Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalography
2020-09-18HamidAbbasiCharlesUnsworth
Hamid Abbasi, Charles P. Unsworth
Department of Engineering Science, The University of Auckland, Auckland, New Zealand
Abstract
Key Words: advanced signal processing; aEEG; automatic detection; classification; clinical; EEG; fetal; HIE;hypoxic-ischemic encephalopathy; machine learning; neonatal seizure; real-time identification; review
Introduction
Perinatal hypoxia-ischemic encephalopathy (HIE) is a severe brain injury that is caused by significant reduction in cerebral oxygen (hypoxia) and reduced perfusion (ischemia) due to various undesirable events at or around the time of birth(i.e., obstruction of the umbilical cord) (Low, 2004; Mwaniki et al., 2012; Jonsson et al., 2014). Neonatal encephalopathy is reportedly shown to be related to HI insults occurred well before or during labor (Takenouchi et al., 2012; Ahearne et al., 2016) putting premature babies under much higher risks(Back, 2015). Although, the survival rates of babies with signs of HIE has been recently improved, neonatal encephalopathy is still shown to significantly contribute to high morbidity rate of 23% of death in newborns, in 2005 worldwide(World Health Organization, 2005) and reported to be improved to ~11% (6.7-16.8%) for under 5 years old neonates in 2013 (Liu et al., 2015). Surviving infants are known to develop neurodevelopmental impairments that is associated to impaired neural network and neuronal loss in white and gray matter cells (Back et al., 2007). Lack of oxygen prevents the energy supply into the cells and disrupts cells function.This happens by progressive cellular depolarization through allowing potassium out of cells and reversely letting calcium,sodium and water into the cells (Kalogeris et al., 2012). This excessive cellular depolarization eventually leads to extracellular accumulation of excitatory amino acids causing a profound drop in the cell activity (Burd et al., 2016). Clinically,it has been shown that most neuronal cells die post HI insult and not during the HI event (Drury et al., 2014; Merchant and Azzopardi, 2015). In fact, perinatal and neonatal HIE is shown to evolve rapidly providing a very short optimal window of opportunity for the neuroprotective hypothermic strategies (Thoresen et al., 2013). Recent clinical reports from asphyxiated newborns strongly emphasize that an early initiation of therapeutic hypothermic protocols within the first three hours of birth, extend out through 48-72 hours,highly contributes in the improvements of the outcomes(Kollmar et al., 2002; Gunn et al., 2005; Edwards et al., 2010;Thoresen et al., 2013; Gunn and Bennet, 2016; Gunn and Groenendaal, 2016).
Conventional electroencephalography (EEG) is a tool to directly monitor and collect the brain’s neuronal activity from the cerebral cortex over scalp. In clinical practice,EEG recording is routinely recorded and digitized in smaller 10-20 minute sections to even longer recordings often lengthy as days; while continuous prolonged recordings are more commonly used hence allowing to identify real-time signatures of various brain disorders such as epilepsy or HIE(Halford, 2009). In practice, interpretation of EEG requires the experience acquired over many years by a technician/clinician (Cooper et al., 2014). This method faces inevitable difficulties to be used on newborn infants; mostly because pediatrics or neonatal EEG specialists are not available at many hospitals for a 24-hour support at the neonatal intensive care units (NICUs) and/or accessibility of equipment can be limited. Instead, due to simplicity of interpretation,EEG recordings through 2-4 limited electrodes are shown to be commonly more practical in newborns (Shah et al.,2008). An early clinical study demonstrated that the intensity of neurological brain injury can be evaluated through analysis of 12 hours of two-channel continuous EEG recordings initiated at 2 hours and 50 minutes, post-birth (Azzopardi et al., 1999). Biagioni et al. (1999) addressed that EEG features evaluation through differentiation between burst- and non-burst suppression EEG intervals could be a potential biomarker to grade severity of HIE. Since then, monitoring of EEG signals is increasingly recognized as a useful methodology to identify potential biomarkers of HIE (Pavlidis et al., 2017). Over the past two decades, a variety of signal processing strategies have been developed by researchers across the world for automatic analysis and diagnosis of various brain disorders through studying of abnormal activity using different types of the EEG recordings such as conventional EEG, amplitude-integrated EEG (aEEG), quantitative EEG and intracranial EEG. Guidelines for neonatal EEG monitoring are comprehensively described in (Tsuchida et al., 2013).
In this review, various strategies (i.e., Google scholar,Scopus and other scientific platforms) were used to extract the research in the literature, we will firstly introduce the reader to HIE EEG seizures then to the automated strategies used for the identification of seizure-like events in human neonates, developed by different research groups across the world. This paper will review manuscripts since 1990-2018 with an emphasis on the most recent developed techniques that use larger datasets which employed better data acquisition technology at higher sampling resolutions.
Hypoxic Ischemic Epileptiform Electroencephalography Seizures
In EEG studies, the deviation from normal activity is known as abnormal EEG events/transients (Engel, 2013; Silverstein and Jensen, 2007). Epileptiform events, in general, contain important neurological information and appear with different morphologies which are often very similar to the normal background EEG or artifacts. Epileptiform seizures have been investigated as EEG signatures for diagnostic of epileptic disorders (Angeles, 1981; Westmoreland, 1996; Binnie and Stefan, 1999). Neonatal seizures are generally defined as abrupt EEG discharges with different profile characterizations in durations (less than a minute to a few minutes), frequencies (0.3-2.0Hz)and amplitudes (25-700 μV) (Bye and Flanagan, 1995; Mizrahi, 1998; Patrizi etal., 2003; Shellhaas and Clancy, 2007; Greene et al., 2008a). Post-HI neonatal EEG recording from 3 hours to days after birth demonstrate that perinatal hypoxic-ischemic encephalopathy is associated with delayed high amplitude epileptiform seizures in a suppressed EEG background (Hill and Volpe, 1981;Williams et al., 1990; Glass et al., 2009). Automated analysis have also shown that the post-ischemic neonatal seizures are strongly correlated to adverse outcomes (Biagioni et al.,1996; Miller et al., 2002; Shalak and Perlman, 2004; Glass et al., 2009; Björkman et al., 2010; Uria-Avellanal et al.,2013; Kang and Kadam, 2015; Pisani and Spagnoli, 2016).Research indicate that recurrent epileptic seizures can be potentially predicted through analysis of epileptiform events
Methods Used for Hypoxia-Ischemic Epileptiform Events Detection
(Litt and Echauz, 2002; Soleimani-B et al., 2012). Current clinical attempts around HI seizure detection have mainly concentrated on the automatic identification and classification of high amplitude neonatal seizures which are shown to emerge in the signal only when the neuroprotective window of opportunity has passed. In fact, appearance of the high amplitude seizures in the EEG, post-HI, debatably indicates that the infant has likely progressed well beyond the optimal window of opportunity for treatment (Gluckman et al.,2005; Gunn and Bennet, 2008). The high amplitude neonatal seizures are reported to be delayed in some neonates(Lynch et al., 2012) and are not always measured accurately since seizures may not always propagate to the cortex (Naim et al., 2015). Thus it would be difficult to determine where exactly in time a newborn with signs of HIE could be, or even if they are still in the optimal window of opportunity for treatment.
In general, to develop an automated strategy, an expert has to visually assess many hours of the recordings and initially annotate the EEG intervals for the desired type of epileptiform event. This difficult and time-consuming but very important task requires essential skills and experiences as the EEG data are usually recorded with lengths up to a few days.Definitions of EEG events has been often seen to vary among EEG experts/interpreters (Webber et al., 1993). Here, automated computational strategies can significantly contribute in saving time in the analysis of prolonged recordings only if they could robustly identify/classify the clinically-important epileptiform events with acceptable accuracies close to an expert. Current automated epileptiform seizure detection algorithms applied to clinical recordings require the identified events to be reviewed by an expert/clinician due to considerable rates of false detections of the algorithms. The importance of automated techniques becomes even more highlighted in HI studies where the window of opportunity for treatment is critically short (Gunn and Drury, 2013;Thoresen et al., 2013). Attempts around automatic identification of HI epileptiform events are detailed in the following.
Automatic neonatal seizure detection strategies in the EEG have been reported since 1992 (Liu et al., 1992). Considerable improvements of the data acquisition tools alongside the advances in computer technology have significantly enhanced the accuracy of automated seizure detection methods in the neonatal HI EEG (Korotchikova et al., 2011). In reality, an ideal automated neonatal seizure detector must result in higher sensitivity measures so that it detects a higher number of true events and satisfies lower false detection rates so that at risk neonates even with a few seizures are not missed. Such objectives are challenging because neonatal EEG seizures are observed with intensely various morphology and background artefacts can highly affect both manual and automatic decision makings. Since early 2000, a valuable collection of analytical attempts have been investigated by the neonatal brain research groups across the world for the automated seizure identification in HI EEG. One important thing to consider when automatic seizure detection algorithms are compared is that there is still a lack of generic definitions on performance measures. However, good detection rates (GDR) and higher sensitivity measures related to manual annotations by expert neurophysiologists have been sought in most of the automatic seizure detector algorithms.False detections are also very important for the validation of algorithms which may affect recordings with longer durations if the original algorithm is trained using shorter lengths of recordings. Clinically, a reliable seizure detector algorithm to be used in NICUs must be able to robustly identify the desired events in data with a length of at least 72 hours (Mathieson et al., 2016).
The rest of the review is now dedicated to describing the neonatal seizure detection approaches investigated by researchers at different groups throughout the world. Complementary information about the discussed studies such as the number and age of subjects, type and total number of studied epileptiform event, length of recording, data collection strategy, sampling frequency as well as performance measures related to each study are detailed in Tables 1 and 2.
Belgium and the Netherlands
In 2004, researchers from the Netherlands investigated the application of a synchronization likelihood approach in raw un-filtered EEGs (without removing artefact) from twenty neonates (3 preterms and 17 terms) for the identification of EEG epochs containing epileptic seizures (Smit et al., 2004).Smit et al. (2004) correlated the results of their non-linear analysis approach with the visual assessments from 3 experts that resulted in sensitivity and specificity measures of 65.9%and 89.8% for seizure detection. They have reported a detection rate of 100% for the longer seizures with lengths of at least 100 seconds (Smit et al., 2004). A comprehensive primer to the synchronization likelihood technique can be found in (Stam and Van Dijk, 2002).
Two lead aEEG is a widespread conventional tool at NICUs that is obtained from highly filtered and rectified standard EEG and displayed on a semi-logarithmic scale.Using a Cerebral Function Monitor device (CFM Lectromed) over sixty eight asphyxiated term babies, Toet et al. (1999) reported that early assessment of aEEG signals from term infants at their first 3 and 6 hours of birth, might provide useful information to identify HIE at risk neonates.Toet et al. (1999) reported positive and negative predictive values (PPV and NPV) of 78% and 84%, respectively, at 3 hours after birth and PPV and NPV measures of 86% and 91%, respectively, at 6 hours of birth. However, aEEG is not suggested to be a suitable framework for time-localization of real time EEG events (i.e., seizure activity or fast seizure-like events that occur at lower amplitudes) as it can only provide limited information on the overall changes of activities (Toet et al., 2002). In 2007, Lommen et al. from the Netherlands investigated the capability of automated trained algorithms for seizure detection with a duration of > 60 seconds in aEEG recordings collected using the Olympics CFM6000 machine. On average their data set was collected at 40.8 hours of birth from 13 babies (10 diagnosed with asphyxia).Their algorithm was trained using recordings from five babies and tested over the recordings from eight other babies.Lommen et al. (2007) reported a sensitivity of ≥ 90% for the identification of seizures in the recording sets containing clear neonatal seizures resulting in a low false positive rate of 1 seizure per hour. Their algorithm was claimed to have potentials as an alarm function for the CFM monitor device.
Deburchgraeve et al. (2008) demonstrated that the human observer-based definition of neonatal seizures characteristics could be defined for the automatic identification of two major neonatal seizure types, namely the spike train seizures and oscillatory seizures. Each measurement in their dataset of 21 term infants was started within 24 hours from birth and continued for 24-48 hours. Deburchgraeve et al.(2008) introduced a combination technique based on autocorrelation, non-linear energy operator (NLEO) and wavelet decomposition to improve the detection ability of their method, resulted in an overall sensitivity of 88% with the false positive rate of 0.66 per hour tested over 217 hours of EEG recordings. A comprehensive primer to the NLEO and the correlation analysis techniques used in Deburchgraeve’s study can be found in Kaiser (1990) and van Putten and van Putten (2007), respectively.
Deburchgraeve et al. (2009) demonstrated that higher-order canonical decomposition or parallel factor analysis was beneficial for the localization of the electrical potential distribution of neonatal cortical seizures that appear in the form of either oscillatory seizures or spike train activity,post-HI event. Similar to their previous study in 2008, measurements in their data set were initiated within 24 hours of birth of six asphyxiated term neonates. Deburchgraeve et al.(2009) demonstrated that their algorithm can be embedded within the current seizure detection devices in the NICUs to monitor information such as seizure burden, length, seizure quantification and spread to contralateral hemisphere. A comprehensive primer to the parallel factor analysis decomposition analysis can be found in (Miwakeichi et al., 2004).
Researchers from the Netherlands have also investigated the superiority of two-channel aEEG recordings over single-channel recordings for automated seizure activity detection using a wave-sequence analysis technique (van Rooij et al., 2010). Using 15 full-term neonates of age 37-41 weeks,Rooij et al. (2010) demonstrated that two channel aEEG recordings will be superior to one-channel aEEG analysis,providing better detailed information for the identification of seizures that are associated to the affected side of the brain. To do so, Rooij et al. (2010) employed Navakatikyan’s wave-sequence analysis algorithm for the automatic detection of neonatal seizures that resulted in a sensitivity of 65%tested over 2150 hours of aEEG recordings (Navakatikyan et al., 2006).
Cherian et al. (2011) from the Netherlands validated the performance of an improved version of Deburchgraeve et al‘s automated seizure detector in 2008 (called NeoGuard) over an independent EEG dataset of 756 hours collected from 24 neonates (22 terms and 2 preterm/near-term) at 30-35 weeks of age in NICU (Cherian et al., 2011). Two parallel running detectors were designed in the NeoGuard for simultaneous identification of spike-trains containing high energy EEG segments as well as intervals containing oscillatory seizure activity resulting in a total sensitivity of 61.9% and false positive rate of 0.28 per hour. Cherian reported that alteration of EEG characteristics such as amplitude, rhythmicity and duration in relation to EEG background deterioration reduces the performance of the automated seizure detector.
Ansari et al. (2016) introduced a multi-stage support vector machine (SVM)-based heuristic neonatal seizure classifier that is equipped with a data-driven post processing unit that uses a novel set of seizure-relevant features for final decision making. Ansari et al. (2016) reported improved performances when the post-processing technique was used in their heuristic algorithm by obtaining lower false alarm rates(FAR) tested over 1023 hours of EEG-polygraphy recordings containing 3493 seizures from 71 neonates. Ansari et al’s seizure detector resulted in a GDR of 88% with a FAR of 3.81/h. A comprehensive primer to the SVM technique can be found in (Scholkopf and Smola, 2001). Continuing their work, in 2016, Ansari et al. (2017) developed a third stage to their automated seizure recognition algorithm that receives a neurologist’s feedback and adaptively retunes a threshold parameter that was shown to improve the performance criteria of their detector (i.e., FAR and PPV) especially in the identification of brief seizures. Using 977 hours of recordings from 17 neonates Ansari et al. (2018b) demonstrated that the good detection measure of their improved algorithm remained unchanged while the FAR was decreased to 2.48/h.Later, Ansari et al. (2018b) addressed the limitations of current metrics such as FAR and GDR when seizure events are rated based on the majority of votes from multiple experts.Using bootstrapping test, Ansari et al. (2018b) suggest that a multi-scoring strategy on the manually identified event can be taken into account in order to consider the agreements of the expert for the detected event by the automated seizure identifier. Ansari et al. (2018b) demonstrated that more realistic results can be obtained through bootstrapping for the commonly used metrics such as FAR, GDR, and PPV, selectivity, sensitivity and specificity when such a strategy had been considered. Assessing 353 hours data including 4980 seizures from 81 neonates, Ansari et al‘s heuristic algorithm contains two parallel algorithm which the first algorithm identifies spike trains by comparing the maximums of nonlinear energy to the background activity in the signal and decides if sufficient amount of spikes are found in a sequence.The second algorithm uses discrete wavelet transform to decompose the EEG and uses the delta and theta frequency bands to mark potential intervals that the energy of the signal peaks allowing to identify oscillatory type seizures. Ansari et al. (2018a) reported a sensitivity, specificity, selectivity,and overall GDR of 78.1%, 90.5%, 59.2%, and 95%, respectively, for their heuristic detector with a FAR of 3.14/h. In a very recent work, Ansari et al. (2018a) introduced a heuristic method based on the combination of convolutional neural networks and random forest to identify neonatal seizures in 74 hours of recordings from 22 neonates of age greater than 36 weeks. It is claimed that the classification ability of the purposed algorithm is improved through substitution of the final classifying layers by a random forest block compared to other conventional classifiers such as linear discriminant analysis and SVM. Ansari’s seizure detector is suggested to minimize the manual interaction of an expert by automatically extracting the required features of the data to be used in training of the network resulting in a total accuracy of 77%with a FDR of 0.63 per hour. A comprehensive primer to the convolutional neural network and random forest techniques can be found in (LeCun and Bengio, 1995) and (Liaw and Wiener, 2002), respectively.
Ireland
Greene et al. (2008) from the University College Cork in Ireland initially reported best performing features from quantitative EEG to be used in a linear discriminant classifier for neonatal seizure detection. A linear discriminant classifier discriminates between classes by finding the optimized linear combination of the defined features. A comprehensive primer to the linear discriminant classifier technique can be found in (Kuncheva, 2004). Using a cohort of 17 full term infants at the age of 39-42 weeks, Greene et al. (2008) have reported that the RMS amplitude, the line length and the number of local extremums are the most useful features to be used to differentiate between seizure and non-seizure segments. However, they claimed optimum sensitivity and specificity results of 81.08% and 82.23%, respectively, when all features were combined and fed together into their developed classifier.
Also, Greene et al. (2008) demonstrated that an optimized early-integration configuration of parameters across all EEG channels in an automated regularized discriminant classifier architecture outperforms for patient-independent neonatal seizure detection compared to their two other developed models based on linear discriminants and quadratic discriminants. Greene et al. (2008) validated their automated regularized discriminant classifier using 14.8 hours of recordings from 17 full term infants at the age of 39-42 weeks which resulted in sensitivity and specificity measures of 33.17% and 95.99%, respectively.
In 2010, Thomas et al. (2010) from the same team investigated the performance of a Gaussian Mixture Model (GMM)-based algorithm for seizure detection in post-HI 256 Hz neonatal EEG of 20 full-term babies. Thomas analysed the choice of parameters for an optimal performance that could maximize the receiver operating characteristic (ROC)area which their designed neonatal seizure detector could provide. Thomas et al’s GMM-based algorithm resulted in an ROC area and GDR of 95.6 ± 2.9% and 79%, respectively,with a FDR of 0.5 per hour. They have also provided a detailed primer to the Gaussian mixture model technique used in their study.
Temko et al. (2011a) from Neonatal Brain Research Group at University College Cork demonstrated that machine learning-based approaches such as SVM provided considerable performance improvement for the classification between seizure and non-seizure EEG epochs that can be used for reliable interpretation of events in the hypoxic-ischemic EEG in in NICUs. Temko et al‘s SVM-based algorithm was tested over 267 hours of recordings from 17 full term neonates at the age of 39-42 weeks resulting in an ROC area of 96.3%, sensitivity and specificity of 90% and an average GDR of 89% with an average FDR of 1 per hour.
Low et al. (2011) from the same affiliation investigated the performance validation of Temko’s neonatal seizure detector algorithm (trained over 17 term neonates) for the interpretation of EEG recordings using a new dataset collected at the NICU from 41 term babies with signs of HIE. Low et al. (2011) claimed that the accuracy level of their neonatal seizure detector algorithm is approaching to the clinical acceptable values in the NICUs resulting in an ROC area of 95.4% with a seizure detection rate of 60% and a FDR of 0.1/h.However, they report that the FARs still needs to be reduced in order to improve the performance.
Temko et al. (2012) aimed to implement the novel clinical understanding of the evolution of post HI neonatal seizures to amend the performance of their previously designed seizure detector algorithms. They investigated this through using probabilistic weights associated to temporal locations of seizures corresponding to time of birth that was fed into their previously developed SVM-based seizure detector using 816.7 hours of data from 18 full term new-borns at the age of 39-42 weeks. Temko et al. (2011a) reported that the ROC area of the improved seizure detector was increased to 96.74% with a correct seizure detection rate of 70% while the FDR was significantly decreased to 0.25/h by adding a “prior”block to the detector’s structure compared to their previous work. In 2013, researchers from the same group demonstrated that the performance of their developed SVM-based seizure detector was increased when an adaptive probabilistic EEG background modelling was embedded into their suggested algorithm in 2011 (Temko et al., 2011a), resulting in an ROC area of 96.1% and a correct seizure detection rate of 70% at the cost of 0.24 false detections per hour (Temko et al., 2013). Their study in 2013 used a dataset of 2540 hour recordings from 51 full-term neonates at the age of 39-42 weeks. Temko et al. (2015) compared the performances of three different strategies namely 1, a binary output; 2, a probabilistic trace; and 3, seizure’s spatio-temporal colormap for neonatal seizure detection and advantages and disadvantages of each method was discussed. They suggested that a combination of a binary output and a probabilistic trace method provides a suitable framework to determine the output of their suggested seizure detector at the clinical acceptable range. Mathieson et al. (2016) from Neonatal Brain Research Group in Ireland investigated the performance validation of Temko’s seizure detection algorithms, developed in 2011 and 2013, on a larger unseen clinical dataset from 70 term babies and collected at Cork University Maternity Hospital (Temko et al., 2011b) and University College London Hospital. From the 70 near-term and term neonates at the age of ≥ 37 weeks,35 babies were selected from a sham control group without seizures and 35 babies with seizures totalling 4060 hours of recordings. Mathieson reported an overall performance of 52.6-75.0% and FDRs of 0.04-0.36/h for the purposed seizure detection algorithms. In 2016, Mathieson et al. (2016)reported performance enhancements to Temko’s original alpha version of automated neonatal seizure classifier (Temko et al., 2011b) when the algorithm was equipped with a novel neurophysiology-based technique for further comprehensive analysis. Mathieson et al. (2016) demonstrated that the extra rigorous analysis allows for better estimation of key seizure features that significantly improves the performance of ANSeR algorithm which led to a beta version of “Algorithm for Neonatal Seizure Recognition” (ANSeR). The ANSeR algorithm is currently being clinically validated across NICUs in Europe. ANSeR was tested using a dataset of 1263 hours of recordings from 20 term neonates (10 from sham group with no seizure and 10 with seizure) resulting in a seizure detection rate of 60.64% at threshold 0.4 with a FDR of 0.52/h.Mathieson et al addressed that the majority of wrong detections of the ANSeR algorithm occurs at the place of artefacts and the extremely rhythmic EEG background.
Finland
Tapani et al. (2017) demonstrated that incorporating EEG features, adapted from spike correlations, using a smoothed non-linear energy operator in conjunction with EEG features from the Temko’s original seizure classifier (Temko et al., 2011b), into an SVM-based neonatal seizure detector results in clinically acceptable performances. Tapani et al’s seizure detector resulted in a median area under curve (AUC)of 98.1% tested on 112 hours of recordings from 79 full-term neonates. Following their work in 2017, Tapani et al. (2018)incorporated combination of adapted estimations of spike correlations (in both time and time-frequency domains)for non-stationary periodic feature extraction to be fed into their SVM-based neonatal seizure detector for the classification between seizure and non-seizure intervals. From a dataset of 79 full term babies, they calculated the performance measures for their seizure detector only for 39 patients with consensus seizures of at least 10 seconds long resulting in an AUC of 98.8% with seizure detection rate of 86.6% at a cost of 1 false detection per hour.
Other groups
In 2003, researchers at the hospital for sick children in Toronto in collaborations with researchers in Japan and Iran demonstrated that wavelet transform can be a suitable spectral analysis tool for the detection of neonatal seizures and characterization of their epileptic component (Kitayama et al., 2003). Using a group of 15 preterm to term new-borns aged 37.1 ± 4.6 weeks, they reported that the Wavelet Transform was able to detect sustained dominant spectral components within 40 EEG seizures out of a total of 69 neonatal EEG seizures with lengths of longer than 10 seconds resulted in a detection rate of 58%. Kitayama et al. (2003) suggest that sustained dominant spectral components can help to predict post-neonatal epileptic seizures with an onset that could vary from birth to many days.
In 2004, researchers from Queensland University of Technology in Australia introduced a time-frequency method based on spectral feature extraction to identify neonatal EEG seizures in both low (< 10 Hz) and high frequency (> 70 Hz)bands in a cohort of 5 neonates (Hassanpour et al., 2004).Their algorithm resulted in a GDR of 92.6% at the cost of 3.8 false detections per hour.
In 2006, researchers from BrainZ instrument Ltd. and Liggins Institute of New Zealand in collaboration with perinatal researchers and paediatricians from Australia and the United states introduced a wave-sequence analysis based automated technique for real-time neonatal seizure detection using a parallel EEG fragmentation strategy (Navakatikyan et al.,2006). The ‘Recognize’ algorithm by Navakatikyan used by the Brainz aEEG monitor (Natus Medical Inc., USA) employs a 2 channel EEG technology. Navakatikyan et al. (2006)reported sensitivity, specificity and selectivity performance measures of 94.5%, 93.7% and 76.6%, respectively, with an FDR of 2 examined over ~5 hours of recordings containing seizures from 17 full term neonates at the age of 39-42 weeks. In a recent work in 2017, researchers from Australia examined the accuracy of neonatal seizure detection between 2-channel amplitude-integrated EEG and the conventional video EEG using at risk infants (35 near to full term infants of age ≥ 35 weeks - only 7 with seizures), suggesting that amplitude-integrated electroencephalography is a poor screening tool for seizure detection (Rakshasbhuvankar et al., 2017). In their study, amplitude-integrated EEG was performed using BrainZ monitor (BRM2; BrainZ Instruments, Auckland, Wellington, New Zealand) equipped with embedded neonatal seizure detector software (RecogniZe,Natus Medical Incorporated, Pleasanton, CA), while video EEG was recorded through Compumedics equipment and analysed using PSG software (Compumedics, Abbotsford,Victoria, Australia). Rakshasbhuvankar et al. (2017) reported a very weak detection rates of 33.7% and 53.2% for sensitivity and selectivity measures, respectively (only 57 out of 169 seizures) using amplitude-integrated EEG.
In 2007, researchers from France demonstrated that a combination technique using a rule-based decision-making system and a multilayer back-propagation artificial neural network classifier could be beneficial for seizure recognition with a promising low false detection rate (Aarabi et al.,2007). They reported sensitivity, specificity, selectivity and GDR measures of 74%, 85.6%, 70.1%, and 79.7, respectively,with an average FDR of 1.55 per hour tested over 86 hours of recordings from 10 full term neonates at the age of 39-42 weeks. A comprehensive primer to the multilayer artificial neural network classifiers can be found in previous studies(Abraham, 2005; Gurney, 2014).
In late 2008, researchers from Sweden demonstrated that a SVM fed with five input extracted features from the EEG signals outperforms for the classification of bursts from suppression EEG intervals compared to an artificial neural network or a Fisher’s linear discriminant (Löfhede et al.,2008). Löfhede et al. (2008) reported an AUC of > 90% for their SVM-based algorithm using data from 6 neonates aged 39-42 weeks.
In 2009, researchers from Houston in the United States demonstrated that a three stage automated algorithm with an EEG measures-based qualification final stage can highly distinguish candidate seizures with widely varying morphology (Mitra et al., 2009). Mitra et al’s multi-stage algorithm was able to detect seizures with durations of at least 10 seconds with an average overall sensitivity of 79.8% and an FDR of 0.78/h along 34 hours of recordings from 28 full term subjects aged 39-42 weeks.
Lawrence et al. (2009) from France investigated the feasibility of a neonatal seizure detector in limited-channel hypoxic-ischemid aEEGs and compared the results to conventional EEG-video recordings. They used 2708 hours of recordings from 40 near- to full-term infants of aged ≥36 weeks. They demonstrated that the performance of the software-based seizure detector improved from 55% for the detection of all seizures to 87% for the detection of seizures with longer durations of > 60 seconds only.
A few other attempts, between 2009 and 2014, have developed automated algorithms for grading HIE through analysis of background EEG as well as identification of neonatal inter-burst EEG intervals in infants with signs of HIE (Deburchgraeve et al., 2009; Matic et al., 2012, 2014, 2015).
Conclusion
This article highlights the recent advances in the automatic identification of neonatal epileptiform seizures in the post HIE EEG recordings developed by different research groups using signal processing and machine learning techniques.Examining literature from 2000 to 2018, this survey discussed a variety of methods ranging from basic techniques such as wave-sequence analysis (Navakatikyan et al., 2006), pattern characteristic-based analysis (Lommen et al., 2007), correlation/autocorrelation (Deburchgraeve et al., 2008) and adaptive thresholding (Ansari et al., 2017) to more complicated techniques such as time-frequency (Hassanpour et al., 2004)and wavelet-based analysis (Kitayama et al., 2003), artificial neural networks (Mitra et al., 2009) and energy and spectral component analysis (Cherian et al., 2011). The article also detailed the application of more advanced techniques such as SVM combinational techniques (Löfhede et al., 2008; Temko et al., 2011a, 2012, 2013, 2017; Low et al., 2011; Ansari et al.,2016; Mathieson et al., 2016; Tapani et al., 2017, 2018), Gaussian mixture model (Thomas et al., 2010) and convolutional neural networks (Ansari et al., 2018a) for the identification of neonatal seizures. Among all, SVM-based methods (Temko et al., 2011a, 2012, 2013, 2017) as well as Ansari’s heuristic detector (Ansari et al., 2018b) alongside with his convolutional neural network-based approach (Ansari et al., 2018a),introduced in 2018, demonstrated superiority in the classification of seizures from non-seizure intervals. These methods resulted in considerable smaller false alarm rates tested over much larger clinical datasets from neonates with signs of HI at birth. Despite the significant advances in the improvement of automated techniques, false detections have been remained a substantial challenge for the automated techniques to be fully accepted by the clinicians. Acquiring lower false detection rates are naturally challenging due to the various morphologies of seizures in neonatal EEG after an HI event. Detailed information of the discussed techniques is supplied in Tables 1 and 2 at the end of the manuscript.
On reflection of the research that has been performed on neonatal EEG with advanced signal processing and machine learning, it should be noted that all the current techniques applied to neonatal EEG studies have been solely concentrated on the identification of high amplitude seizures. One consideration for the future is to investigate other forms of transients that exist in the EEG such as spike waves, sharp waves, slow waves, micro-scale transients and complexes that have currently not been explored in human neonates but have been found to provide useful biomarkers correlated with injury in animal models (Abbasi et al., 2014, 2015,2016, 2017, 2018, 2019a, b c). In addition, typical sampling rates used in neonatal EEG are usually no higher than 256 Hz which could serve to limit the resolution, reduce detection and dramatically increase FAR of such biomarkers and other transients that exist in the neonatal EEG. Thus, another suggestion would be to move current sampling rates to be greater than 1024 Hz which has been shown to provide considerable performance improvement in EEG biomarker identification in animal studies (Abbasi et al., 2014, 2015, 2016, 2017, 2018,2019a, b, c). Finally, all seizure detection in the neonatal EEG has been performed post initial seizure, namely after the latent phase of injury has occurred (and the window of opportunity is passed). Whilst data is sparse in the latent phase of injury for human neonates, it would still be useful to endeavor to analyze EEG transients in this region which has been shown to provide useful information in animal models(Abbasi et al., 2014, 2015, 2016, 2017, 2018, 2019a, b, c) for the advanced prediction of initial seizure onset.
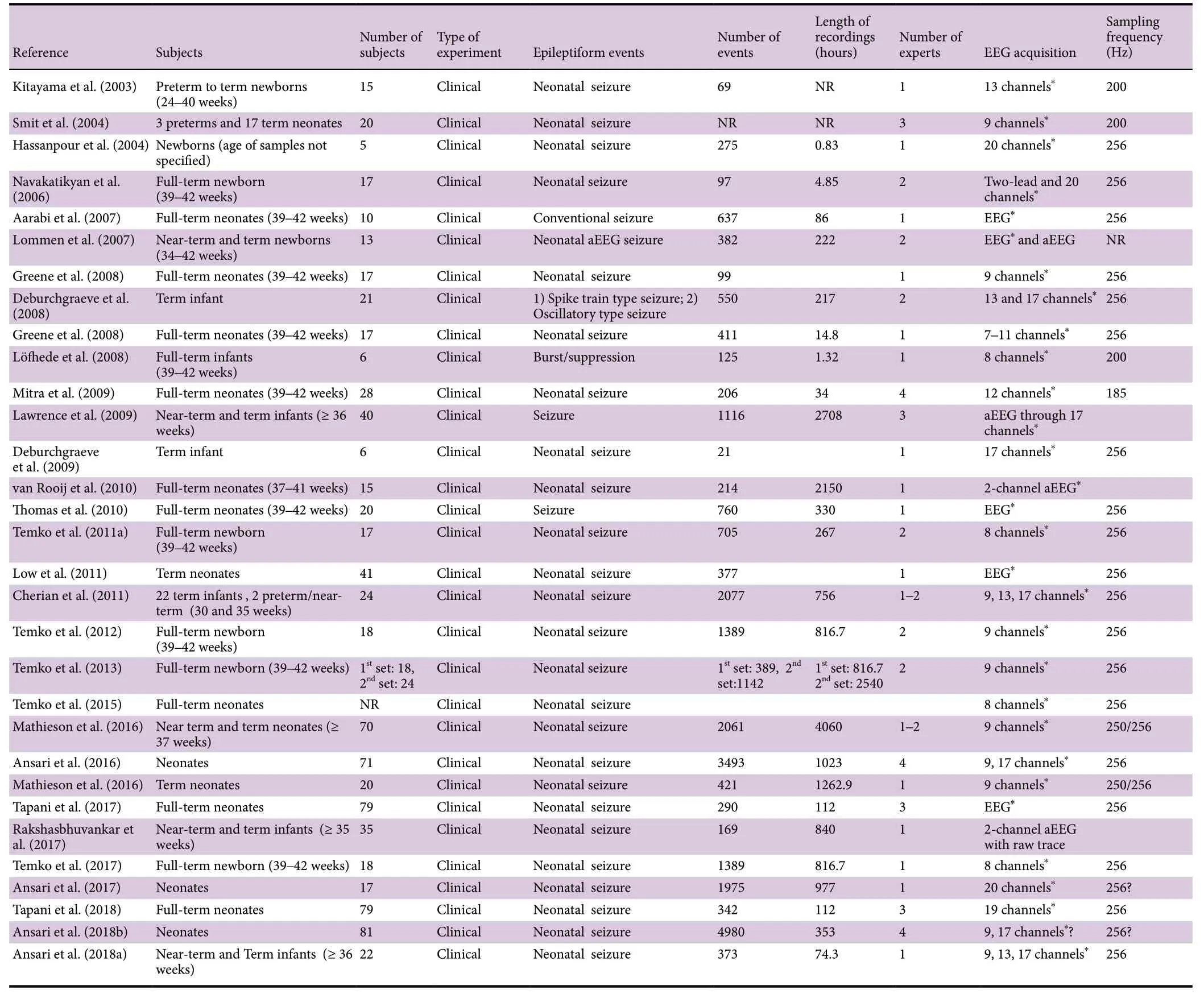
Table 1 Automated strategies on the detection of epileptiform seizures post a hypoxic-ischemic event (continued in Table 2)
Author contributions:Literature retrieval and data collection: HA;manuscript preparation: HA, CPU; manuscript writing: HA; manuscript review: CPU. The final submitted article has been revised and approved by the authors.
Conflicts of interest:We declare no conflicts of interest.
Financial support:This work was supported by the Auckland Medical Research Foundation, No. 1117017 (to CPU).
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
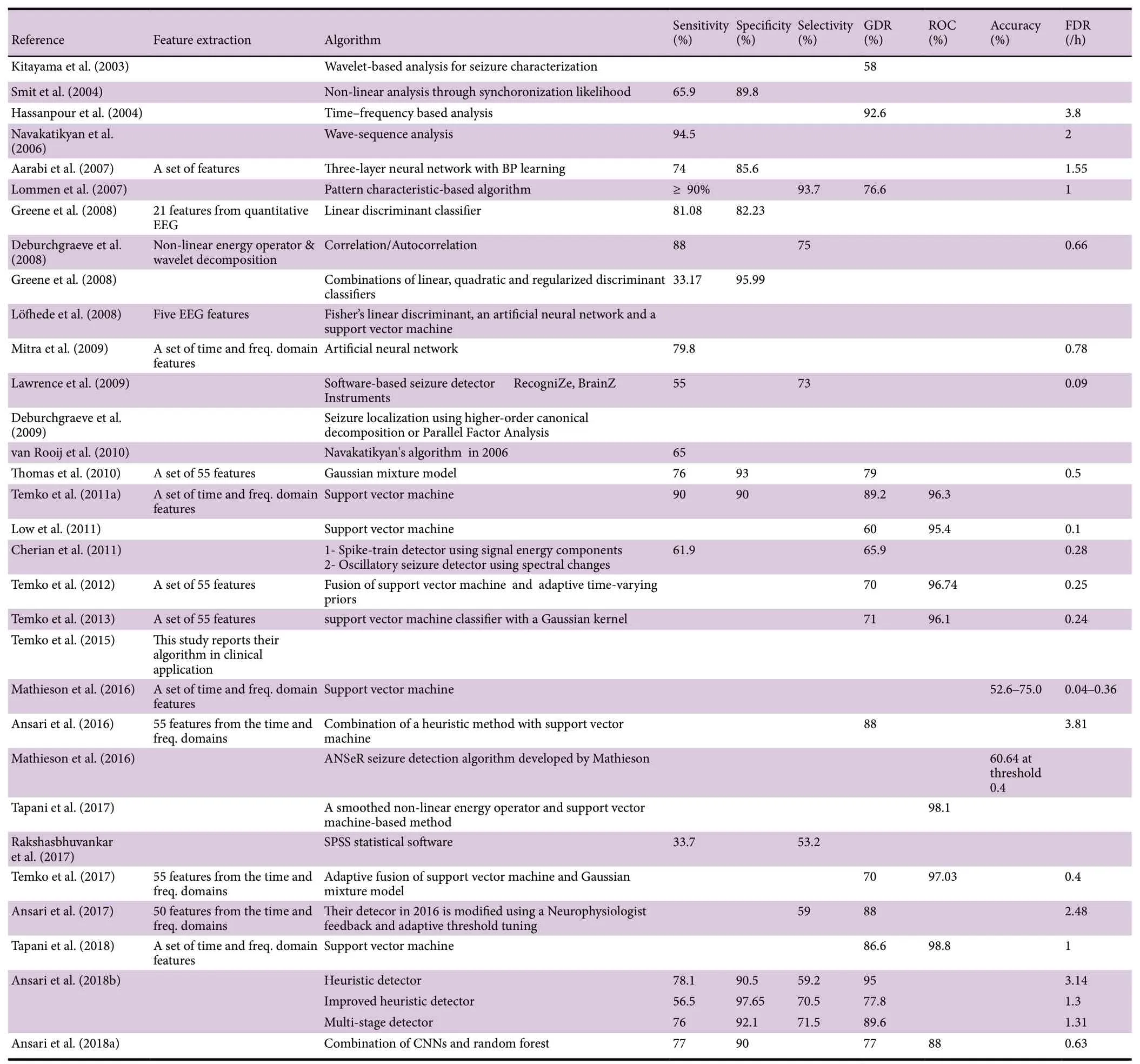
Table 2 Automated strategies on the detection of epileptiform seizures post a hypoxic-ischemic event (continued from Table 1)
杂志排行
中国神经再生研究(英文版)的其它文章
- Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein
- Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration
- Genetic targeting of astrocytes to combat neurodegenerative disease
- Pathological significance of tRNA-derived small RNAs in neurological disorders
- Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases
- Current status and future prospects of stem cell therapy in Alzheimer's disease
