稀土-钛氧簇合物EuTi6,EuTi7和La2Ti14的可控合成
2020-09-28杨亚梅伦会洁龙腊生孔祥建郑兰荪
杨亚梅,伦会洁,龙腊生,孔祥建,郑兰荪
厦门大学化学化工学院化学系,固体表面物理化学国家重点实验室,能源材料化学协同创新中心,福建 厦门 361005
1 Introduction
The nanoparticles (1–100 nm) have received much attention owing to their special physical or chemical properties1–8.Meanwhile, the unclear surface structure and the polydispersity of nanoparticles make it difficult to precisely regulate the surface electronic structure and understand the relationship between structure and chemical reactivity at the atomic level9–11.Compared with nanoparticles, the atomically precise metal clusters possess the well-defined surface and crystal structure to study structure-activity relationships12–14. Hence, the metal clusters are attracting widespread current interest and have become one of the most interesting research frontiers.
Titanium oxo clusters (TOCs) are the ideal molecular modes for TiO2to clarify the relationships between the surface structures and photocatalytic properties of TiO215. Up to now,lots of TOCs with fantastic architectures and interesting physical and chemical properties, especially for the photocatalytic activities, have been reported16–22. However, the large band gap of TOCs similar to that of TiO2leads to the low photocatalytic activity of TOCs under visible light illumination23,24. To expand the utilization of sunlight, various metal ions are introduced into TOCs to reduce their band gaps25–33. Recent studies show that the heterometallic lanthanide-titanium oxo clusters LnTOCs not only show high photocatalytic activity but also possess the high stability26. However, due to the intense hydrolysis of Ti4+ions and the competitive coordination of Ln3+ions, the controlled synthesis of atomically precise LnTOCs remains great challenge34–36. Owing to the synthetic difficulty, highnuclearity LnTOCs are still very rare35,36, which obstructs further studies of their properties.
Because chelating ligands can reduce the degree of hydrolysis of Ti4+ions, choosing appropriate chelating ligands should be an effective way to construct LnTOCs. Herein, based on the chelating ligands of 3,5-di-tert-butylsalicylic acid (H2dtbsa),four LnTOCs, EuTi6(μ3-O)3(OC2H5)8(dtbsa)6(Hdtbsa)].(C2H5OH)(1), [EuTi7(μ3-O)3(μ2-OH)2(OiPr)9(dtbsa)6(Hdtbsa)Cl].(HOiPr)3(2), [EuTi7(μ3-O)3(μ2-OH)2(OiPr)8(dtbsa)7(Hdtbsa)].(HOiPr)3(3)and [LaTi7(μ3-O)3(μ2-OH)2(OC2H5)8(dtbsa)7(Hdtbsa)]2.(C2H5OH)4(4) were obtained by solvothermal method. Crystal structure analysis shows 1 contains a heptanuclear EuTi6metal core, in which six Ti4+ions form a trigonal prismatic structure and one Eu3+locates in the center of prism. The EuTi6 in 1 connected another Ti4+on one side of triangular prism forms the octanuclear metal core EuTi7in 2 and 3. The metal core of Ln2Ti14in 4 can be viewed as a dimer of EuTi7. The band gap values of clusters are significantly small than anatase, which may bring potential applications in the fields of solar energy utilization and photocatalysis. Light-driven H2 productions of water-splitting studies and photoelectric response tests both indicate that these three clusters are good photocatalysts.
2 Experimental and computational section
2.1 Materials and physical measurements
All chemicals were of reagent grade and were used without further purification. The powder X-ray diffraction (PXRD)patterns were recorded on Rigaku Ultima IV diffractionmeter using CuKαradiation at 25 kV and 100 mA. Infrared spectra were recorded on a Nicolet AVATAR FT-IR360 spectrophotometer with pressed KBr pellets. The thermogravimetric analysis (TGA)curves were prepared on a Netzsch TG209F1 thermal analyzer.The diffuse absorption spectra were recorded on a Carry 5000 spectrophotometer.
2.2 Syntheses of compounds 1–4
2.2.1 EuTi6(μ3-O)3(OC2H5)8(dtbsa)6(Hdtbsa)]·(C2H5OH) (1)
Analytically pure Ti(OiPr)4(0.06 mL, 0.2 mmol),Eu(NO3)3.6H2O (0.03 mmol) and 3,5-di-tert-butylsalicylic acid(H2dtbsa, 0.2 mmol) were dissolved in a mixed solvent of anhydrous ethanol (4 mL) and acetonitrile (2 mL). The mixture was stirred with ultrasonic machine for 30 min and heated at 80 °C for three days. After cooling to room temperature, green rod-like crystals were obtained. The crystals were washed with acetonitrile for several times, then saved in a vacuum drying oven (71% yields based on Eu(NO3)3.6H2O). IR (cm−1): 2954(s), 2867 (s), 1534 (s), 1443 (s), 1388 (s), 1255 (s), 1123 (s), 858(s), 809 (s), 735 (s), 554 (s).
2.2.2 [EuTi7(μ3-O)3(μ2-OH)2(OiPr)9(dtbsa)6(Hdtbsa)Cl]·(HOiPr)3(2)
Analytically pure Ti(OiPr)4(0.24 mL, 0.8 mmol),EuCl3.6H2O (0.3 mmol) and 3,5-di-tert-butylsalicylic acid(H2dtbsa, 0.6 mmol) were dissolved in isopropyl alcohol (5 mL).The mixture was stirred with ultrasonic machine for 30 min and heated at 80 °C for three days. After cooling to room temperature, orange sheet crystals were obtained. The crystals were washed with isopropyl alcohol for several times, then saved in a vacuum drying oven (17% yields based on EuCl3.6H2O). IR(cm−1): 2955 (s), 2867 (s), 1534 (s), 1443 (s), 1388 (s), 1255 (s),1123 (s), 858 (s), 809 (s), 735 (s), 554 (s).
2.2.3 [EuTi7(μ3-O)3(μ2-OH)2(OiPr)8(dtbsa)7(Hdtbsa)]·(HOiPr)3(3)
Analytically pure Ti(OiPr)4 (0.15 mL, 0.5 mmol),Eu(NO3)3.6H2O (0.20 mmol) and 3,5-di-tert-butylsalicylic acid(H2dtbsa, 0.5 mmol) were dissolved in isopropyl alcohol (5 mL).The mixture was stirred with ultrasonic machine for 30 min and heated at 80 °C for three days. After cooling to room temperature, orange rod-like crystals were obtained. The crystals were washed with isopropyl alcohol for several times, then saved in a vacuum drying oven (13% yields based on Eu(NO3)3.6H2O).IR (cm−1): 2955 (s), 2867 (s), 1534 (s), 1443 (s), 1388 (s), 1255(s), 1123 (s), 858 (s), 809 (s), 735 (s), 554 (s).
2.2.4 [LaTi7(μ3-O)3(μ2-OH)2(OC2H5)8(dtbsa)7(Hdtbsa)]2·(C2H5OH)4(4)
Analytically pure Ti(OiPr)4(0.03 mL, 0.1 mmol),La(NO3)3.6H2O (0.02 mmol) and 3, 5-di-tert-butylsalicylic acid(H2dtbsa, 0.1 mmol) were dissolved in a mixed solvent of anhydrous ethanol (1 mL) and acetonitrile (0.5 mL). The mixture was stirred with ultrasonic machine for 30 min and heated at 80 °C for three days. After cooling to room temperature, bright green rod-like crystals were obtained. The crystals were washed with acetonitrile for several times, then saved in a vacuum drying oven (56% yields based on La(NO3)3.6H2O). IR (cm−1): 2955(s), 2868 (s), 1532 (s), 1442 (s), 1389 (s), 1254 (s), 1121 (s), 856(s), 809 (s), 732 (s), 552 (s).
2.3 X-ray crystallography
Data of 1 was collected on an Oxford Gemini S Ultra diffractometer using graphite monochromatized MoKαradiation(λ= 0.71073 Å (1 Å = 0.1 nm)) at 173 K. Data of 2, 3, and 4 were collected on an Agilent SuperNova X-Ray single crystal diffractometer using MoKαradiation (λ= 0.71073 Å) microfocus X-ray sources at 100 K. Absorption corrections were applied by using the multiscan program. The structures were determined and refined using full-matrix least squares based onF2with SHELXS-97 and SHELXL-97 within Olex237,38. As shown in Table S1 (see Supporting Information (SI)), bond valance sum(BVS) calculations showed that all rare earths have a +3 valence and all titanium ions have a +4 valence. Crystal data and details of the data collection and refinement of these four clusters were summarized in Table S2 (see SI)). Selected bond distances and bond angles of 1–4 were shown in Table S3–S6 (see SI).Cambridge Crystallorgaphic Data Centre (CCDC) numbers of 1968828–1968831 for 1–4, respectively, contain the supplementary crystallographic data for this paper. The data can be obtained free of charge from the Cambridge Crystallorgaphic Data Centre.
3 Results and discussion
3.1 Crystal structures analysis
Single crystal X-ray diffraction shows that 1 consists of one Eu3+, six Ti4+ions, threeμ3-O2−, eight C2H5O−, six dtbsa2−and one Hdtbsa−ligands. As shown in Fig. 1a, the metal-oxo core of[EuTi6(μ3-O)3]21+can be viewed as that three triangular[EuTi2(μ3-O)]9+units linked together by sharing a central Eu3+ion. So, six Ti4+form a trigonal prismatic structure, in which one Eu3+locates in the center. The [EuTi6(μ3-O)3]21+metal core is stabilized by 6 dtbsa2−, 1 Hdtbsa-and 8 OEt−ligands (Fig. 1b).As shown in Fig. S1 (see SI), the Eu3+is nine-coordinated by six O atoms from six dtbsa2−ligands and threeμ3-O2−. Six Ti4+ions are six-coordinated and show slightly distorted octahedral geometries. The bond length ranges of the Ti-O and Eu-O bonds are range of 1.736–2.189 Å and 2.313–2.577 Å,respectively, close to the reported values in LnTOCs26. Adjacent EuTi6clusters are linked togetherviathe hydrogen bonding andπ–πstacking (Fig. S2, see SI).

Fig. 1 Ball-and-stick view of the metal-oxo core of 1 (a) and the crystal structure of 1 (b).

Fig. 2 Ball-and-stick view of the metal oxo core of 2 and 3 (a) and the crystal structures of 2 (b) and 3 (c).
As shown in Fig. 2a, the compound 2 contains one [EuTi7(μ3-O)3(μ2-OH)2]23+cluster core, which can be viewed as the[EuTi6(μ3-O)3]21+in 1 is connected to another Ti4+ion through twoμ2-OH-on one side of the trigonal prism. The additional Ti4+ion shows slightly distorted octahedral geometries and coordinated by twoμ2-OH−, three OiPr−and one Cl−, as shown in Fig. S3 (see SI). The octanuclear metal core EuTi7is protected by 6 dtbsa2−, 1 Hdtbsa−and 9 OiPr−ligands (Fig. 2b). Notably,another octanulcear EuTi7 (3) with same metal-oxo core structure was prepared by replacing EuCl3with Eu(NO3)3and slightly increasing the ratio of the ligand. Different from compound 2, the metal-oxo core is stabilized by 7 dtbsa2−, 1 Hdtbsa−and 8 OiPr−ligands, as shown in Fig. 2c. The coordination environments of metal ions are displayed in Fig. S4(see SI).The coordination environment of Eu3+in 2 and 3 is same as that of cluster 1, just with slightly different Eu-O bond length of 2.316–2.616 Å for 2 and 2.320–2.591 Å for 3. The Ti-O bond length is 1.760–2.202 Å for 2 and 1.750–2.223 Å for 3. The packing patterns of 2 and 3 are shown in Fig. S5 and Fig. S6, respectively (see SI).
Compound 4 is a centrosymmetric La2Ti14 structure. As shown in Fig. 3a, two [LaTi7(μ3-O)3(μ2-OH)2]23+units are connected by a two bridging C2H5O−ligands, forming the[LaTi7(μ3-O)3(μ2-OH)2(OC2H5)]244+metal-oxo core. The[LaTi7(μ3-O)3(μ2-OH)2]23+unit displays the same geometry with that in 2 and 3, except for the bridging Ti4+is coordinated by 2μ2-OH−, 2 OEt−and 2μ2-OEt−. As shown in Fig. 3b, each of the LaTi7metal-oxo core is stabilized by 7 dtbsa2−, 1 Hdtbsa-and 8 OEt−ligands. The coordination environments of metal ions are displayed in Fig. S7 (see SI). The ligand (H2dtbsa) displays same coordination modes in 1–4, as shown in Fig. S8 (see SI). The Ti-O and La-O are range of 1.752–2.195 Å and 2.408–2.637 Å, respectively. The packing pattern of 4 is shown in Fig. S9 (see SI).
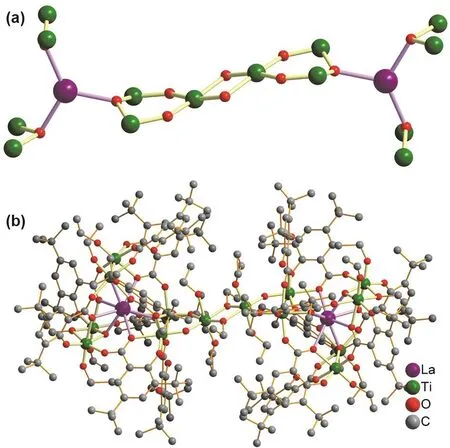
Fig. 3 Ball-and-stick view of the metal oxo core of 4 (a) and the crystal structures of 4 (b).

Fig. 4 Ball-and-stick view of the metal-oxo core of [EuTi6(μ3-O)3]21+in 1, [EuTi7(μ3-O)3(μ2-OH)2]23+ in 2 and 3, and[La2Ti14(μ3-O)6(μ2-OH)4(OC2H5)2]42+ in 4.

Fig. 5 The TGA curves of 1–4 under atmosphere.
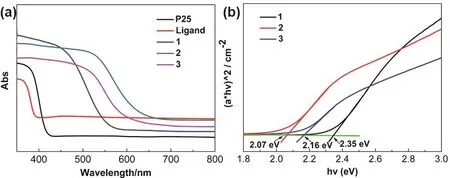
Fig. 6 UV-Vis diffuse-reflectance spectra of P25, H2dtbsa, 1–3 (a); band gap values of 1–3 (b).
Structure analysis shows that the metal-oxo core structures of the 1–4 are related to each other. As shown in Fig. 4, the[EuTi6(μ3-O)3]21+in 1 connected to one Ti4+ion on one side of the trigonal prism forms the [EuTi7(μ3-O)3(μ2-OH)2]23+in 2 and 3. While the metal-oxo core of [La2Ti14(μ3-O)6(μ2-OH)4(OC2H5)2]42+in 4 can be viewed as a dimerization of [EuTi7(μ3-O)3(μ2-OH)2]23+in 2 and 3, except for the different lanthanide ions.
3.2 General characterization
In order to test the purity of the sample, PXRD test was carried out. As shown in Fig. S10 (see SI), PXRD curves display that the peak positions of the clusters are consistent with the simulated peak position from the crystal data. To examine the thermal stability, the thermogravimetric analyses of 1–4 were measured in the temperature range 20–800 °C. As shown in Fig.5, the TG curves show that 1 and 4 have similar thermal stability.They both display a slightly weight loss between room temperature to 230 °C, indicating that the clusters are stable over this temperature range. After 230 °C, the coordinated solvent molecules and organic ligands gradually decompose and the framework begins to collapse until complete decomposition. The remaining mass is attributed to the final products TiO2and Ln2O3. Clusters 2 and 3 have a weight loss of about 6.6% and 5.8% from room temperature to 230 °C, which corresponds to the loss of the guest molecules isopropanol (Calculated: 5.9% for 2 and 5.6% for 3). In the temperature range of 230–320 °C,cluster 2 and 3 have a weight loss of about 11.4% and 12.8% due to the dissociation of coordinated isopropanol. When the temperature is higher than 320 °C, the organic ligands gradually decompose.
UV-Vis diffuse-reflectance spectra show that 3 starts to absorb from 700 nm, 2 starts from 670 nm and 1 starts from 590 nm, as shown in Fig. 6a. Their absorption ranges are much larger than that of commercial P25 and H2dtbsa ligand. The band gap values for 1, 2 and 3 are 2.35, 2.07, and 2.16 eV, respectively(Fig. 6b), which are substantially smaller than 3.2 eV for anatase.This significant redshift of band gaps for 1–3 leads to an increase of absorption range in the UV-Vis region, which may bring potential applications in the fields of solar energy utilization and photocatalysis. In order to explore the application in the field of photocatalysis, H2production of water-splitting studies were carried out under irradiation by a 300 W Xe lamp (300–800 nm)in an aqueous methanol solution (20 mL, 10%). As shown in Fig.S11 (see SI), the H2 production rates for 1, 2 and 3 are 112, 106 and 87 μmol∙h−1∙g−1, respectively, which are larger than the commercial P25. To detect the optical stability of the clusters,the samples after 8h of illumination were tested by PXRD. As shown in Fig. S12 (see SI), the curves before and after the experiment are consistent with the simulated values, suggesting that 1, 2 and 3 are stable during the photocatalytic experiments.The photoelectric tests show that the three clusters have obvious photoelectric response (Fig. S13, (see SI)). The charge separation efficiency of 1 and 2 are similar, and both better than that of 3, which is consistent with the result of light-driven H2production of water-splitting studies. These results suggest that the lanthanide-titanium oxo clusters are potential photocatalyst in water splitting.
4 Conclusions
In summary, four lanthanide-titanium oxo clusters have been prepared by using the chelated organic ligand. Crystal analysis shows that 1 contains a heptanuclear EuTi6metal core featuring a Eu-centered trigonal prismatic structure. The EuTi6 unit linked another Ti4+on one side of prism generates the octanuclear metal core EuTi7in 2 and 3. The metal-oxo core of La2Ti14in 4 can be viewed as a dimer of EuTi7. The regularity of the structures has certain reference significance for understanding the formation of lanthanide-titanium oxo clusters. This work not only provides a chelating ligand strategy for the synthesis of lanthanidetitanium-oxo clusters, but also suggests that lanthanide-titaniumoxo clusters have potential light-driven photocatalytic activity because of the relatively low band-gap.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
