Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein
2020-09-18HyunJinParkTingTingZhaoSeungHwanKimChongKilLeeBangYeonHwangKyungEunLeeMyungKooLee
Hyun Jin Park , Ting Ting Zhao , Seung Hwan Kim, Chong Kil Lee Bang Yeon Hwang Kyung Eun Lee Myung Koo Lee
1 Department of Pharmacy, College of Pharmacy, Chungbuk National University, Cheongju, Republic of Korea 2 Research Center for Bioresource and Health, College of Pharmacy, Chungbuk National University, Cheongju, Republic of Korea 3 Department of Social Physical Education, Songwon University, Gwangju, Republic of Korea
Abstract
Key Words: A53T α-synuclein genetic mice; ERK1/2; gynostemma pentaphyllum; gypenosides; Parkinson's disease; retention transfer latency time
Introduction
Parkinson’s disease (PD) is caused by the degenerative neuronal cell loss of dopamine in the substantia nigra pars compacta (SNpc). Patients with PD mainly exhibit the typical motor symptoms such as tremor, rigidity, and bradykinesia,and some non-motor symptoms, including affective disorders and dementia. In addition, the Lewy body formation in the degenerating neurons is observed in the brain of PD patients. α-Synuclein is a major component of the protein inclusions (Lewy bodies) (Spillantini et al., 1997; Lee and Trojanowski, 2006). α-Synuclein is expressed at presynaptic nerve terminals and accumulates as insoluble, proteinase K-resistant protein aggregates (Spillantini et al., 1997). The presynaptic accumulation of α-synuclein precedes the formation of Lewy bodies in distinct regions of the brain. Lewy bodies can be found in both sporadic PD and PD due to genetic mutations (Tanji et al., 2010).
Missense mutations in the α-synuclein gene, which is encoded by SNCA, including A53T, A30P, and E46K, have been found in PD patients (Kruger et al., 1998; Zarranz et al.,2004; Tanji et al., 2010). In the animal model of PD based on α-synuclein overexpression, the neuronal α-synucleinopathy in the brain is followed by the Lewy body formation and this is similar to the neuropathology of Lewy bodies of the human brain (Xu et al., 2015). In the brains of mice, A53T mutant human α-synuclein overexpression causes the Lewy body formation of the SNpc region, and this in turn causes the progressive dopaminergic neuronal cell loss (Kramer and Schulz-Schaeffer, 2007). Furthermore, α-synuclein has been reported to give rise to neuronal cell death and mitochondrial dysfunction by apoptosis in human neuroblastoma(SH-SY5Y) cells and rat adrenal pheochromocytoma (PC12)cells, and that cell death may trigger PD pathogenesis in vivo(Cai et al., 2010; Liu et al., 2016).
Gynostemma (G.) pentaphyllum (Cucurbitaceae), a wellknown natural herbal medicine, contains various gypenosides (GPS), flavonoids, and polysaccharides (Razmovski-Naumovski et al., 2005). The extract of G. pentaphyllum by ethanol (GP-EX) shows a protective effect on dopaminergic neuronal cells in the rat model of PD induced by 6-hydroxydopamine (6-OHDA) (Choi et al., 2010). Studies involving the same rat models have found that GP-EX protects against neuronal cell death in tyrosine hydroxylase (TH)-expressing neurons and alleviates L-3,4-dihydroxyphenylalanine(L-DOPA)-induced dyskinesia (Choi et al., 2010; Shin et al.,2015). GPS have also been shown to have protective effects on the cell death of dopaminergic neurons in the mouse model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Wang et al., 2010). In addition, GP-EX and GPS have ameliorative functions in MPTP lesion-induced anxiety and neuroprotective effects on dopaminergic neuronal cells of MPTP-lesioned mice (Shin et al., 2014).
PD is a multi-symptomatic condition. However, both the neurotoxin-based and genetic animal models are not able to replicate all of the motor and pathophysiological symptoms of PD patients (Jackson-Lewis et al., 2012). To address this limitation, PD-related studies should combine neurotoxin models and genetic models.
This study, therefore, investigated the ameliorative effects of GP-EX, including GPS, on the dopaminergic neuronal cell death in the A53T α-synuclein genetic PD mouse models to confirm the neuroprotective activities of GP-EX.
Materials and Methods
GP-EX and GPS preparation
G. pentaphyllum was purchased from SLOGA Co. (Daegu,Korea). A voucher specimen (herbal leaves) of G. pentaphyllum was kept at the herbarium (College of Pharmacy,Chungbuk National University, Cheongju, Korea). G. pentaphyllum (air-dried leaves, 1 kg) was extracted using ethanol (80%, v/v). The extract was then evaporated under the reduced temperature and pressure to obtain a dry powder(GP-EX, yield: 9.5% w/w, 95.3 g).
To control the quality of GP-EX, high-performance liquid chromatography (HPLC) was performed in a Waters system[two 515 pumps with a 996 photodiode array detector (Waters Co., Milford, MA, USA) and a YMC J’sphere ODS-H80 column (150 × 20 mm internal diameter, 4 μm) (YMC Co.,Ishikawa, Japan)] using the mixed solvent system CH3CNH2O (15:85 to 30:70, 0-15 minutes; 30:70 to 100:0, 15-40 minutes) at a flow rate of 1.0 mL/minute.
The HPLC chromatograms of GP-EX and ombuoside have been provided (Additional Figures 1 and 2). Ombuoside was used as the marker compound. It was isolated from GP-EX and identified by comparison with the authentic compound, which was purchased from the BioBioPhar Co. (purity: > 98.6%) (Kunming, Yunnan Province, China)(Additional Figure 2). GPS was obtained from the Ankang Dongke Maidisen Nature Pharmaceutical Co. (purity: >99%) (Xi’an, China) (Shang et al., 2006; Wang et al., 2010).
Experimental design
Homozygous transgenic mice expressing A53T mutant human α-synuclein (A53T) (004479-B6; C3-Tg (Prnp-SNCA*A53T) 83Vle/J, male, 8 weeks of age, 25-30 g) and control mice [100010 B6C3F1/J, male, wild-type (WT)] were obtained from the Jackson Laboratory (Bar Harbor, ME,USA). All animals were fed with standard water and diet ad libitum and raised under standard conditions (temperature,23 ± 2°C; humidity, 60 ± 5%; illumination, a 12-hour light/dark cycle). This study was reviewed by the Animal Ethics Committee of Chungbuk National University (approval No.CBNUA-956-16-01) on September 21, 2016. The experiments were conducted according to the National Institutes of Health guidelines for the care and use of laboratory animals and the guidelines of Chungbuk National University Laboratory Animal Research Center.
The experiments were planned and conducted in two separate subsets of animals comprising six groups (4-5 animals/group). The experiments included step-through passive avoidance and elevated plus-maze tests, immunohistochemistry for α-synuclein, and western blot analyses.Mice in the control groups received saline (0.9%) administration and the GP-EX- (or GPS)-treated A53T mice received GP-EX or GPS (both at 50 mg/kg, oral) daily for 20 weeks. After the last treatments, the mice were subjected to behavioral tests. After anesthesia with Zoletil (50 or 100 mg/kg, intraperitoneal) (Virbac, Carros, France), rat brains were dissected for α-synuclein immunohistochemistry and western blot analysis.
α-Synuclein immunohistochemical staining
Intracardial perfusion in mice was conducted using a paraformaldehyde solution (4% in 0.1 M PBS, pH 7.4), and the removed brains were stored in a 30% sucrose solution. The brain samples (coronal sections, 30-μm thick) were prepared to examine the dopaminergic neuronal cell bodies of the midbrain using a Vibratome (Leica Microsystems GmbH,Wetzelar, Germany). Tissue sections were subjected to α-synuclein immunohistochemistry using mouse anti-human-specific Syn204 (1:250) (Abcam, Cambridge, UK) and mouse anti-α-csynuclein Syn-1 (1:1000) (BD Transduction Laboratories, San Jose, CA, USA) antibodies overnight at 4°C and a biotinylated goat anti-rabbit secondary antibody(1:250) (Cell Signaling Technology Inc, Beverly, MA, USA)for 2 hours at room temperature. Then, the sections were incubated with an ABC kit (Vector Laboratories, Burlingame,CA, USA), and immunoreactivities were measured using a DAB kit (Vector Laboratories, Burlingame, CA). The photomicrograph of α-synuclein immunoreactivity and brightfield digital image were captured by an Axiophot optical microscope (100×) (Carl Zeiss MicroImaging GmbH, Jena,Germany). Quantitation of α-synuclein-immunopositive cells was performed by an image analysis system with an Axiophot optical microscope (Axiovision software, Carl Zeiss MicroImaging GmbH). The data was quantified by an average value of five slices of the whole midbrain of each mouse.
α-Synuclein and TH phosphorylation
Western blot analysis was performed to examine the phosphorylation of α-synuclein (Ser129), that of TH (Ser40),and β-actin levels. Proteins in samples (5 μg) were electrophoresed on sodium dodecyl sulfate-poly acrylamide gels(SDS-PAG, 10-15%) and they were transferred to a membrane of polyvinylidene difluoride. The blots were incubated overnight at 4°C in primary antibodies (rabbit source)against α-synuclein, phosphorylated α-synuclein (at Ser129)(p-α-synuclein), TH, phosphorylated TH (at Ser40) (p-TH), and β-actin were purchased from the Cell Signaling Technology Inc. (Beverly, MA, USA) (dilutions, 1:1000)in Tris-buffered saline containing Tween-20 (TBS-T) and bovine serum albumin (BSA, 5%). Then the blots were kept in goat anti-rabbit secondary antibodies (1:5000 in TBS-T containing 5% BSA) (Cell Signaling Technology Inc.) for 1 hour at room temperature in accordance with the standard procedure.
After washes, the transferred proteins were incubated with the substrate solution of electrochemiluminescence (ECL)(Amersham Pharmacia Biotech, Piscataway, NJ, USA) in accordance with the instructions of the manufacturer. Reactive bands were visualized on radiographic film and digitalized using MP Navigator EX 2.0 (Canon, Tokyo, Japan).
western blot analysis
Midbrain samples from the mice were harvested and the protein of each sample (10 μg) was separated using an electrophoresis (10% SDS-PAG). Then the protein was transferred to a polyvinylidene difluoride membrane. Immunoblots of the lysates were probed using antibodies against p-ERK1/2(Thr202/Tyr204), p-Bad (Ser112), and p-JNK1/2 (Thr183/Thr185), as well as antibodies against β-actin (Park et al.,2013; 2016b). The blots were blocked in a fresh blocking buffer (TBS-T containing 5% BSA) for 1 hour at room temperature, then kept overnight at 4°C using primary antibodies(rabbit source) against extracellular signal-regulated kinase(ERK1/2), phosphorylated ERK1/2 (at Thr202/Tyr204)(p-ERK1/2), c-Jun N-terminal kinase (JNK1/2), phosphorylated JNK1/2 (at Thr183/185) (p-JNK1/2), Bcl-2-associated death promoter (Bad), phosphorylated Bad (at Ser112)(p-BadSer112), and β-actin were purchased from the Cell Signaling Technology Inc. (dilutions, 1:1000) in TBS-T with 5% BSA. Finally, the blots were incubated for 1 hour at room temperature with goat anti-rabbit secondary antibodies (dilutions, 1:5000 in TBS-T with 5% BSA) (Cell Signaling Technology Inc.,) in accordance with the standard procedure.Antibody binding detection was analyzed by incubation of the membranes in ECL substrate (ATTO Corporation,Tokyo, Japan). The band of reactive protein was then visualized using radiographic films and imaged using the imaging system (ImageQuant 400 Scan CCD) and ImageQuant 400 Capture software (version 1.0.0) (GE Healthcare; Piscataway,NJ, USA).
Measurement of latencies of step-through passive avoidance and elevated plus-maze tests
On the first day after the habituation period, the mice were placed in the illuminated chamber of the apparatus for stepthrough passive avoidance test (Med Associates Inc., St. Albans, VT, USA). This apparatus consisted of an illuminated chamber connected to a dark chamber by a guillotine door.Each mouse was delivered an inescapable electric shock (0.5 mA, 3 seconds). The initial latency was recorded when the mouse entered into the dark chamber, and the initial latency that was longer than 180 seconds was not included in the final analysis. Twenty-four hours later, the retention latency was measured by placing each mouse in the illuminated chamber (Ögren and Archer, 1994).
The apparatus for the elevated plus-maze test was composed of two open and two closed arms of the same size (30 cm × 5 cm, with 16 cm-high black walls), which were elevated to 45 cm above the floor. Each mouse was placed in an open, outward-facing arm. The time taken for entering the closed arm during the first trial was measured as the initial transfer latency. Twenty-four hours after the first trial, a second trial was performed and the retention transfer latency was measured. The results represent the ratios of the retention transfer latency to the initial transfer latency (%ITL)(Kumar et al., 2011).
Statistical analysis
To assess differences in the initial and retention trials between groups in the step-through passive avoidance, repeated-measures analysis of variance (ANOVA) with a multiple comparison test followed by post hoc Pillai’s Trace was used.One-way ANOVA followed by post hoc Tukey’s test was performed to examine the effects of GP-EX and GPS on the%ITL using the elevated plus-maze test. One-way ANOVA followed by Dunnett’s test was also performed to examine the effects of GP-EX or GPS on immunohistochemical and western blot results. Data analyses were performed using a statistical software package (GraphPad Prism, version 6.00,San Diego, CA, USA). The results represent the mean ± SD or ± SEM, and P-values < 0.05 were considered statistically significant.
Results
α-Synuclein-immunopositive cells in the midbrain
In the representative photomicrographs (Figure 1), there were more α-synuclein-immunopositive cells (150%) (P <0.01) in the A53T mice than in the WT mice. There were fewer α-synuclein-immunopositive cells in the A53T mice treated with 50 mg/kg GP-EX than in the untreated A53T mice (112%, vs. WT) (P < 0.05) (Figure 1). GPS (50 mg/kg)treatment led to the same effect as that of GP-EX (50 mg/kg)(Figure 1).
Phosphorylation of ERK1/2 in the midbrain
The p-ERK1/2 levels in the A53T mice were 0.65-fold (P< 0.05) that in the WT mice (Figure 2A). Treatment with 50 mg/kg GP-EX elevated p-ERK1/2 levels by 1.10-fold (P< 0.05) in the A53T mice, compared to the untreated mice(Figure 2A). GPS (50 mg/kg) had similar effects to those of 50 mg/kg GP-EX (Figure 2B).
Phosphorylation of α-synuclein and TH in the midbrain
The level of p-α-synuclein in the midbrain was increased by 2.54-fold (P < 0.05) in the A53T mice, compared to that in the WT mice (Figure 3A). On the contrary, treatment of A53T mice with 50 mg/kg GP-EX reduced the p-α-synuclein level to 1.62-fold that in the WT levels (P < 0.05, vs. untreated A53T mice) (Figure 3A).
The level of p-TH in the A53T mice was 68.1% (P < 0.01)of that in the WT group (Figure 3B). Treatment of A53T mice with 50 mg/kg GP-EX increased p-TH level to 85.8%(P < 0.05, vs. untreated A53T mice) of that in the WT group(Figure 3B).
Phosphorylation of Bad and JNK1/2 in the midbrain
The level of p-BadSer112 in the A53T mice was 58.2% (P< 0.05) of that in the WT mice (Figure 4A). Treatment of A53T mice with 50 mg/kg GP-EX induced the p-BadSer112 level to 81.3% (P < 0.05, vs. untreated A53T mice) of that in the WT group (Figure 4A).
The p-JNK1/2 level in the A53T mice was 1.67-fold (P <0.05) that in the WT mice (Figure 4B). Treatment of A53T mice with 50 mg/kg GP-EX reduced the p-JNK1/2 level 1.35-fold (P < 0.05, vs. untreated A53T mice) that in the WT group (Figure 4B).
Latency of step-through passive avoidance test
Table 1 shows the latency of the step-through passive avoidance test. Baseline differences were observed between the WT and A53T mice. The initial latency was slightly longer in the A53T mice (43.3 seconds) than that in the WT group(26.6 seconds). The retention latency in the A53T group was not significantly different from the initial latency. The shorter retention latency in A53T mice indicates more severe impairment when compared to that in the WT mice(42.1 seconds vs. 139.4 seconds, df = 5, F = 15.1, P < 0.05).In the A53T mice, the retention latency was shortened to be 99.2 seconds (df = 7, F = 34.5, P < 0.01) by treatment with 50 mg/kg GP-EX when compared to that in the untreated A53T mice. GPS (50 mg/kg) had a similar effect (df = 7, F = 7.08,P < 0.05) (Table 1), although it had a lower efficacy than 50 mg/kg GP-EX.
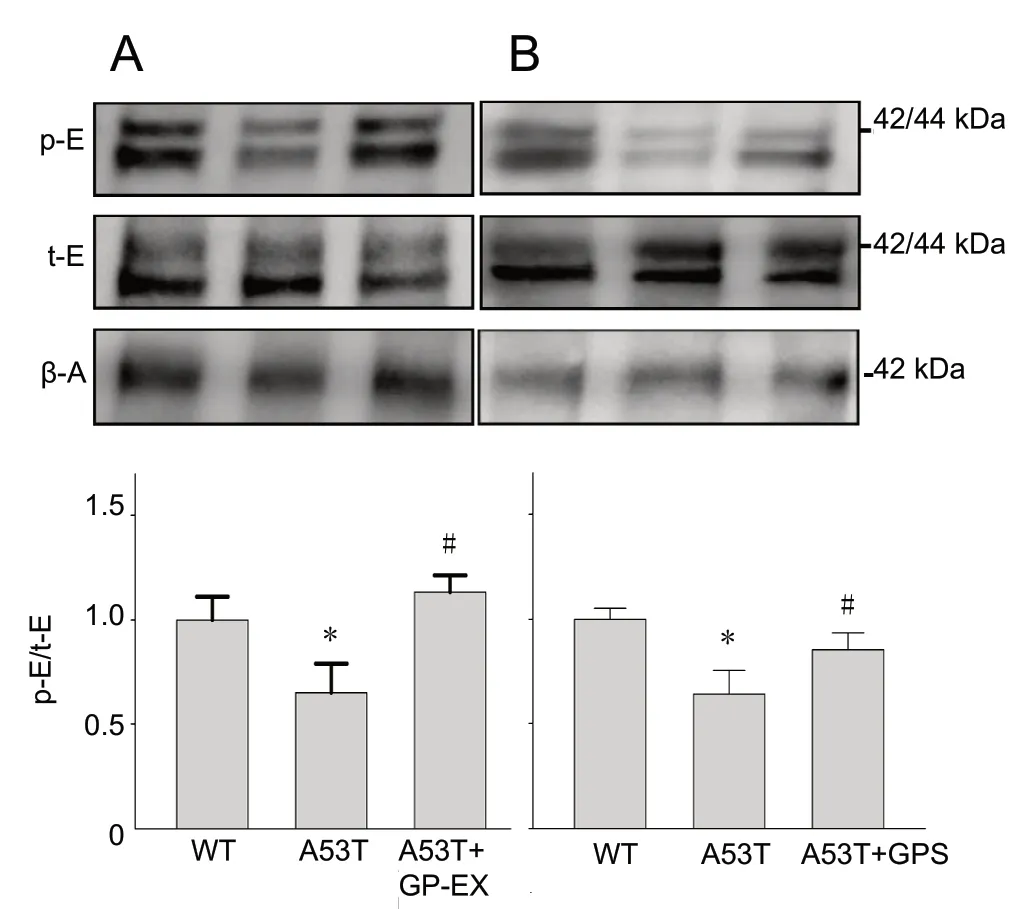
Figure 2 Modulations of ERK1/2 phosphorylation by GP-EX and GPS in the midbrain of A53T mice.
Transfer latency of the elevated plus-maze test
The %ITL was significantly higher (by 282%) in the A53T mice than in the WT mice (df = 6, F = 9.09, P < 0.01) (Figure 5).Treatment of A53T mice with 50 mg/kg GP-EX reduced %ITL(184% of that of WT, df = 8, F = 8.16, P < 0.05) when compared to the untreated A53T mice (Figure 5). GPS at 50 mg/kg had effects and efficacy similar to those of 50 mg/kg GP-EX (211%of that of WT, df = 8, F = 6.04, P < 0.05) (Figure 5). Furthermore, the basic locomotor activity by the distance traveled was reduced in the A53T transgenic mice (data not shown).
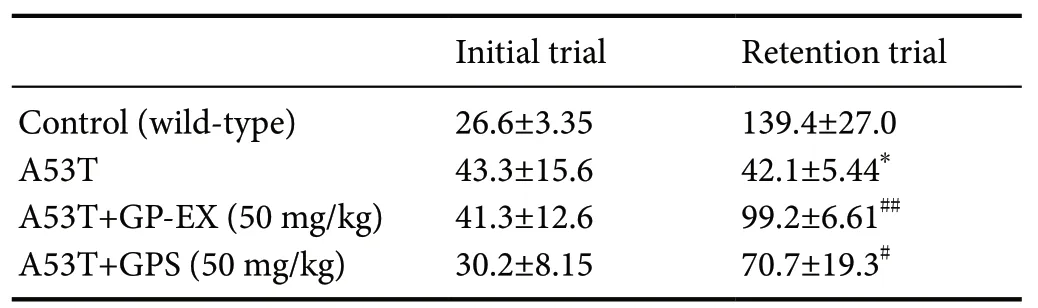
Table 1 Modulation of retention latency (s) by treatment with GP-EX and GPS in the step-through passive avoidance test

Figure 3 Modulatory effects of GP-EX on α-synuclein and TH phosphorylation in the midbrain of A53T mice.

Figure 4 Modulation of Bad and JNK1/2 phosphorylation by GP-EX in the midbrain.
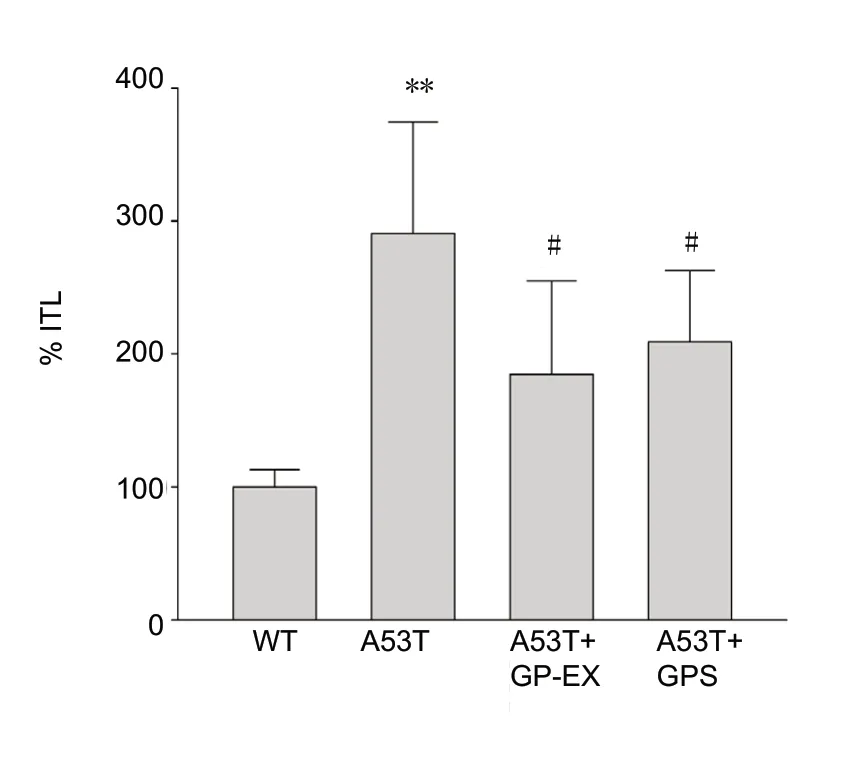
Figure 5 Inhibition of increased retention transfer latency by GP-EX and GPS in the elevated plus-maze test.
Discussion
GP-EX (25 and 50 mg/kg per day) and GPS (50 and 100 mg/kg per day) were shown to have ameliorative effects on rodent models of PD induced by MPTP- and 6-OHDA (Choi et al., 2010; Shin et al., 2014, 2015). In the MPTP-lesioned mice, both GP-EX and GPS treatments at 50 mg/kg per day for 21 days improved retention latency and reduced %ITL(Zhao et al., 2017). In this study, we used both GP-EX and GPS at 50 mg/kg per day, based on recommendations from previous findings (Shin et al., 2015; Zhao et al., 2017).
The A53T mouse model is a suitable model of early-onset PD (Lu et al., 2015) characterized by A53T α-synuclein overexpression (Giasson et al., 2002). The A53T mouse model has been shown to exhibit the protein aggregation, Lewy body-like inclusion formation, α-synuclein phosphorylation, and dopaminergic neuronal cell loss (Lu et al., 2015).α-Synuclein is phosphorylated at the Ser87, Ser129, and Tyr125 residues in the brain. α-Synuclein phosphorylation at Ser129 induces dopaminergic neuronal toxicity of the brain(Xu et al., 2015). α-Synuclein phosphorylation at the sites of Ser87 and Tyr125 also leads to neuronal toxicity, although the effects of phosphorylation at these sites are not as severe as those associated with phosphorylation at Ser129 (Xu et al., 2015). Rats expressing A53T α-synuclein have insoluble inclusions of α-synuclein and high α-synuclein phosphorylation levels, leading to loss of dopaminergic neurons (Lu et al., 2015).
In this study, we observed α-synuclein-immunopositive cells and p-α-synuclein in the A53T mice. α-Synuclein-immunopositive cells and p-α-synuclein levels were partially reduced by 50 mg/kg GP-EX treatment. The p-TH levels were also significantly decreased in A53T mice, indicating dopaminergic neuronal toxicity. Treatment with 50 mg/kg GP-EX improved the α-synuclein expression-induced reduction in p-TH in the A53T mice. The above results suggest that GP-EX protects from the cell death of dopaminergic neurons which was mediated by α-synuclein overexpression in the A53T mice.
The level of p-ERK1/2 has been shown to undergo dual patterns of changes. Neurotoxic agents, including 6-OHDA,have been shown to induce the cell death of dopaminergic neurons in the animal model of PD (Shin et al., 2015; Park et al., 2016b, 2017). In the context of 6-OHDA toxicity, the p-ERK1/2 and p-BadSer112 levels are both decreased and the p-JNK1/2 level is increased. In contrast, the p-ERK1/2 level is induced in the midbrain of the rat lesioned by 6-OHDA following L-DOPA administration. The increase in p-ERK1/2 level is evident when the animal is euthanized early after L-DOPA administration, which leads to dopaminergic neuronal cell death (Park et al., 2016b). This suggests that ERK1/2 activity may be reduced as dopaminergic neurons die during chronic treatment with L-DOPA.The level of p-BadSer122 is slightly reduced or sometimes even unaltered in the presence of 6-OHDA at the high/toxic levels, although in PC12 cells it increases in the presence of ameliorative agents (Park et al., 2013, 2017). In our study, the mutated α-synuclein expression in mice led to reductions in the p-ERK1/2 and p-BadSer112 levels, and an increase in the p-JNK1/2 level in the midbrain. Treatment of A53T mice with 50 mg/kg GP-EX modulated the p-ERK1/2, p-BadSer112, and p-JNK1/2 levels, suggesting that the ameliorative activities of GP-EX against cell death in α-synuclein-overexpressing dopaminergic neurons may involve the ERK1/2 and JNK1/2 signaling pathways.
A neurotoxic metabolite of MPTP, 1-methyl-4-phenylpyridinium, increases both the expression of α-synuclein and phosphorylation of ERK/MAPK in SH-SY5Y cells, and it causes cell death (Gómez-Santos et al., 2002). α-Synuclein overexpression causes the increased oxidative stress and intracytoplasmic dopamine levels in PC12 cells, and then this does damage to mitochondria (Zhang et al., 2013). In SH-SY5Y cells, the expression of α-synuclein containing the A53T mutation leads to neurite injury (Liu et al., 2016). The overexpression of α-synuclein following exposure to manganese induces p-ERK1/2 of PC12 cells (Cai et al., 2010). The level of p-JNK1/2 has been also reported to slightly increase with A53T α-synuclein overexpression in SH-SY5Y cells(Liu et al., 2016) and this in turn has been shown to promote the autophagic degradation of A53T α-synuclein. The above findings show that α-synuclein expression causes cytotoxicity via the oxidative stress-mediated ERK1/2-JNK1/2 signaling systems in SH-SY5Y and PC12 cells. This may also partially account for the α-synuclein-induced neurotoxicity in A53T mice.
The step-through passive avoidance examines learning and memory deficits in the mice lesioned by MPTP (Ögren and Archer, 1994). The transfer latency of the elevated plusmaze test is also applied for studying spatial memory deficits(Kumar et al., 2011). Late-adult A53T transgenic mice exhibit hypoactivity-like behaviors in the elevated plus-maze test (Graham and Sidu, 2010). Our study showed that the latency in the retention trial was significantly decreased and the %ITL was significantly increased in A53T mice. GP-EX increased the retention latency of the step-through passive avoidance test and also reduced the %ITL of the elevated plus-maze test in A53T mice. This suggests that GP-EX ameliorated the α-synuclein-induced deficits in memory function by decreasing the cell death of dopaminergic neurons in the midbrains of A53T mice. The major protein inclusion component of Lewy bodies is α-synuclein in PD patients(Kramer and Schulz-Schaeffer, 2007; Tanji et al., 2010; Xu et al., 2015). Lewy body formation leads to deficits in memory in idiopathic PD patients (Hall et al., 2015). α-Synuclein overexpression also leads to severe and complex motor impairment (Giasson et al., 2002). However, no significant learning or memory deficits have been reported in 6 to 7-month-old human α-synuclein-expressing transgenic mice containing the A30P mutation (Gomez-Isla et al., 2003).
α-Synuclein leads to oxidative and nitrative neurotoxicity,which lead to neurodegenerative synucleinopathies, including PD (Giasson et al., 2000). α-Synuclein overexpression does damage to mitochondria caused by oxidative stress to PC12 cells (Zhang et al., 2013). A low dose (10 mg/kg) of L-DOPA is beneficial, as it increases the level of dopamine in the brain (Park et al., 2016; Zhao et al., 2017). On the contrary, long-term/repeated treatments with L-DOPA at a low dose or treatments with L-DOPA at a high dose (20-30 mg/kg) may cause oxidative neurotoxicity in dopaminergic neurons in the rodent lesioned by MPTP or 6-OHDA (Blunt et al., 1993; Shin et al., 2014; Park et al., 2016). The cell death of dopaminergic neurons induced by oxidative stress is a prominent pathogenic component of PD, thus it has been suggested to take scavenging agents capable of depleting ROS to protect dopaminergic neurons from oxidative stress-induced cytotoxicity (Yacoubian and Standaert, 2009).Oxidative stress reducing agents, including coenzyme Q10 and selegiline, have been applied for the clinical therapies(Yacoubian and Standaert, 2009). In addition, the effects of various bioactive components from natural herbal products on dopaminergic neuronal cell death of an animal model of PD have been discussed (Park et al., 2016a), but the clinical applications for PD remain undeveloped.
GP-EX has anxiolytic functions in the chronic-stressed mice (Zhao et al., 2015) and MPTP-lesioned mice (Shin et al., 2014). GP-EX had prophylactic effects on cell death induced by oxidative stress in the 6-OHDA-induced animal model of PD (Shin et al., 2015). GPS also has protective effects on oxidative neurotoxicity induced by glutamate in rat cortical cultured cells (Shang et al., 2006). Treatment with GP-EX (at 50-400 mg/kg) does not have adverse drug effects, like vomiting, weight loss, and death (Attawish et al.,2004). In this study, GPS had ameliorative effects on neurotoxicity induced by α-synuclein in A53T mice, similarly to those of GP-EX. Taken together, we hypothesize that GPEX, including GPS, has protective effects on neurotoxicity induced by oxidative stress and plays an important role in the amelioration of α-synuclein overexpression-induced cell death of dopaminergic neurons in A53T mice. G. pentaphyllum contains mainly GPS, flavonoids and polysaccharides.The flavonoids and polysaccharides exhibit anti-neurodegenerative functions (Razmovski-Naumovski et al., 2005). In the MPTP-lesioned mice, water extract from G. pentaphyllum also reduces the cell death of dopaminergic neurons,but this effect is lower than that of GP-EX (Shin et al., 2014).The mechanisms of bioactive compound(s), including GPS,flavonoids and polysaccharides, need further study. In addition, there are several limitations to this study: first, small sample size was applied to perform statistical analysis; second, it took a time to maintain the animal conditions during the 20 weeks of experiment to perform the behavioral tests.
In conclusion, GP-EX, including GPS, has ameliorative effects on cell death in α-synuclein-expressing dopaminergic neurons through modulating the ERK1/2-BadSer112-JNK1/2 signaling pathway in the midbrains of A53T mice. It is necessary to further investigate the potential use of GP-EX as a treatment for neurodegenerative synucleinopathies such as PD.
Author contributions:HJP and MKL designed the experiment. HJP and TTZ did the biochemical laboratory works, were responsible for animal experiments and data analysis, and wrote the manuscript with MKL.BYH and SHK supported the chemicals and conducted high-performanceliquid chromatography. CKL and KEL supervised the works. The final submitted manuscript was read and approved by all authors.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This study was supported by the National Research Foundation of Korea, grant No. 2016R1D1A3B03930722, 2017-2018 (to MKL), Republic of Korea.
Institutional review board statement:The study was approved by the Animal Ethics Committee of Chungbuk National University (Approval No. CBNUA-956-16-01) on September 21, 2016, and the experiments were performed in accordance with the National Institutes of Health guidelines for the care and use of Laboratory Animals and those of Chungbuk National University Laboratory Animal Research Center.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Anjali Aggarwal, Post Graduate Institute of Medical Education and Research, India; Clarissa Cavarsan, UFPR, USA.
Additional files:
Additional Figure 1:High-performance liquid chromatography-DAD chromatogram of 80% ethanol extract of Gynostemma pentaphyllum leaves.
Additional Figure 2:High-performance liquid chromatogram of ombuoside, marker compound isolated from the extract of dried leaves of Gynostamma pentaphyllum.
Additional file 1:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- Endothelin increases the proliferation of rat olfactory mucosa cells
- Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration
- Genetic targeting of astrocytes to combat neurodegenerative disease
- Pathological significance of tRNA-derived small RNAs in neurological disorders
- Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalography
- Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases
