Endothelin increases the proliferation of rat olfactory mucosa cells
2020-09-18BertrandBrycheAudreySaintAlbinClaireLePouponSchlegelChristineBalyPatriceCongarNicolasMeunier
Bertrand Bryche , Audrey Saint-Albin Claire Le Poupon Schlegel Christine Baly Patrice Congar Nicolas Meunier ,
1 Neurobiologie de l’olfaction, Institut National de Recherche Agronomique (INRA), Université Paris-Saclay, Jouy-en-Josas, France 2 Université de Versailles Saint-Quentin en Yvelines, Département de Biologie, Versailles, France
Abstract
Key Words: autocrine factor; cell culture; cellular dynamics; endothelin; olfaction; olfactory basal cells;olfactory epithelium; olfactory mucosa primary culture Chinese Library Classification No. R452; R364; R741
Introduction
The first step of odour detection takes place in the olfactory epithelium. This sensory neuroepithelium is mainly composed of olfactory sensory neurons (OSNs) surrounded by sustentacular cells. It lies on a lamina propria composed of Bowman’s glands, blood vessels, fibroblasts and OSNs axons wrapped in olfactory ensheathing cells. The lamina propria and the olfactory epithelium form the olfactory mucosa(OM). OSNs possess cilia-holding olfactory receptors capable of binding odorants. These cilia are bathed in mucus directly in contact with the environment. They are exposed to oxidative stress, noxious substances and pathogens that gradually damage the OSNs. While neuronal injury in the central nervous system cannot be repaired, the olfactory epithelium undergoes regular apoptosis and proliferation throughout life to maintain the sense of smell (Graziadei,1973; Schwob, 2002; Brann and Firestein, 2014). This unique capacity has given rise to interest in this mechanism as a potential way to regenerate other injured neuronal tissue especially as it involves the targeting of newly formed OSN axons to the central nervous system (Yao et al., 2018). The renewal of the OM relies on the proliferation of two stem cells type:globose and horizontal basal cells which are engaged differently in the process according to the severity of the OM damages (Schwob et al., 2017). Numerous growth and survival factors are involved in this process and studies of their characterization are increasing in number. Many such factors have been described since the review by Mackay-Sim and Chuah (Mackay-Sima and Chuahb, 2000), from pituitary adenylyl cyclase-activating polypeptide (Hansel et al., 2001),to C-type natriuretic peptide, along with the brain-derived neurotrophic factor (Simpson et al., 2002), neurotrophin(Simpson et al., 2003), the leukemia inhibiting factor acting on immature OSNs (Moon et al., 2009), and more recently BDNF (Ortiz-Lopez et al., 2017).
Endothelin (ET) was first characterized as the strongest vasoconstrictive peptide (Yanagisawa et al., 1988) but has since been described as a pleiotropic peptide, acting on various cellular processes including cellular population dynamics in different tissues (Schinelli, 2006). Three peptides of 21 amino acids named ET-1 to ET-3 (Edn1 to Edn3) have been characterized, ET-1 being the most widely distributed (Khodorova et al., 2009). These peptides are secreted as pro-peptides (big-ETs), which are matured locally through peptidase endothelin-converting enzymes (mainly Ece1).They then act autocrinally and/or paracrinally on two different G protein-coupled receptors, named ETAand ETB(Ednra and Ednrb, respectively). The peptides along with their converting enzymes and receptors are expressed in the olfactory epithelium. This expression is particularly strong in the basal cell and neuronal cell layers in 10-dayold rats, with a similar level of expression for ETAand ETB(Le Bourhis et al., 2014). Endothelin acts as an anti-apoptotic factor in the OM (Laziz et al., 2011) but ET-1 is also known to act as a proliferative factor in the central nervous system(Vidovic et al., 2008). In the present study, we explored this potential role in the rat OM using primary culture and in vivo approaches.
Material and Methods
Animals
Male Wistar rat pups (Rattus norvegicus) were obtained from multiparous females, and housed in our local animal care facilities in 12-hour light, 12-hour dark cycles. Breeders were fed standard chow ad libitum. We performed nasal instillations on pups as described previously (Francois et al.,2013), either for one-week starting on day 3 or for 24 hours at the age of 9 days. For the one-week treatment, instillations were performed twice a day (at 9 a.m. and 6 p.m.) with 5 to 8 μL (increasing the volume by 0.5 μL every day) per nostril of either a mixture of endothelin receptor antagonists (BQ123and BQ788, Sigma Aldrich, Saint-Quentin Fallavier, France)at 10-5M in PBS; referred to BQs in the following; n = 13/6 for proliferating cell nuclear antigen (PCNA) and quantitative polymerase chain reaction (qPCR) experiments, respectively) or PBS with 10-4% of NaOH (pH 7.6; vehicle taking into account the initial dilution of BQ788 in 10-2% NaOH; n= 15/7 for PCNA and qPCR experiments, respectively). The higher concentration used in the nasal instillation compared to the treatment of the primary culture took into account the dilution in the mucus, as well as the low amount reaching the olfactory epithelium. Similar differences in treatment effectiveness have been observed among others for vasopressin treatments (Ludwig et al., 2013). For the one-day treatment starting at the age of 9 days, instillations were performed at 9 a.m., 6 p.m. and again at 9 p.m. the next day (n = 5/6 for 10-4% of NaOH/10-5M BQs respectively). Pups were randomly allocated to treatments taking into account their weight (17.4 ± 0.53 g at the age of 9 days) and sex. Pups were euthanized 1h after the last instillation. For the OM primary culture, we used 2 pups for the quantification of OM primary culture confluence, six pups to prepare two independent experiments for the quantification of cellular population by lactate dehydrogenase content, two pups for the ET-1 quantification by enzyme immunoassay (EIA), two pups for reverse transcriptase-qPCR (RT-qPCR) and 6 pups for the calcium imaging.
All animal experiments were conducted in accordance with the European Communities Council Directive 2010/63/EU on the protection of animals used for scientific purposes,and approved by the Comité d’éthique en expérimentation animale COMETHEA (COMETHEA C2EA -45; protocol approval #12-058) on November 28, 2012. Investigators hold the Individual Authorization for Performing Experiments in Animals, including the animal’s experiments conducted in the present study (agreements #78-154/R-94ENVA-F1-12).
OM primary cultures
We used a long-term primary culture of rat OM cells as previously described (Gouadon et al., 2010). Briefly, for each experiment, two to three Wistar rats (7 to 10 day old) were euthanized by decapitation and their entire OM was dissected from the turbinates and the olfactory part of the septum. After enzymatic digestion and mechanical dissociation, the suspension was passed through a 40 μm filter to remove undigested material. After centrifugation through 10% new-born calf serum gradient (NCS; 150 × g,10 minutes at room temperature), cells were suspended in sterile Dulbecco’s Modified Eagle Medium/ HamF12 based medium (DMEM/Ham F12; Eurobio, les Ulis, France) containing 10% NCS (Eurobio). They were plated at a density of approximately 120 cells/mm2on 6-well plates (for qPCR assay), 12-well plates filled with a glass coverslip previously coated with 0.01% poly L-lysine (for confluence evolution measurement and calcium imaging), or 96-well plates (for lactate dehydrogenase (LDH) measurements). Cells were grown at 37°C under 5% CO2. NCS level was reduced to 2%to limit the influence of external growth factors in order to evaluate the impact of ET-1 and endothelin receptor antagonists on cell proliferation.
Quantification of OM primary culture confluence
To measure the degree of confluence of the cellular culture,we took an average of 18 images per glass coverslip for each day of culture from day 2 to day 8. We used ImageJ (Rasband,W.S., ImageJ, U. S. National Institutes of Health, Bethesda,MD, USA, http://imagej.nih.gov/ij/, 1997-2012) to quantify the percentage of total area filled by cells (n = 3 coverslips for each day).
Quantification of cellular population by LDH content
We assessed the growth of the OM primary culture following the different treatments by quantifying the content of LDH in the cells as described previously (Allen et al., 1994;Stander et al., 2018). The LDH concentration is proportional to the rate of NADH oxidation, and we thus measured activities of both the extracellular (related to the level of cellular death) and the intracellular LDH (related to the cellular population). This was respectively measured in the extracellular culture medium and in the primary cultured adherent cells extemporaneously lysed with PBT (PBS + 0.5% Triton X-100). Results were normalized to the control condition(CTL) for each culture (n = 8 from two independent primary cultures).
ET-1 measurement by EIA
ET-1 concentration in the OM primary culture medium was measured using a commercial EIA kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer instructions. Plates were read at 405 nm and compared to a standard curve. Data are presented either as the raw values of ET-1 concentrations or divided by the slope of NADH oxidation in the presence of intracellular LDH content. This last measure accounts for the production of ET-1 relative tothe cellular population (n = 5/4/5 for day in vitro 3 (DIV3),DIV5 and DIV7 respectively from two independent primary cultures).
qPCR
Total RNA was extracted from OM primary culture cells using the Trizol method and subsequently treated with DNase I.OligodT first strand cDNA were synthesized from 5 μg total RNA by the Superscript II reverse transcriptase (Invitrogen,Cergy Pontoise, France) following the manufacturer recommendations. 5 μL of 200-fold diluted cDNA templates were added to a 15 μL reaction mixture containing 200 nM primers (sequences are shown in Additional Table 1) and SYBR Green GoTaq®qPCR Master Mix (Promega, Charbonnieres,France). The expression levels of target genes were measured using the CFX Connect qPCR platform (BioRad, Hercules,CA, USA). A dissociation curve was carried out at the end of the PCR cycle to verify efficiency of primers to produce a single and specific PCR amplification. Quantification was achieved using the ΔΔCt method, and mRNA expression was normalized to the expression level of β-actin (Francois et al., 2016). An efficiency corrective factor was applied for each primer pair.
Calcium imaging
OM primary culture responses to stimulations with ET-1 (10-7M) and adenosine triphosphate (ATP, 10-4M) were monitored using microscopic epifluorescence Ca2+imaging. Cells were loaded with Fura-2 AM, using a dedicated Xcellence RT imaging station (Olympus Soft Imaging Solutions - OSIS,Münster, Germany) as previously described (Gouadon et al.,2010). Standard image pairs of Fura-2 fluorescence, excited at 340 and 380 nm, were acquired at 510 nm at a frequency of 1 Hz, with short exposure times (200 ms) to minimize bleaching, using an UPlanApo oil immersion objective with a 10× magnification. Images were background subtracted and kinetics of Fura-2 fluorescence ratios 340/380 nm of defined regions of interest (ROI) were calculated offline and normalized to the baseline averaged ratio. Changes in fluorescence(F) were calculated relative to the averaged baseline (from 30 seconds before stimulation) and expressed as %ΔF/F ([(FFbaseline)/Fbaseline] × 100) using Xcellence RT software (Olympus Soft Imaging Solutions OSIS, Münster, Germany).
Immunohistochemistry
Immunohistochemistry of OM tissue sections was performed as described previously (Laziz et al., 2011). Briefly,the nasal septum and turbinates were removed as a block and post-fixed overnight at 4°C in 4% paraformaldehyde PBS. Blocks were cryoprotected with sucrose (30%) and cryo-sectioned sagitally (14 μm thickness). Sections were kept frozen at -80°C until use. PCNA staining requires an antigen retrieval in a citrate buffer (pH 6) at 95°C for 30 minutes. Non-specific staining was blocked by incubation with 10% non-immune goat serum diluted in PBS containing 2% bovine serum albumin and 0.3% Triton X-100. The sections were then incubated overnight at 4°C with primary antibodies directed against PCNA (1:200; mouse monoclonal PC10, GeneTex; Tebu-bio SAS, Le Perray-en-Yvelines,France). Fluorescence staining was performed with Alexa-Fluor-488-conjugated goat secondary antibodies (1:1000;Molecular Probes; Invitrogen, Cergy Pontoise, France) for 150 minutes at room temperature. Immunohistochemistry was performed on medial transversal sections of the OM.We took 6 to 8 images located either dorso-medially at the base of the septum or on lateral turbinates similar to a recent study (Hasegawa-Ishii et al., 2017). Images were taken blind to the animal origin at 100× magnification using an Olympus IX71 inverted microscope equipped with an Orca ER Hamamatsu cooled CCD camera (Hamamatsu Photonics France, Massy, France). Images were quantified blind to the animal origin using ImageJ to threshold specific PCNA staining (Laziz et al., 2011). As the antigen retrieval treatment required for PCNA staining does not allow sufficient nuclear staining, we used the auto fluorescence of the olfactory epithelium at 555 nm excitation to measure its area. On each image, we measure the PCNA staining from an OE area averaging ~0.08 mm2. These measurements allowed us to quantify the percentage of PCNA staining area in the olfactory epithelium reflecting the level of proliferation.
Statistical analysis
Group data presented in the text and figures are expressed as mean ± standard error of the mean (SEM). Statistical significance was assessed using non-parametric Mann-Whitney U tests, one-way or two-way analysis of variance (ANOVA)followed by Bonferroni multiple comparison post-hoc tests(GraphPad Prism 5.0, GraphPad software Inc., La Jolla, San Jose, CA, USA). A probability value of P < 0.05 was set for significant differences.
Results
ET-1 acts as a proliferative factor on OM primary culture cells
In order to confirm and quantify a potential proliferative action of ET-1 on OM cells, we treated an OM primary culture with ET-1 at 10-9M every 48 hours from day 0 to day 4 in vitro (DIV; Figure 1A). We used a previously developed primary culture which is growing rapidly for one week in vitro before reaching confluence around DIV7 (Figure 1B).At DIV6, we measured the LDH content related to the cell population (Allen et al., 1994; Laziz et al., 2011). We observed a slight but significant increase in the LDH content after ET-1 treatment (Figure 1C; Mann-Whitney U test, P= 0.016) showing that ET-1 could modify the cellular dynamic of the mucosa, resulting in increased cell density in the culture. Proliferative factors can be provided directly by the primary culture cells (Price, 2017) and big ET-1 and its converting enzyme to ET-1 are expressed by an OM primary culture (Gouadon et al., 2010). We thus hypothesized that a substantial local production of ET-1 could contribute to the primary culture growth. In order to test this possibility,we treated the primary culture with a mixture of BQ123(ETAreceptor antagonist) and BQ788(ETBreceptor antagonist) inthe same conditions from DIV0 to DIV4. For simplicity, this mixture known to efficiently block both ET-1 receptors will be referred to BQs in the following. Treatment with BQs at 10-9M significantly decreased the LDH content after 6 days of in vitro development (Figure 1D; Mann-Whitney U test, P= 0.023). This decrease in intracellular LDH could be related to a BQs-induced antagonism in ET-1 proliferative action.Another possibility relies on a BQs-induced cellular death in the OM primary culture cells. Such cellular death would lead to an increase in LDH present in the extracellular medium (Laziz et al., 2011). We thus measured the level of LDH present in the primary culture medium after BQs treatment,finding great variability in the measurement as it was at the limit of detection (Figure 1E). LDH levels were also similar to the control condition and in the same range as basal LDH activity measured in the cell-free culture medium containing 2% NCS. The absence of cellular LDH release shows that the BQs treatments did not impact the cellular death level in the primary culture. Overall, these results suggest that ET-1 can be produced locally by the OM primary culture cells and actively promote cell growth.
OM primary culture produces ET-1 until reaching confluence
In order to characterize the level of local ET-1 production by the OM primary culture cells, we measured the presence of ET-1 in the medium of culture at DIV3, DIV5 and DIV7 directly by EIA (Figure 2A). ET-1 was not present in the primary culture medium without cells (0.03 ± 0.1 pg/mL, i.e.,0.012 pM; n = 4), but was present at DIV3 (36.9 ± 3.4 pg/mL,i.e., 14.8 pM; n = 5) and DIV5 (37.1 ± 3.1 pg/mL, i.e., 14.9 pM; n = 4). However at DIV7, ET-1 was no longer detectable(0.2 ± 0.5 pg/mL; n = 5). We normalized the level of ET-1 in the medium relative to the cellular density estimated from LDH content to express the production of ET-1 relative to the cellular density (Figure 2B). One-way ANOVA followed by Bonferroni multiple comparison test showed that the ET-1 production significantly decreased from DIV3 to DIV7(P < 0.001). This was consistent with RT-qPCR analysis showing that big-ET1 expression similarly decreased from DIV3 to DIV7 (Figure 2B; P = 0.003). By contrast, the expression of ETAand ETBreceptors increased gradually with culture age (Figure 2C; P = 0.017 and 0.003, respectively).
OM primary culture cells do not respond to ET-1 stimulation until its endogenous production level decreases
Primary culture of OM cells are strongly desensitized after ET-1 stimulation (Gouadon et al., 2010). If ET-1 was secreted in the culture medium by OM cells, then they should not be responsive to ET-1 stimulation until ET-1 level decreases.We recorded from DIV1 to DIV8 the response of the OM primary culture cells to exogenous stimulation by ET-1 (10-7M) and ATP (10-4M) used as a positive control. While some OM cells were already sensitive to ATP at DIV1, they were not responsive to ET-1 before DIV6 and the largest differences between these two agonists were observed at DIV5(Figure 3A and B). Two-way ANOVA analysis revealed significant differences between the percentage of cells responding according to the age of the culture (F(7,43)= 45.74,P < 0.001). The percentage of cells responding between ATP and ET-1 were also significantly different (F(7,43)= 11.46, P< 0.001) especially at DIV5 and DIV6 (Bonferroni multiple comparison post-hoc tests, P < 0.01). While the percentage of responding cells clearly varied according to the culture ages, no obvious effect of treatment was observed for the intensity of the calcium rise in the sensitive cells following ATP or ET-1 stimulation (Figure 3C). Globally, the calcium response intensity increased with culture age which is consistent with the increasing degree of differentiation and maturity of the OM primary culture cells.
Treatment of the OM in vivo with ET receptor antagonists impact on cellular proliferation
To evaluate the potential proliferative role of ET-1 in vivo,we next treated 3-day-old rat pups with intranasal instillations of BQs at 10-5M during 1 week. We then evaluate the proliferation level by immunohistochemistry against PCNA,a marker of dividing cells (Ohta and Ichimura, 2000). We focused on two different zones of the olfactory epithelium based on a recent study showing that nasal instillation was differentially effective according to the OM localization(Hasegawa-Ishii et al., 2017). This epithelium can be divided in three sub-areas with the lowest one containing basal cells and young neurons (Moon et al., 2009). As expected,we observed a staining mainly above the lamina propria(Figure 4A). However, the percentage of PCNA stained area in the olfactory epithelium was not significantly modified by a treatment with a saline solution or the mixture of ET receptors antagonists in both zones evaluated (Figure 4B;Mann-Whitney U test; P = 0.317 and 0.903 for zones 1 and 2, respectively). Wondering if our treatment could impact the local production of ET-1, we measured by RT-qPCR the expression of big ET-1 and ET receptors in these conditions.Big ET-1 mRNA was indeed increased after the BQs treatment (Figure 4C; Mann-Whitney U test; P = 0.004). Expression of ET receptors was not significantly altered despite a slight tendency for a decrease in the expression of ETB in BQ-treated animals (Mann-Whitney U test; P = 0.522 and 0.072 for ETAand ETB, respectively). This result would indicate that the ET system in the OM adapted unexpectedly fast to the BQs treatment, so we next examined the impact of a 24-hour treatment with BQs on 9-day-old rat pups, which were euthanized at the same age as the previous group, i.e.,at 10-day-old (Figure 5A). With this shorter treatment duration, we observed that the proliferation rate was decreased by the BQs treatment in the OM in both zones evaluated(Figure 5B; Mann-Whitney U test; P = 0.004 and 0.017 for zones 1 and 2, respectively).
Discussion
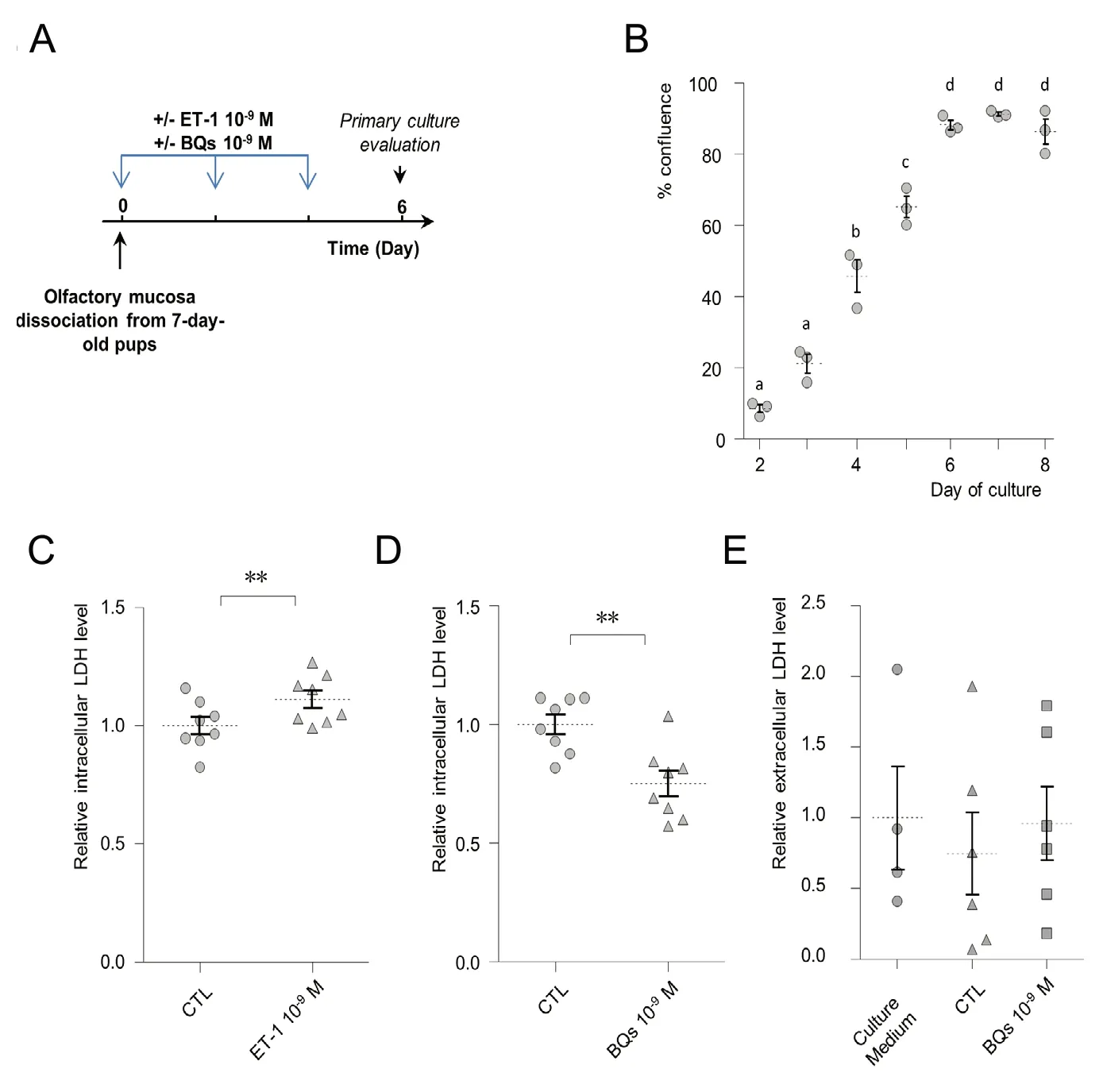
Figure 1 Endothelin-1 (ET-1) is involved in olfactory mucosa (OM) primary culture cell growth.

Figure 2 Endothelin-1 (ET-1) is produced by olfactory mucosa primary culture cells until confluence.
Since the first description of ET-1 as a potent vasoconstrictive peptide (Yanagisawa et al., 1988), multiple roles have been identified for this pleiotropic peptide (Davenport et al.,2016). Its action on cellular dynamics has been extensively described (Komuro et al., 1988), especially in relation to cancer (Bagnato et al., 2011). In the nervous system, endothelin acts as a proliferative factor on astrocytes during gliosis (Gadea et al., 2008) and on neuronal precursors in the cerebellum during rat pups development (Vidovic et al.,2008). ET-1 receptors and its precursor big ET-1 along with its converting enzymes are strongly expressed in the OM (Le Bourhis et al., 2014). As the OM is permanently renewing,we explored here a potential proliferative role of endothelin on OM cells.
Using primary cultures of OM cells, we first found that ET-1 supplementation in the culture medium increased the LDH level related to cellular population. We performed several experiments showing that ET-1 was produced directly by the primary culture. First, we observed that the treatment of the culture with a mixture of ET receptors antagonists(BQ123and BQ788) impaired the culture growth. In previous studies, these antagonists were effective to specifically block the cellular response to ET-1 mediated by both ETAand ETBreceptors (Gouadon et al., 2010). The inhibition of the cellular culture growth by ET antagonist treatments suggests an endogenous production of ET-1 by the primary culture cells.Using EIA, we observed that while absent from the initial culture medium, ET-1 appears in the extracellular medium as soon as DIV3 and its concentration remains stable at DIV5 before decreasing at DIV7. The level of ET-1 concentration at DIV3 and DIV5 was consistent with other studies(Celik et al., 2013; Maffei et al., 2014) supporting the likelihood of its importance in OM cellular dynamics. This was also consistent with RT-qPCR results showing an important expression of big ET-1 at DIV3 and DIV5 which decreased at DIV7. Using calcium imaging, we took advantage of the strong desensitization of OM cells to ET-1 stimulation to indirectly explore whether endogenous ET-1 was present in the primary culture medium. Our results indicate that ET-1 was produced by the OM cells as soon as DIV1 and until DIV6 when the concentration dropped as revealed by the increased sensitivity of the OM cells to external ET-1 stimulation. This increased sensitivity could also arise from an increased expression level of endothelin receptors. However,although their expression increased already at DIV5, the primary culture cells were unresponsive to ET-1 while being sensitive to ATP stimulation. This increase in endothelin receptor levels may reflect the progressive differentiation of OM cells in the culture (Gouadon et al., 2010). The fact that ET-1 was effective to modulate cellular dynamics prior to DIV6 but could not elicit calcium imaging response may seem counterintuitive. However, calcium imaging may not be sensitive enough to record a small increase in cellular activity when supplementary ET-1 was added in the culture medium. Overall, these results are consistent with a production of ET-1 by the OM primary culture until cells reach confluence. The production of proliferative factors by cell culture is a classic feature of conditioned cultured medium(Price, 2017), which has already been described for ET-1(Maffei et al., 2014) and this raises the question of its importance in the OM in vivo.
To answer this question, we used a protocol consisting of 7 days of nasal instillations of endothelin receptor antagonists in rat pups (Francois et al., 2013). Surprisingly, the proliferation rate in the OM was not affected by this treatment.RT-qPCR analyses revealed, however, that the local production of endothelin was increased showing an adaptation of the ET-1 production in the OM following the endothelin receptor antagonists treatments. Shorter BQs treatments were effective in reducing the proliferation cell rate in the OM, which confirms our in vitro observations of a proliferative role of ET-1 in the OM. We used the saline vehicle as a control and thus the observed effect could be a result of a non-specific action of BQs nasal instillations. This is unlikely as the 7-day BQs treatment did not affect the proliferation of the OM while consistently increasing the expression of big ET-1. A recent study showed that nasal instillations were much more effective in the lateral turbinates corresponding to the zone 2 evaluated here (Hasegawa-Ishii et al., 2017).However, we found no differences of the one-day BQs treatment between the zones evaluated indicating that our nasal instillation protocol was effective in reaching both zones. The difference in the results may be related to the turbinate organization and the experimental protocol, as Hasegawa-Ishii and co-workers used anesthetized adult mice while we used awake young rats. The localization of PCNA staining corresponds to the basal cells and young neurons as described previously (Moon et al., 2009; Francois et al., 2016). As horizontal basal cells are usually involved in OE proliferation(Schwob et al., 2017), they are probably the cells impacted by the ET-1 treatment but further experiments are required to confirm it.
ET-1 acts as an anti-apoptotic factor in the OM (Laziz et al., 2011). Therefore the ET-1 impact on the OM primary culture may not be caused by a proliferative action but instead to a decreased apoptosis level following ET-1 treatment. Conversely, the impact of the treatment with endothelin receptor antagonists (BQs) could be related to an increased level of apoptosis. While we cannot completely rule out this possibility, it seems unlikely for several reasons. Firstly, treatment with BQs was effective in reducing the primary culture cell density at DIV6 without increasing the LDH level in the primary culture medium. Extracellular LDH concentration reflects the level of cellular death, indicating that BQs treatment did not increase significantly the apoptosis level while strongly decreasing the cellular population. Secondly, a treatment of 7 days of BQs nasal instillations increases the apoptosis level in the OM (Francois et al.,2013) but we did not observed any impact of BQs on proliferation probably because endogenous production of ET-1 was increased to limit BQs actions. However, we observed a strong decrease of proliferation in the OM after one day of BQs treatment which is consistent with a proliferative role of endogenous endothelin. As all the present experiments were performed on young animals and primary culture, this proliferation may be more related to the development of the animal than regeneration of the OE. Further studies are required to answer this question.

Figure 3 Calcium imaging responses to endothelin-1 (ET-1) stimulation of the olfactory mucosa primary culture.
The regulation of endothelin secretion still remains to be explored. Our observation that BQs treatments are sufficient to increase the level of endogenous expression of big ET-1 suggests that there is probably an autocrine mechanism involved in its regulation in the OM. Such autocrine actions of ET-1 have already been described on keratinocytes (Bagnato et al., 1995) and dendritic cells (Guruli et al., 2004).
Overall, these results show that endothelin acts pleiotropically in the OM as an anti-apoptotic (Laziz et al., 2011)and proliferative factor as well as a GAP junction blocker(Le Bourhis et al., 2014). As the OM is a potential source for cellular grafts to regenerate injured neuronal tissue, the use of endothelin may thus help to improve this therapeutic approach.
Acknowledgments:We would like to thank Birte Nielsen (Neurobiologie de l'olfaction, Institut National de Recherche Agronomique (INRA),Université Paris-Saclay, Jouy-en-Josas, France) for her help in improving the manuscript, Patrice Dahirel and Aurelien Raynaud (Neurobiologie de l'olfaction, Institut National de Recherche Agronomique (INRA), Université Paris-Saclay, Jouy-en-Josas, France) for animal care and treatments;Stéphanie Rimbaud, Denise Grébert, Sacha Muszlak (Neurobiologie de l'olfaction, Institut National de Recherche Agronomique (INRA), Université Paris-Saclay, Jouy-en-Josas, France) and Nicolas Martin (MSc Student, Université Versailles St Quentin, France) for technical help.
Author contributions:Study design and experiment implementation,data analysis and manuscript writing: NM; study design and experiment implementation: BB; experiment implementation and data analysis: ASA;experiment implementation: CLPS; study design: CB, PC; manuscript editing: BB, CB, PC. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was funded by the Institut National de la Recherche Agronomique (INRA).

Figure 4 Impact of 1 week of endothelin receptor antagonists (mixture of BQ123 and BQ788) instillation on the olfactory mucosa.

Figure 5 Impact of 1 day of endothelin receptor antagonist (mixture of BQ123 and BQ788) instillation on the olfactory mucosa.
Institutional review board statement:All animal experiments were conducted in accordance with the European Communities Council Directive 2010/63/EU on the protection of animals used for scientific purposes, and approved by the Comité d'éthique en expérimentation animale COMETHEA (COMETHEA C2EA -45; protocol approval #12-058) on November 28, 2012.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Assitional Table 1: Primers used for qPCR reactions.
杂志排行
中国神经再生研究(英文版)的其它文章
- Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein
- Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration
- Genetic targeting of astrocytes to combat neurodegenerative disease
- Pathological significance of tRNA-derived small RNAs in neurological disorders
- Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalography
- Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases
