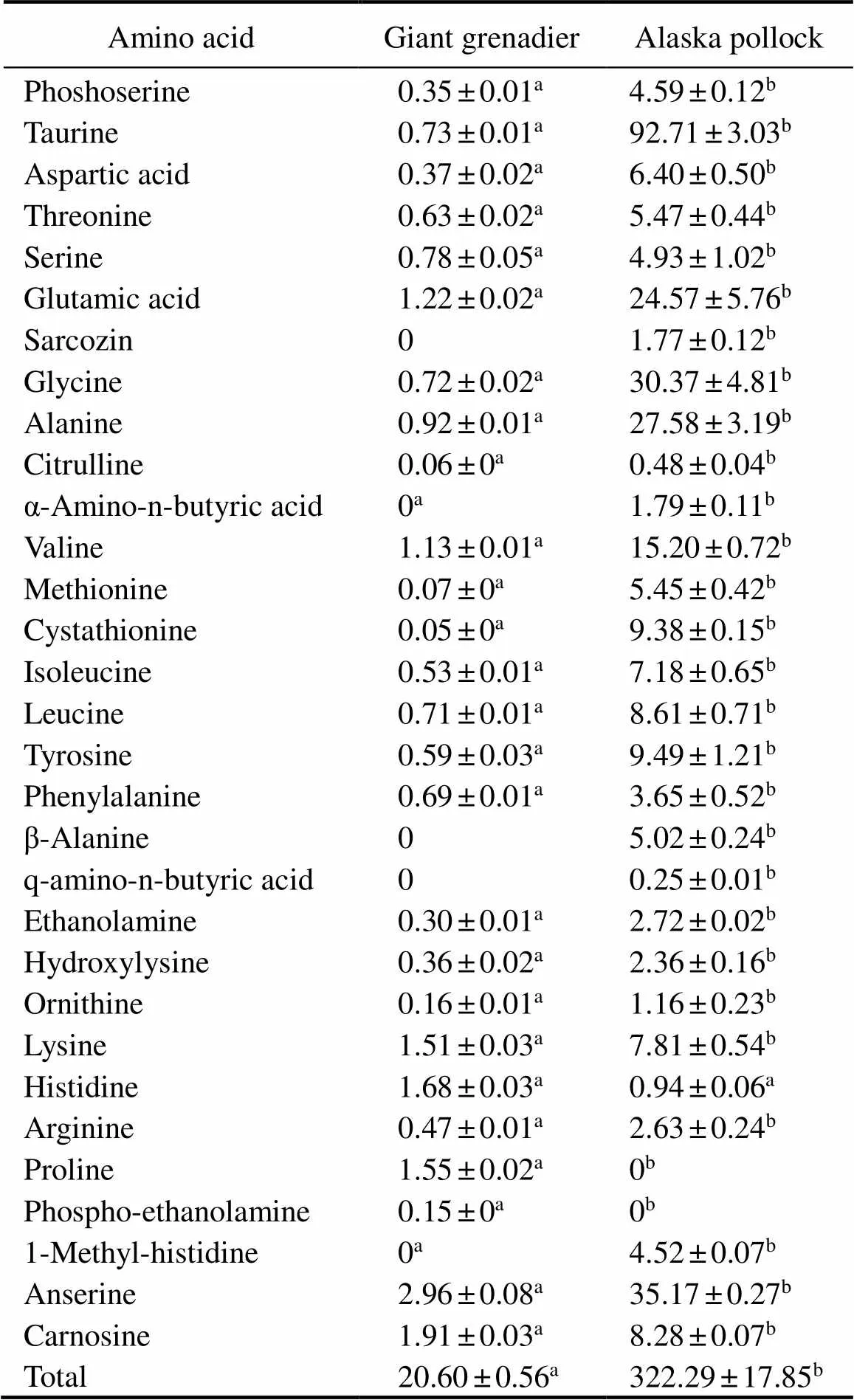
Table 3 Contents of free amino acids and dipeptides in the muscle tissues of giant grenadier and Alaska Pollock (mg(100g tissue)–1)
Note: Different superscript letters in the same column represent significant difference between the two fishes with same treatment (<0.05) (=6).

Table 4 Enzyme activities of fish muscle tissue
Note: Different superscript letters in the same column represent significant difference between two fishes with the same treatment (<0.05) (=6).
The proteases in fish muscle tissue are localized mainly in lysosomes and cytosol. After the disintegration of tissues most enzymes are released into the sarcoplasm. The post-mortem muscle softening involves lysosomal cathep- sins (optimum pH 5.5–6.5) and calpains (optimum pH 7) that provide a synergistic contribution to this process (Was- son, 1992; Tolano-Villaverde., 2016). The results show- ed that the levels of protease activity in the muscle tissues of the fish species under study remain similar at various pH values (<0.05). Their activities are low, and the pro- bability that they may cause grenadier muscle tissue softening is also extremely low.
Activity of ATPase is often used to assess the quality of frozen pollock surimi. High Ca2+-ATPase activity well correlates with gel strength (Ando., 1996). In live fish muscle contraction is provided by the energy release as a result of ATP breakdown under the action of Ca2+- ATPase, controlled by the influx and outflux of Ca2+ions in sarcoplasm. With postmortem changes Ca2+concentration in sarcoplasm drops below 0.5µm. Thus ATP can no longer be hydrolyzed due to the inactivation of Са2+-AT- Pase, and ATP functions as muscle softener. The variations in Са2+-ATPase activity of live tissue are associated with the differences in rate of muscle contraction and the mobility of the organism. From the viewpoint of technological research, the maintenance of ATPase activity is associated with the retention of the native structure of myofibrils and with the viscosity of tissue. The value of relative viscosity of actomyosin solutions correlates with the myosin/actin ratio and the ATPase activity (Foegeding., 1991). According to the results a low myosin/actin ratio and a low viscosity corresponding to the low value of ATPase activity, which was almost 3-fold lower than in pollock were observed in grenadier muscles.
Another factor that determines the texture characteristics of fish muscle tissue during its processing is the activity of tissue TGase. The main substrate for TGases in muscle systems is myosin (Araki and Seki, 1993; Dong and Holley, 2011). Heavy-chain myosin (HCM) polymers are created and strong and elastic gels are formed under the action of this enzyme (Jiang and Yin, 2004). For fish muscle tissue, a high variability in TGase activity is observed during processing, which is associated with the function of different factors (Nozawa., 1997; Ashie and Lanier, 2000). Therefore, it cannot be concluded that a high level of TGase activity will correspond to a high gel-forming ability. The results in Table 4 show that TGaseactivity of the muscle tissue in grenadier is more than three times higher than that in pollock (<0.05). How- ever, it does not provide any advantages for obtaining strong gel structures. It is shown that the accumulation of myosin polymerization products in some deep-sea fishes is possibly only with certain ratios of TGase activity and myosin concentration (Karaulova., 2017). From the results of previous studies, the optimum value of TGase activity to create a strong structure of minced muscle tis- sues in deep-sea fish was 0.3–0.7U(100mg myosin)−1. An increase of TGase activity led to a decrease in strength and elasticity. With this activity, there was a depolymeri- zation of HCM with a simultaneous increase in the pro- portion of HCM monomer.
The results indicated that the main factors affecting the structure of grenadier muscle tissue, along with the high moisture content, are as follows: 1) a low level of non-pro- tein nitrogenous substances; 2) a low proportion of myo- sin toward actin in myofibrillar proteins; 3) the activities of ATPase and TGase.
3.4 Substantiation of Technology to Produce a Foodstuff from Grenadier Muscles
After studying the properties in grenadier muscle tissue that hamper further technological processing and obtaining a complete food item, the following research was aim- ed at enriching the protein composition and obtaining a strong structure.
The low protein content in grenadier muscles makes it necessary to use additional protein enrichers for the production of the nutrition product with high nutritional value. Contents of proteinogenic essential amino acids (EAA) in grenadier and pollock muscles, as well as their scores (AS) are provided in Table 5. AS value is equal to the ratio of the certain AA content (mg) in 1g of the studied protein to the same AA content (mg) in 1g of an ideal protein. The acid with the lowest score is referred to as the first limiting amino acid. The score value of such AA determines the nutritional value and the degree of proteins up- take.

Table 5 Contents of EAA (mg (g protein)−1) in muscle tissues of grenadier and pollock and their score
The first limiting AA for the grenadier muscle protein is methionine. Valine has the lowest value of amino acid score, but is not in the limiting range. For this reason, when developing a production of the food item from gre- nadier muscle, we proposed to supplement it with a protein-rich raw fish material which has a score of methionine and valine higher than that of a perfect protein. Pollock muscle tissue was selected as an additional source of methionine and valine. The ratio of raw fish materials is 2.5:1 (grenadier:pollock), which allows to achieve the ne- cessary values of AA score.
3.5 The Influence of Binary Structure-Forming Agent on Products Quality
To create a stable structure of the product, we used the gelatin-chitosan system, which is a binary structure-form- ing agent (BSA). As a result, we obtained a product from the category of jellied food items, defined by us as fish jelly.
Traditionally, gelatin is used in the production of ready- to-eat jellied food. To improve its physical and mechanical properties, we used it together with high-molecular chi- tosan (590kDa). This combination provides gels with in- creased strength. Chitosan can influence the TGase-catalyzed cross-linking of fish HCM. Thus, the breaking strength of gels obtained from Alaska pollock surimi increases as the concentration of chitosan rises. However, the mechanism of this phenomenon need further study (Benjakul., 2003;Gómez-Guillén, 2005). The results of the studyof the gel-forming ability of BSA, consisting of gelatin and solution of chitosan, in mixed minced fish meat are shown in Fig.1. The concentration of gelatin was 2%, while the con- centration of chitosan solution varied from 0.5% to 2.0%.

Fig.1 Effect of chitosan concentration on the temperature of gelation (1) and melting (2) of protein gels. Bars present standard deviation from 3 replicate experiments. The difference is considered significant when P<0.05. For approximation, a polynomial trend line (R2=0.6738) was used.
It is obvious that the increasing concentration of chitosan causes the rise of gelation and melting temperatures. The time of formation of a dense gel-like structure was 45 min in the samples containing BSA. In the control sample, that only contained gelatin, it was 3.5h. The content of BSA components in protein gel, corresponding to 2% dry gelatin and 2% chitosan solution (0.04% dry chitosan), provided the maximum gel-forming ability during the for- mation of a dense elastic structure of the muscle protein.
In the process of producing of finished food the mincing of fish muscle tissue was accompanied by the mechanical and chemical changes that cause water to bind to the protein, thereby improving the structure and consistence of the minced mass, and also increasing its viscosity and adhesiveness. Fine mincing provides crushing, cutting, squeezing, and abrasion of the mixture components and their complex interaction. As a result, the product has a homogeneous structure that is different from of the initial raw material. This in turn influences the interaction with structure-forming agents and the gel formation. The relationship between the viscosity of the protein gel and the cutting time is presented in Fig.2. A sharp increase in viscosity occurred as the duration of the process was extended to 240s. When the sample acquired the maximum viscosity and the components were distributed homogenously with a maximum comminution of raw materials. When the cutting time expanded to 300s or more the viscosity decreased due to the destruction of the formed aggregates and the transition of the absorbed water into a free state.
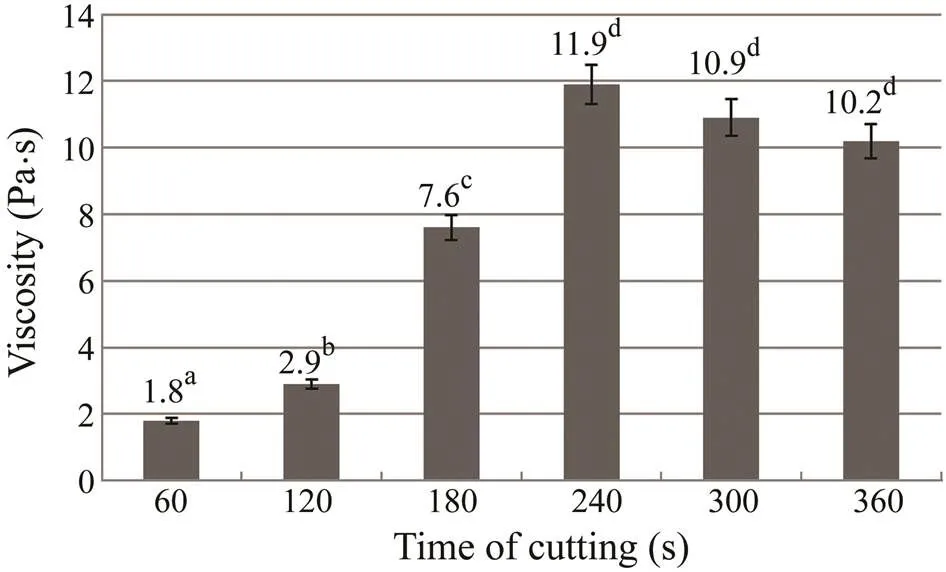
Fig.2 Viscosity of the protein gel composed of grenadier muscle tissue, gelatin, and chitosan, with different cutting time. Bars present standard deviation of 3 replicate experiments. Different superscript letters indicate significant differences between treatments (P<0.05).
3.6 Protein Denaturation in the Process of Heating
To evaluate the changes of nutritional value of the pro- ducts during thermal processing, the degree of protein de- naturation was estimated. The myofibrillar proteins respon- sible for the formation of a dense reticular structure in the finished products of shredded fish tissue can denature during heat treatment. The denatured muscle proteins can be digested and absorbed much easier. This changes their solubility, the degree of hydration and the pattern of in- teraction of these proteins, the ratio of hydrophilic and hydrophobic groups, and the transformation the structure of the protein gel. Such protein gel, when exposed to heat- ing, is characterized by a lower volume and weight and a greater elasticity and mechanical strength (Xiong, 1997). Fig.3 shows the relationships between protein denaturation degree and the heating temperature of the samples.
With the rise of the temperature of heating, the degree of protein denaturation increased, reaching a maximum at 70–80℃. The maximum rate of denaturation changes oc- curred when the product was heated in the range from 40 to 50℃. With the further increase in temperature, the further increase in temperature denaturation process slowed down.
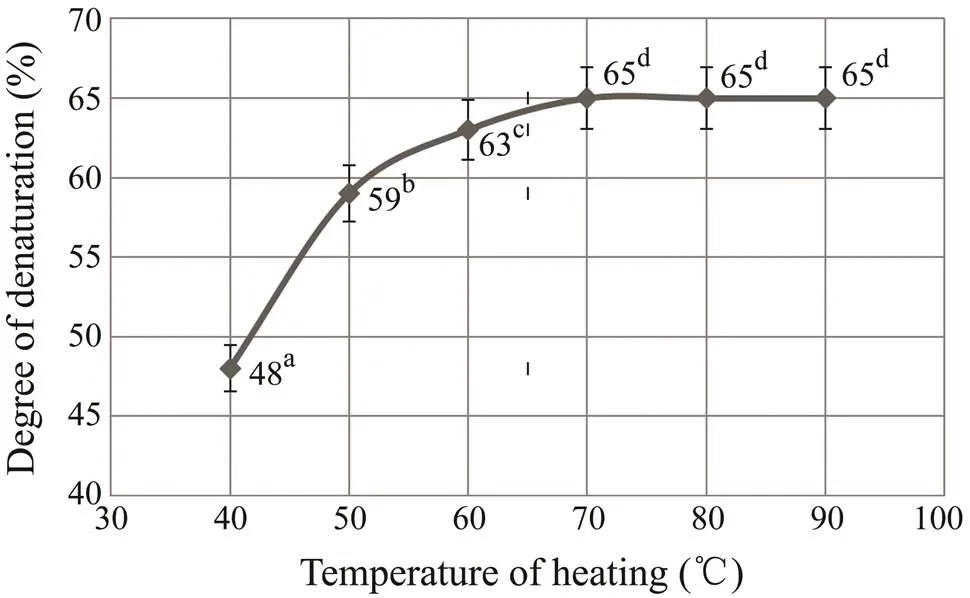
Fig.3 Effects of heating temperatures on the denaturation degree of proteins from grenadier and pollock muscle tis- sues. Bars present standard deviation of 6 replicates. Dif- ferent superscript letters indicate significant differences be- tween treatments (P<0.05).
3.7 Biological Assay of Food Items Using the Ciliate T. pyriformis
To study the nutritional function of denatured proteins, the ciliatewas employed in this research,considering its main metabolic processes are similar to those in higher organisms (Wheatley., 1994; Hala- vach., 2015). The function of the protein can be assessed by the change of ciliates number per day in growth media with the product tested. The higher the biological function of the product, the better it is absorbed, and the more active growth of ciliates is observed.Fig.4 shows the growth ofin the culture media with jellied minced fish obtained at different temperatures of pro- cessing.

Fig.4 Growth of the ciliate Tetrahymena pyriformis in me- dia with jellied minced fish samples obtained at different processing temperatures. Bars present standard deviation of 3 replicate experiments. Different superscript letters in figure indicate significant differences between treatments (P<0.05).
The maximum cell growth was recorded in the medium with the product that was heated for 5min at 45℃. The denaturation changes of proteins that occur at this tem- perature can have a positive effect on digestibility of the product. The temperature from 50℃ to 70℃ can create fa- vorable conditions for growth of endogenous thermophi- lic microflora, which can inhibit the vital activity of the ciliate population. This can explain the decline of ciliates’ growth in this temperature range. However, as the processing temperature increased above 70℃, the growth activity of the associated microflora is inhibited, and, as a result, the availability of nutrients for the test organism is restored. At the same time, the denaturation changes of muscle tissue proteins intensify, which can also help the growth ciliates. Thus, processing the product at a temperature of 85℃±2℃ provides a high level of protein availability for ciliates. The number of ciliates cells grown when such product was added to the medium was 65% higher compared to a product without heat treatment.
3.8 The Technology Scheme for Manufacturing Ready-to-Eat Jelly-Like Foodstuffs
Based on the results, we propose a sequence of tech-nological operations for manufacturing ready-to-eat jelly-like food on the basis of protein-enriched minced grenadier meat using a binary structure-forming agent.
The main raw materials were fresh-frozen grenadier and pollock. Preparatory operations for them included defrosting to the temperature of −5℃ in the deep layers of fish, filleting, and washing. Minced grenadier fillet was cut for 4min. Then it was supplemented with salt, BSA, pollock fillet, and flavorings. Gelatin was introduced in a dry form. After swelling, dispersion of gelatin particles, their uniform distribution was at the stage of cutting the highly hydrated grenadier muscle tissue, and the final dissolution was during the gradual thermal processing of the product. The resulting mixture was stuffed into poly-amide impermeable coatings to form loaves (300g). Next it was put into boiling tanks with the temperature gra- dually increasing up to 85℃ at the center of loaf. Then the mixture was kept at this temperature for 5min. Finally the heat-processed product was cooled by barbotage in tanks with an ice-water mixture to a temperature of 5℃ and then stored at 0–4℃.
The specific features of composition of the finished food item: the presence of chitosan with proven antimi- crobial activity, the minimum possible bacterial conta- mination of the product during technological processing, and the use of barrier impermeable coating–allowed extending its date of expiry from 2 to 5 days. This was confirmed by microbiological tests. On day 9 of storage of the product samples hermetically sealed in a barrier coating at the temperature of 4℃±2℃ and a higher temperature of 9℃±1℃, the number of mesophilic aerobic and facultative anaerobic microorganisms (NMAFAnM) reach- ed 1.8×102and 1.2×104CFUg−1, respectively. They were below the requirements of the Technical Regulation of Eurasian Economic Union (TR EAEU 040/2016), while the maximum permissible level of NMAFAnM is 5×104CFUg−1for fish food products.
4 Conclusions
The comparative study of the composition of giant gre- nadier muscle tissue suggests that the main factors caus- ing its softening and increased dehydration during proc- essing are low total protein content and low myosin/actin ratio. The softening process is aggravated by the osmo- regulation corresponding to the low content of low-mo- lecular-weight nitrogenous compounds, which makes the grenadier tissue labile to external influences. Autolytic pro- cesses cannot cause tissue softening in this fish, while the presence of highly active TGase accompanied by low concentration of proteins can lead to a reduction in the strength and elasticity of tissues due to the HCM deploy- merization. Weak ATPase activity in grenadier muscles agrees with the low myosin/actin ratio and low viscosity. To avoid significant losses of product weight, a technol- ogy was proposed to obtain jellied minced fish using a binary structure-forming agent (BSA). Its composition in- cluding gelatin and high-molecular chitosan provides en- hanced strength characteristics of the gel, higher melting temperature, higher gelation rate and retention of gel-form- ing capabilities during thermal processing. Pollock fillet was used to enrich the protein in the ready-to-eat food. The tests of denatured myofibrillar proteins on the growth ofconfirmed the biological function of this ready-to-eat food. The developed technology of processing giant grenadier can prolong the storage time of the food significantly, which might also be applied in other food products.
Acknowledgement
The authors are grateful to Dr. L. Ya. Tishchenko from Analytical Laboratory of G. B. Elyakov Pacific Institute of Bioorganic Chemistry (PIBOC) of Far-Eastern Branch of the Russian Academy of Sciences (RAS) for her help in the analysis and identification of amino acids.
Ando, M., Banno, A., Hitani, M., Hirai, H., Nakagawa, T., and Ma- kinodan, Y., 1996. Influence on post-mortem rigor of fish body and muscular ATP consumption by the destruction of spinal cord in several fishes., 62: 796-799.
Araki, H., and Seki, N., 1993. Comparison of reactivity of trans- glutaminase to various fish actomyosins., 59: 711-716, DOI: 10.2331/suisan.59.711.
Ashie, I. N., and Lanier, T. C., 2000.. Marcel Dekker, Inc., New York, 147- 190.
Ayushin, N. B., Petrova, I. P., and Epstein, L. M., 1999. Nitro- genous extractive compounds in the Far Eastern mollusks tis- sues., 125: 52-56.
Benjakul, S., Visessanguan, W., Phatchrat, S., and Tanaka, M., 2003.Chitosan affects transglutaminase‐induced surimi gelation., 27 (1): 53-66, DOI: 10.1111/j.17454514.2003.tb 00.266.x.
Bockus, A. B., and Seibel, B. A., 2018. Synthetic capacity does not predict elasmobranchs’ ability to maintain trimethylamine oxide without a dietary contribution.Comparative Biochem- istry and Physiology. Part ,217(3): 35-42, DOI: 10.1016/j.cbpa.2017.12.008.
Borderías, A. J., and Sánchez‐Alonso, I., 2011. First processing steps and the quality of wild and farmed fish., 76: R1-R5, DOI: 10.1111/j.1750-3841.2010.01900.x.
Brown, W. D., 1986.Academic Press, Inc., Elsevier Inc., 405-451.
Cochón, A. C., Mino, L. A., and San Martín de Vi, L. C., 2010. Early increases in transglutaminase activity and polyamine levels in a Mallory-Denk body mouse model., 199: 160-165, DOI: 10.1016/j.toxlet.2010.08.018.
Crapo, С., Himelbloom, B., and Pfutzenreuter, R., 1999a. Causes for soft flesh in giant grenadier () fillets.Journal of Aquatic Product Technology,8 (3): 55-68, DOI:10.1300/J030v 08n03_05.
Crapo, C., Himelbloom,B., Pfutzenreuter,R.,and Lee, C., 1999b.Texture modification processes for giant grenadier () fillets.Journal of Aquatic Product Technol- ogy,8(4):27-40, DOI:10.1300/J030v08n04_04.
Devinea, J. A., Watlingb, L., Caillietc, G., Drazenb, J., Durán Muñozd, P., Orlove, A. M., and Bezauryf, J., 2012. Evalua- tion of potential sustainability of deep sea fisheries for grena- diers (Macrouridae)., 52 (10): 709-721.
Dong, S. X., and Holley, R. A., 2011. Factors influencing gel formation by myofibrillar proteins in muscle foods.Compre- hensive Reviews in Science and Safety,10: 33-51, DOI: 10.1111/j.1541-4337.2010.00137.x.
Foegeding, E. E., Brekke, C. J., and Xiong, Y. L., 1991.Ameri- can Chemical Society, Washington, D. C., 257-267.
Folk, J. E., and Cole, P. W., 1966. Transglutaminase: Mecha- nistic features of the active site as determined by kinetic and inhibitor studies.ica, 122: 244-264.
Food and Agriculture Organization, 2013.. FAO, Rome.
Gokcek, K., Szabo, T., Alptekin, C., Tore, Y., and Urbanyi, B., 2016. A preliminary study on protease activity of Russian sturgeon,Brandt and Ratzenburg, 1833, at early life stages., 16: 1025-1029, DOI: 10.4194/1303-2712-v16_4_29.
Gómez-Guillén, C., Montero, P. M., Solas, T., and Pérez-Mateos, M., 2005. Effect of chitosan and microbial transglutaminase on the gel forming ability of horse mackerel (spp) muscle under high pressure.,38 (1): 103-110, DOI: 10.1016/j.foodres.2004.09.004.
Halavach, T. N., Kurchenko, V. P., Zhygankov, V. G., and Evdo- kimov, I. A., 2015. Determination of physicochemical, im- munochemical and antioxidant properties, toxicological and hygienic assessment of whey protein concentrate and its hy- drolysate., 3 (1): 105-114, DOI: 10. 12737/13127.
Ishikawa, H., 1983.Plenum Press, New York, 1-40.
Jiang, S. T., and Yin, L. J., 2004. Surimi enzymology and bio- technology.. Lexington, 57-65.
Karaulova, E. P., Levan’kov, S. V., Yakush, E. V., and Akulin, V. N., 2003. Qualitative and quantitative composition of myofi- bril proteins of some deep-sea fish species., 134: 309-320.
Karaulova, E. P., and Yakush, E. V., 2017. The comparative study of myofibrillar proteins of skeletal muscles of some deep-sea fish species., 11 (2): 1-8.
Kube, S., Gerber, A., Jansen, J. M., and Schiedek, D., 2006. Pa- tterns of organic osmolytes in two marine bivalves,, andspp., along their European distribution., 149: 1387-1396, DOI: 10.1007/s00227-006- 0303-7.
Listrat, A., Lebret, B., Louveau, I., Astruc, T.,Bonnet, M., Le- faucheur, L., Picard, B., and Bugeon,J., 2016. How muscle structure and composition influence meat and flesh quality., 2016: 1-14, DOI: 10.1155/2016/ 3182746.
Morita, T., 2010. High‐pressure adaptation of muscle proteins from deep‐sea fishes,and., 1189 (1): 91- 94, DOI: 10.1111/j.1749-6632.2009.05181.x.
Nozawa, H., Mamagoshi, S., and Seki, N., 1997. Partial purify- cation and characterization of six transglutaminases from or- dinary muscles of various fishes and marine invertebrates.Comparative Biochemistry and Physiology. Part , 118B: 313-317, DOI: 10.1016/ S0305-0491(97)00062-x.
NPFMC, 2011. Inclusion of grenadiers in the fishery manage- ment plans for the Bering Sea and Aleutian Islands and/or the Gulf of Alaska. Discussion Paper. North Pacific Fishery Management Council. Anchorage, Alaska, 31pp.
Tolano-Villaverde, I. J., Torres-Arreola, W., Ocaño-Higuera, V. M., and Marquez-Rios, E., 2016. Thermal gelation of myofi- brillar proteins from aquatic organism s.–,14 (3): 502-508, DOI: 10.1080/19476337.2015.1116024.
Treberg, J. R., and Driedzic, W. R., 2002. Elevated levels of trimethylamineoxide in deep-sea fish: Evidence for synthesis and intertissue physiological importance., 293: 39-45, DOI: 10.1002/jez.10109.
Tuponogov, V., and Novikov, N. P., 2016. Grenadier as an important reserve of Far Eastern deep-sea fisheries., 6: 54-60.
Tuponogov, V., Orlov, A., and Kodolov, L., 2008. The most a- bundant grenadiers of the Russian Far East EEZ: Distribution and basic biological patterns. In:. Orlov, A. M., and Iwamoto, T., eds., Bethesda, Maryland, 285-316.
Upreti, G. C., 1984. Colorimetric estimation of inorganic phos- phate in colored and/or turbid biological samples: Assay of phosphohydrolases., 137 (2): 485-492,DOI: 10.1016/0003-2697(84)90117-9.
Wekell, J. C., and Barnett, H., 1991. New method for analysis of trimethylamine oxide using ferrous sulfate and EDTA., 56: 132-138, DOI: 10.1111/j.1365-2621. 1991.tb07993.x.
Wheatley, D. N., Rasmussen, L., and Tiedtke, A., 1994. Tetra- hymena: A model for growth, cell cycle and nutritional stud- ies with biotechnological potential.,16 (5): 367- 371, DOI: 10.1002/bies.950160512.
Xiong, Y. L., 1997.. Chapman and Hall, New York, 111- 140, DOI: 10.1007/978-1-4615-5975-7_8.
Yansey, H, 2007. Adaptations to hydrostatic pressure in protein structure and organic. Osmolytes in deep-sea animals. High pressure bioscience and biotechnology., 1: 90-95.
. Tel:0079242302534
E-mail: tnpivnenko@mail.ru
June 27, 2019;
October 21, 2019;
January 13, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Preparation of Clay/Biochar Composite Adsorption Particle and Performance for Ammonia Nitrogen Removal from Aqueous Solution
- Propulsion Performance of Spanwise Flexible Wing Using Unsteady Panel Method
- Simulation of the Power Take-off System for a Heaving Buoy Wave Energy Converter
- Air Temperature and Emersion Time Can Affect the Survival Rate and Ammonium Loading of Swimming Crab Portunus trituberculatus Exposed to Air
- Estimation of the Reflection of Internal Tides on a Slope
- Study on Wave Added Resistance of a Deep-V Hybrid Monohull Based on Panel Method
