金二元纳米晶超晶格的自组装和结构表征
2020-09-28赵亚楠何敏刘晓芳刘斌杨建辉
赵亚楠,何敏,刘晓芳,刘斌,杨建辉
西北大学化学与材料科学学院,教育部合成与天然功能分子化学重点实验室,陕西省物理无机化学重点实验室,西安 710127
1 Introduction
The ordered arrays or superlattices (SLs) composed of monodisperse nanocrystal (NC) are important materials and have been widely used in light-emitting devices, biomarkers,catalysts and solar cells1,2. These NC SLs can exhibit physical and chemical properties different from both individual NC and their bulk assemblies3. The development of SLs synthesis has attracted significant research attention and is emerging as a new frontier in the field of nanotechnology4,5. The preparation of NC SLs has involved the controllable synthesis and assembling them into ordered-NC SLs6. Among various kinds of nanocrystals(NCs) as building blocks, noble metallic nanocrystal is one of the mostly studied materials due to their unique/strong interactions with extra electromagnetic field7–9. Noble metallic NCs, especially Au is the excellent candidate material because of their exceptional chemical stability, catalytic activity, process ability and metallic nature, which provide them unique sizedependent optical and electronic properties and a wide variety of applications in sensing, imaging, electronic devices, medical diagnostics and cancer therapeutics owing to their strong interactions with external electromagnetic field10.Monodisperse Au NCs can self-assemble into SLs with cubic close packing (ccp) or hexagonal close packing (hcp)11. It has been shown that thiol-stabilized Au NCs with broad size distribution (polydisperse) can form bimodal ordering AB2structure by a process involving spontaneous size segregation12.After that, the assembly of mixtures of two-component NCs,such as metal, magnet and semiconductor, into binary nanocrystal superlattices (BNSLs) provides a route to fabricate novel classes of materials. BNSLs with interesting structures exhibited combined and collective properties and were widespread used in the field of electronics and magnetic devices13–15. The theoretical approach based on the hard-spheres model and experiments all demonstrated that mixtures of large(A) and small (B) NCs with suitable size ratio and stoichiometry can self-assembly into stable AB, AB2and AB13type BNSLs16–19.While most studies focused on the combined two-component NCs with different sizes for the self-assemble BNSLs. There are only a few studies on the single-component NCs, especially Au NCs, with different sizes for the construction of BNSLs20,21. In contrast to two-component BNSLs, single-component BNSLs allow exploitation of their structures and collective properties without regard to the compositional effect. For example, Mirkinet al. reported five distinct symmetries of Au BNSLs through programmable DNA interactions20. Therefore, it is important to develop a simple and efficient procedure to build BNSLs with single nanocomponent of different sizes.
In this work, we synthesized monodispersed 6.0, 7.3 and 9.6 nm Au NCs using dodecanethiol stabilized 3.7 nm Au NCs as seeds through the seed growth method in oleylamine. The oleylamine functionalized 6.0, 7.3 and 9.6 nm Au NCs as “A”were mixed with 3.7 nm Au NCs serves as “B” at a certain concentration ratio, respectively. Au BNSLs with AB2type(hexagonal AlB2structure), AB13(NaZn13structure) and AB type (cubic NaCl structure) were obtained through the solvent evaporation method. The investigation of such single nanocomponent as building block is noteworthy in structures and properties study of BNSLs as well as potential development of novel meta-materials.
2 Experimental
2.1 Chemicals
Chlorotriphenylphosphine gold (98%), chloroauric acid(HAuCl4.4H2O), borane tert-butylamine complex (97%),dodecanethiol (98%), oleylamine (OAm, C18: 80%–90%),toluene and ethanol were obtained from Sinopharm Chemical Reagent Beijing Co. Ltd. All chemicals were used as received without further purification.
2.2 Synthesis of Au NCs with different size
3.7 nm Au NCs were synthesized by a modified procedure according to the previous report22. The precursor solution consists of 0.1237 g of chlorotriphenylphosphine gold and 0.5 mL of dodecanethiol dissolved in 25 mL of toluene. 0.4345 g of borane tert-butylamine complex was then dissolved in 5 mL of toluene as a reducing agent. The above two solutions were preheated in a 100 °C oil bath. The reducing agent was quickly injected into the precursor solution. The mixture was kept 100 °C for 5 min under stirring. The color of the suspension changed from colorless to dark brown and finally to wine red as time elapsed. The Au NCs was purified and obtained by the centrifuged process using ethanol. The supernatant was removed and the precipitate was dispersed in toluene.
6.0 , 7.3 and 9.6 nm Au NCs were prepared by the seed growth method using the above 3.7 nm Au NCs as seeds23. Briefly, the growth solution (4 mL, 1.65 mmol∙L−1) was prepared by dissolving chloroauric acid in oleylamine under stirring. 0.6, 0.4 and 0.1 mL of Au seed solution (3.92 mmol∙L−1) were added to the growth solution, respectively. The mixture was kept at 90 °C for 6 h under stirring. The color of the solution changed from light red to deep wine red. Finally, the Au NCs was washed with ethanol and dispersed in toluene.
2.3 Self-assembly of Au BNSLs
The Au BNSLs were prepared by the solvent evaporation method. 3.7 nm Au NCs were mixed with 6.0, 7.3 and 9.6 nm Au NCs at different concentration ratios in the toluene,respectively. The concentrations of the Au NCs were calculated by Lambert-Beer law24,25. The molar absorption coefficients (ε)areε3.7nm= 3.62 × 106L.mol−1.cm−1,ε6.0nm= 1.26 × 107L.mol−1.cm−1,ε7.3nm= 2.03 × 107L.mol−1.cm−1andε9.6nm= 6.15 ×107L.mol−1.cm−1. 50 µL of toluene solution containing a mixture of two sizes of NCs with the desired concentration ratio(from 10/1 to 1/1) was placed in a small glass vial with a carboncoated copper grid on its bottom26. The toluene was completely evaporated under room temperature.
2.4 Characterization
UV-Vis absorption spectra of Au NCs diluted with toluene and recorded by a SP-756P UV-Vis spectrometer (Shanghai Spectrum Instruments Co., Ltd., China). The concentration of Au NCs can be calculated from its absorbance value and molar absorption coefficient. The morphology, sizes of Au NCs and assembly of Au BNSLs can be observed by transmission electron microscopy (TEM). TEM images were taken on a Talos F200X (FEI/Thermo Fisher Scientific Inc., USA) operating at 200 kV accelerating voltage. The samples for TEM measurements were prepared by dropping Au dispersions on carbon-coated copper grids. Manual measured the TEM images with Image Tool generating the average size and standard deviation of Au NCs.
3 Results and discussion
As described in the synthesis section, Au NCs are synthesizedviaa facile one-step one-phase synthetic route by using amineborane complexes as reducing agents22. As shown in Fig. 1a, b,the average size is 3.7 nm with 8.1% in diameter distribution. 3.7 nm Au NCs can be used as seeds for the growth of larger NCs with desired size in oleylamine23,27. The resulting size of Au NCs can be controlled by adjusting the volume of Au seed solution. As the volume of Au seed solution decreases, the average sizes increase from 6.0 nm (Fig. 1c) to 7.3 nm (Fig. 1e)and 9.6 nm (Fig. 1g) with 6.7% (Fig. 1d), 4.1% (Fig. 1f) and 6.3% (Fig. 1h) in diameter distribution, respectively. The obtained Au NCs with low size distribution could self-assembly into hexagonal monolayer on TEM grid. The growth on the surfaces of Au seeds avoids any new nucleation, which results Au NCs kept similar morphology and dispersity with Au seeds.Fig. 2a, b show high-resolution TEM images of 3.7 and 9.6 nm Au NCs with single-domainand polycrystalline, respectively.

Fig. 1 TEM images and the corresponding size distributions of 3.7 nm (a, b), 6.0 nm (c, d), 7.3 nm (e, f) and 9.6 nm (g, h) Au NCs.
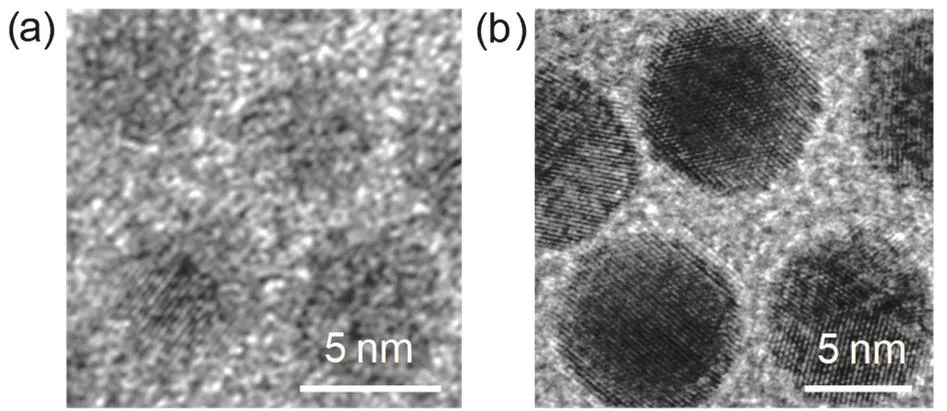
Fig. 2 High-resolution TEM images of (a) 3.7 nm and(b) 9.6 nm Au NCs.
Fig. 3 shows the UV-Vis absorption spectra of Au NCs with different sizes. All absorption spectra of Au NCs have an absorption peak around 520 nm, which is attributed to the characteristic Au surface plasmon resonance (SPR) absorption in the visible region. 3.7 nm Au NCs exhibits broad SPR peak at 519 nm28. The electron conductivity decreases of the NCs due to the outer layer chemically bound to the organic molecules. For the NCs with size below 5 nm, the influence of the outer Au surface is more obvious eventually inducing a damped peak even to featureless due to the reduced mean free path of the electrons25.As the size of Au NCs increases to 6.0, 7.3 and 9.6 nm, the SPR peaks slightly red-shifted to 524, 526 and 526 nm with narrower profile, respectively. The red-shift commonly happened because of the increased electron density of larger NCs29.
In this work, the ligand used in the synthesis of 3.7 nm Au NCs was dodecanethiol, whereas in the seed growth synthesis of 6.0, 7.3 and 9.6 nm Au NCs it was oleylamine. In both cases, the Au NCs were sterically stabilized with hydrophobic molecules and could be dispersed in the organic solvents, such as hexane,chloroform and toluene. Here we selected toluene as the solvent to disperse the Au NCs due to the slowly evaporation rate, which is beneficial to the growth uniform nanocrystal SLs. 3.7 nm Au NCs were mixed with 6.0, 7.3 and 9.6 nm Au NCs at desired concentration ratio, respectively. Binary Au NCs self-assembly was carried out by naturally evaporation on carbon-coated TEM grid. After drying the toluene solution, the structural characterization and analysis of Au BNSLs were performed by TEM.

Fig. 3 UV-Vis absorption spectra of Au NCs with different sizes dispersed in toluene.
For the 3.7 and 9.6 nm Au NCs combination, NaCl type BNSLs was formed, as shown in Fig. 4. The 9.6 nm Au NCs were arranged in a square lattice, and the 3.7 nm Au NCs were filled into the gap formed by the lattice in a ratio of 4 : 1 (Fig.4a). In order to understand the arrangement of the two sizes NCs more clearly, we drew the three dimensional sketch of NaCl unit cell and the depictions of the (100) plane (Fig. 4b). The ordered region in Fig. 4a was found to be structurally consistent with(100) lattice projection of an AB SLs.
Fig 5 shows the TEM image and models of NaZn13type Au BNSLs assembled from 3.7 and 7.3 nm Au NCs. As shown in Fig. 5a, large 7.3 nm Au NCs are located at the center of eight small 3.7 nm Au NCs. Fig. 5b shows a crystallographic model of the superlattice built from a NaZn13unit cell and the depiction of the (001) plane. The ordered domain in Fig. 5a is structurally consistent with (001) projection of NaZn13-type SLs.
For the 3.7 and 6.0 nm Au NCs combination, AlB2type BNSLs was formed, as shown in Fig. 6. Fig. 6a presents the TEM image of superlattice projected along the (001) crystal plane of AlB2type structure. Fig. 6b exhibits the sketch of AlB2unit cell and the depiction of the (001) plane. 6.0 nm Au NCs stacked in hexagonal pattern and small 3.7 nm Au NCs are arranged in the gaps.
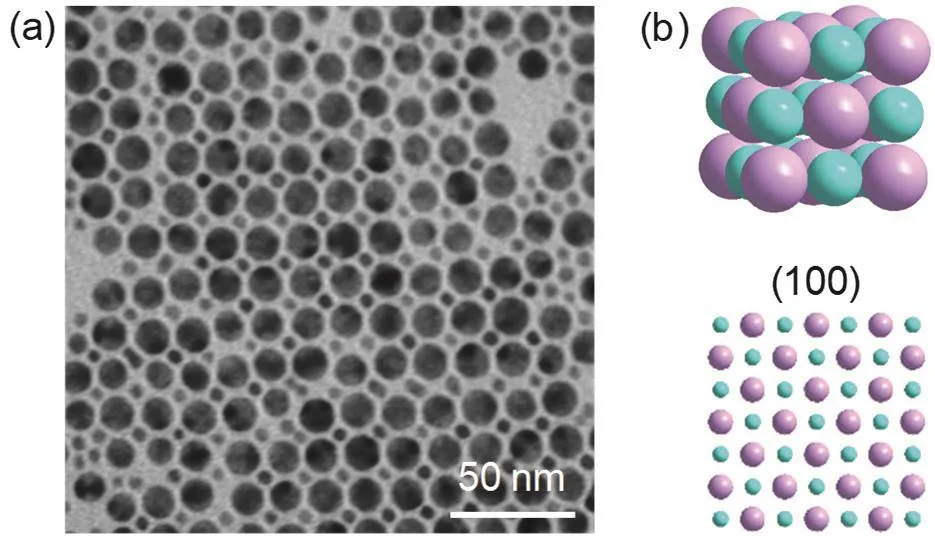
Fig. 4 (a) TEM image of BNSLs assembled from 3.7 and 9.6 nm Au NCs, (b) three dimensional sketch of NaCl unit cell and the depiction of the (100) plane.
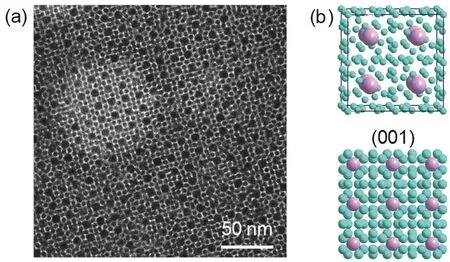
Fig. 5 (a) TEM image of BNSLs assembled from 3.7 and 7.3 nm Au NCs, (b) three dimensional sketch of NaZn13 unit cell and the depiction of the (001) plane.

Fig. 6 (a) TEM image of BNSLs assembled from 3.7 and 6.0 nm Au NCs, (b) three dimensional sketch of AlB2 unit cell and the depiction of the (001) plane.
Many theoretical studies have predicted the possibility of formation of various ordered binary structures and compared their stability16–19. Murray and Sanders developed the relevant space filling principle30, That is, the BNSLs can exist stably only when the space filling factor (ρ) of the constituent binary nanocrystals exceeds the ccp or the hcp (ρ =0.7405) of its single composition31. The effective particle size ratios (γ=Dsmall/Dlarge)serve as the critical factor determining the formation of the BNSLs16. The space-filling curves of AB (NaCl), AB13(NaZn13) and AB2(AlB2) were obtained for the range of radius ratio 0 <γ< 1 by Murray and Sanders30. Fig. 7 shows the calculated space filling curves (ρversusγ) of the NaCl, NaZn13and AlB2type BNSLs. The effective particle size of the NCs is equal to the sum of the metal core diameter and twice the thicknesses of the surface ligand. For the dodecanethiol coated 3.7 nm Au NCs, the thickness of the ligand is assumed to be 1.0 nm, which is measured previously32,33. Therefore, the effective particle sizeDsmallis 5.7 nm. For the oleylamine modified 9.6,7.3 and 6.0 nm Au NCs, the thickness of the ligand is assumed to be 1.5 nm32,33. The effective particle sizesDlargeare 12.6, 10.3 and 9.0 nm, respectively. It is calculated that the effective particle size ratioγis 0.45, 0.55 and 0.63, respectively.According to the previous reports, the NaCl lattice with the highest packing density (0.793 atγ= 0.414) is expected most stable. For all size ratios below 0.458, the packing density of the NaCl lattice exceeds the packing density (0.7405) of fcc packing and the NaCl-type lattice is predicted to be stable. For the 3.7 and 9.6 nm Au NCs combination in the current research, the effective particle size ratioγis 0.45 and NaCl type BNSLs are formed, which agreed with such predictions. Murray and Sanders predicted the stability of a NaZn13lattice built of hard spheres in the range ofγfrom 0.54 to 0.625 and AlB2-type structure should be stable forγin range of 0.482–0.62430. For the 3.7 and 7.3 nm Au NCs, the effective particle size ratioγis 0.55, which is located in the range from 0.54 to 0.625. NaZn13type BNSLs are formed. For the 3.7 and 6.0 nm Au NCs, the effective particle size ratioγis 0.63, which slightly deviate from the range of 0.482–0.624 due to the thickness error of the surface ligand.

Fig. 7 Space filling curve of NaCl, NaZn13 and AlB2 BNSLs.The horizontal dotted line indicates the space filling factor of single component face centered cubic. The vertical dotted lines indicate the values of the Au NCs effective size ratio (γ = 0.45, 0.55 and 0.63) in this study.
4 Conclusions
In this work, we synthesized 6.0, 7.3 and 9.6 nm Au NCs using dodecanethiol stabilized 3.7 nm Au NCs as seeds through the seed growth method in oleylamine. The Au NCs exhibited monodispersity with the size distributions smaller than 10%. The oleylamine functionalized 6.0, 7.3 and 9.6 nm Au NCs as “A”were mixed with 3.7 nm Au NCs serves as “B” at a certain concentration ratio. Au BNSLs with AB2type (hexagonal AlB2structure), AB13(NaZn13structure) and AB type (cubic NaCl structure) were obtained through the solvent evaporation method. The experimental results matched very well the relevant space filling principle. The investigation of such single nanocomponent as building block is noteworthy in structures and properties study of BNSLs as well as potential development of novel meta-materials.
