Current status and future prospects of stem cell therapy in Alzheimer's disease
2020-09-18FuQiangZhangJinLanJiangJingTianZhangHanNiuXueQiFuLinLinZeng
Fu-Qiang Zhang , Jin-Lan Jiang , Jing-Tian Zhang, Han Niu, Xue-Qi Fu, , Lin-Lin Zeng,
1 Scientific Research Centre of China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China 2 School of Life Sciences, Jilin University, Changchun, Jilin Province, China
Abstract
Key Words: Alzheimer's disease; β-amyloid; drug development; embryonic stem cells; induced pluripotent stem cells; mesenchymal stem cells; nerve regeneration; neural regeneration; neural stem cells; neurodegenerative disorders; stem cell therapy
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder clinically characterized by loss of memory and cognitive dysfunction. It is the most common neurodegenerative form of dementia, accounting for 50-70% of these cases. The number of patients with dementia was estimated to be 46.8 million worldwide in 2015, and this number is expected to reach 131.5 million in 2050 (Prince et al., 2016). AD is classified as familial AD (fAD) or sporadic AD (sAD), with fAD mainly presenting with mutations in any of three genes: amyloid-β precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2), each encoding their respective proteins (Zhan et al., 2017; Filadi and Pizzo, 2019). The hallmark feature of AD is the accumulation of extracellular β-amyloid (Aβ) in senile plaques, followed by intracellular deposition of neurofibrillary tangles (NFTs) of abnormally hyperphosphorylated tau proteins (Xu, 2009). The mechanisms underlying AD remain unknown and there is no curative method or drug.With the increase in the number of the elderly individuals in society, AD is proving to be one of the greatest issues in clinical medicine. Efforts to target AD-related factors have shown promise in animal models but have failed during clinical trials (Huang and Mucke, 2012). Therefore, there is an urgent need to identify the mechanisms underlying AD and develop new therapeutic strategies for this disease.
Recently, a novel technique, stem cell therapy, has shown great potential in treating AD patients. This review will introduce the current treatment approaches for AD, and discuss the progress, challenges, and perspectives of stem cell therapy for AD. An online search of the PubMed database was performed for articles and reviews published from 1998-2018 with the terms “Alzheimer’s disease”, “stem cells therapy”, “neural stem cells”, “embryonic stem cells”, “mesenchymal stem cells”, and “induced pluripotent stem cells”.Articles relating to the mechanism of AD, and its treatment with neural stem cells (NSCs), mesenchymal stem cells(MSCs), and induced pluripotent stem cells (iPSCs) were included. These articles and reviews in the same field were published recently (1998-2018) and a total of 425 articles were retrieved, 108 of which were included according to inclusion criteria, and 317 eliminant ones were old or repeated.
Pathophysiology of Alzheimer's Disease
With respect to onset, AD is divided into two types: late-onset AD, where the patients are usually older than 65 years;and early-onset AD, characterized by the appearance of marked AD symptoms at an early age (Rygiel, 2016). The two pathological features of AD are the deposition of Aβ and NFTs (Xu, 2009; Lin et al., 2018). Aβ is proteolytically derived from APP, which can be cleaved via two alternative pathways (Kikuchi et al., 2017): the amyloidogenic pathway and the non-amyloidogenic pathway (Figure 1). In the non-amyloidogenic pathway, βAPP is cleaved within the Aβ sequence byα-secretase, leading to the production of a membrane-anchored C-terminal fragment, CTFα. Alternatively, in the amyloidogenic pathway, APP is cleaved by beta-secretase to produce CTFβ. Both CTFα and CTFβ are subsequently cleaved by gamma-secretase to generate the short peptide p3 from CTFα, and Aβ from CTFβ (Xu, 2009).Soluble APP enhances proliferation of NSCs in vitro and in vivo and as a regulator of subventricular zone progenitor proliferation in the early central nervous system (Ohsawa et al., 1999; Caillé et al., 2004; Gakhar-Koppole et al., 2008; Li et al., 2019).
Another key factor, tau, is a neuronal microtubule-associated protein that plays a crucial role when phosphorylated.In the neuronal cytoplasm it can aggregate microtubules,which are major constituents of NFTs (Iqbal et al., 1998;Zhang et al., 2019). Although it is not certain whether tau pathology is dependent on Aβ aggregation in AD, tau protein is required for the toxic effects of Aβ aggregation, because no neurodegeneration is observed upon depletion of tau protein. Furthermore, tau deletion decreases intracellular Aβ clearance and increases extracellular Aβ plaques (Zhagn and Li, 2014).
As mentioned previously, three genes have been implicated in fAD: APP, PSEN1, and PSEN2, which are involved in the function of the γ-secretase complex, Aβ aggregation, and neurodegeneration (Cacquevel et al., 2012). The main risk factors for sAD include apolipoprotein E (APOE), which may affect Aβ clearance, contributing to the development of AD. The triggering receptor expressed on myeloid cells 2(TREM2), which is selectively expressed by microglia in the brain, induces phagocytosis and influences the inflammatory response (Kanekiyo et al., 2014; Yeh et al., 2016). Further, the TREM2-APOE pathway can regulate microglial phenotypic changes in neurodegenerative diseases, and is crucial for the restoration of microglial homeostasis (Krasemann et al.,2017). To date, analyses of millions of polymorphisms in the human genome from thousands of people have revealed a number of new loci associated with AD risk, including Cluster of differentiation 33 (CD33), clusterin (CLU), Fermitin family homolog-2 (FERMT2), HLA-DRB5-DBR1, and Inositol polyphosphate-5-phosphatase (INPP5D) (Karch and Goate, 2015).
General Treatment for Alzheimer's Disease
The research and development of anti-AD drugs or antibodies mainly focuses on three targets: 1) anti-oxidation,2) removal of Aβ deposits in the brain, delaying cognitive impairment, and 3) regulation of the phosphorylation of tau protein and reduction in misfolding and abnormal agglomeration (Godyń et al., 2016; Wisniewski and Drummond,2016; Ibrahim and Gabr, 2019). At present, clinical treatment using anti-AD drugs mainly involves the use of acetyl cholinesterase inhibitors to improve cognitive ability, and N-methyl-D-aspartate receptor antagonists, such as memantine, or other inhibitors to ameliorate the patient’s symptoms, but none of these agents can cure the condition (Coley et al., 2015; Zhang et al., 2019).
A human monoclonal antibody, aducanumab, from Biogen Inc. (Cambridge, MA, USA) can selectively bind aggregated Aβ, and enter the brain to reduce the Aβ level in a dose-dependent manner in a rodent AD model and in patients with AD (Budd Haeberlein et al., 2017). Although high doses of this drug may lead to brain fluid transfer and increase the risk of hematencephalon, this adverse effect can be detected in the initial stages using magnetic resonance imaging. The finding obtained with this drug is currently being validated in ongoing phase 3 clinical trials (Sevigny et al., 2017).
Another drug, ANAVEX 3-71, from Anavex Life Sciences(New York City, NY, USA) can attenuate the cognitive defect and pathological process in AD via the induction of Sigma-1 and M1 receptors. A recent study demonstrated that Sigma-1 receptor (S1R) agonists can induce oxidative stress in mitochondria and enhance complex I activity-induced by Aβ1-42.Sigma-1 receptor is highly expressed in the central nervous system, and is required for Aβ deposition and tau protein hyperphosphorylation, which occur in the late stage of AD(Goguadze et al., 2019). Goguadze et al. (2019) showed that the attenuation of AD symptoms may require vaccines targeting Aβ and tau protein simultaneously. Vaxine Pty Ltd. (Adelaide, Australia) developed a new technology, Vaxine Advax adjuvant, which could be the key point in vaccines for AD,and serves as a promising strategy for ongoing preclinical evaluation and human clinical trials (Davtyan et al., 2016).
Screening of small molecule compounds may be another focus area in AD drug development, because small molecule compounds regulate neuronal injury and enhance nerve regeneration. One study showed that allopregnanolone, an endogenous neurosteroid, improved neuronal degeneration,induced nerve differentiation both in vitro and in vivo, and restored learning and memory performance in mice (Singh et al., 2012). Apigenin and related compounds extracted from food products enhanced neuronal generation and improved learning and memory performance in rat models and adult humans (Taupin, 2009). As reported, several small molecule compounds, including daucosterol, protocatechuic acid, and fluoxetine, can induce proliferation, differentiation,and migration of neural progenitor cells (NPCs), prevent NPC apoptosis, promote nerve regeneration, and alleviate learning deficits in rodent models (Guan et al., 2008; Jiang et al., 2014; Khademi et al., 2018). However, how these small molecules affect the signaling of NSCs remains unclear. It is a complex process to regulate the biological behavior of NSCs, and further study is required to confirm the exact beneficial concentrations and treatment times.
Stem Cell Treatment for Alzheimer's Disease
Drug treatment is a vital clinical strategy and, to date, it mainly delays neuronal degeneration and is mainly of thera-peutic benefit only in patients with early-onset AD.Stem cell therapy provides new potential in the treatment of AD due to the self-renewal ability and high differentiation potential of stem cells. Cell transplantation as a therapy for neuro-degenerative disorders was first explored in Parkinson’s disease several years ago (Lindvall et al., 1988). Based on the preclinical effect in Parkinson’s disease, the technique was applied to other neurodegenerative disorders, such as AD, amyotrophic lateral sclerosis, and Huntington’s disease.Moreover, stem cells are unspecialized cells with the capacity to differentiate into neural cells in the brain microenvironment, and can restore neuroplasticity and neurogenesis via neurotrophic factors (Enciu et al., 2011). On the basis of their ability to differentiate into various cellular types (Lee et al., 2016b), stem cells can be classified in four types: NSCs,MSCs, embryonic stem cells (ESCs), and iPSCs. Each of these cell types shows unique properties that could be utilized in stem cell therapy regimes in a variety of ways (Figure 2).
Neural stem cells
Capable of generating all the differentiated neural cells in the central nervous system, NSCs can be sourced from primary tissues, such as the fetal, neonatal, and adult brain, or from ESCs and iPSCs (Tong et al., 2015). In AD models, a growing number of studies have suggested that NSC therapy is a promising therapeutic strategy, showing both in vitro and in vivo improvements in AD pathologies and behaviors (Zhao,2016; Wang et al., 2017).
Blurton-Jones et al. (2009) first discovered the potential benefits of NSCs in delivering neu-rotrophic support in an AD mouse model. They demonstrated that transplantation of mouse NSCs into the hippocampus of AD mice rescued hippocampus-dependent learning and memory. They further showed that NSCs generated high levels of brain-derived neurotrophic factor in vitro and also increased brain-derived neurotrophic factor protein levels in the brain upon NSC transplantation, which was required for behavioral and synaptic responses in AD mice (Wu et al., 2016; Marsh and Blurton-Jones, 2017). Zhang et al. (2017) also demonstrated that NSC transplantation can rescue cognitive and synaptic deficits in specific regions. Recovery of neuronal function is crucial for learning and memory in AD mouse models(Marsh and Blurton-Jones, 2017; Wang et al., 2017).
Another study indicated the potential of NSCs to produce neurotrophins for treatment of AD, because modified human NPCs could rescue Aβ42-induced cell death by secreting neuronal trophic factors, including brain-derived neurotrophic factor, insulin-like growth factor 1 or glial-cell derived neurotrophic factor, which were capable of enhancing cholin-ergic functions (Kitiyanant et al., 2012).
As mentioned above, neurotrophins can promote survival and differentiation of grafted NSCs. A decrease in neuro-trophin expression and impaired neurotrophin function in axonal transport have been observed in patients with AD and animal models, indicating that neurotrophins play a significant role in neuronal death and axon degeneration(Poon et al., 2011). Modified NSC-hNGF-eGFPs can survive and integrate into the brain of AD rat models, secrete neurotrophic factors, and replace the loss of neurons. Further,transplantation of NSCs can improve cognitive performance in the presence of nerve growth factor (NGF), and offer feasible therapeutic approaches for AD (Wu et al., 2008).However, there are many limiting factors associated with the establishment of NSC lines in vitro. To overcome these problems, one approach is to generate NSCs as floating spherical aggregates, termed neurospheres, which are a mixture of NSCs and progenitors that lead to the expansion of NSCs(Kim et al., 2009). Generally, neurospheres are generated in vitro after 10 days of culturing in the presence of epidermal growth factor or basic fibroblast growth factor, which seem more sensitive to the microenvironment and improve host neural repair upon triggering the secretion of neurotrophins more than fibroblasts (Bonnamain et al., 2012). Another method involves the combination of epigenetic and genetic im-mortalization strategies, by which cells are triggered with an immortalizing gene (e.g., v-myc, c-myc, SV40T, or TERT),and the proliferation and differentiation is impaired in the presence of growth factors (Villa et al., 2009).
Grafted NSCs can also transfer potential therapeutic agents or proteins into host tissues in AD mouse models,such as neprilysin, insulin-degrading enzyme, plasmin, and cathepsin B, to reduce Aβ levels (Kim and de Vellis, 2009).Injected fibroblasts have been shown to reduce Aβ plaque production in AD mice models in the presence of neprilysin,and NSCs overexpressing the same neprilysin gene can induce greater reduction of Aβ plaques in AD mice models(Chen et al., 2012b; Choi et al., 2014).
However, transplantation of NSCs does not always yield predictable outcomes, and migration and differentiation may be significantly affected by the recipient’s brain microenvironment. Overexpression of human APP changes the cell fate of human NSCs leading to the generation of more astrocytes than neurons, indicating the negative influence of APP processing on the therapeutic effect of the grafted NSCs(Kwak et al., 2006).
Human NSCs grafted into 3xTg-AD mice differentiated into neural cell types of the central nervous system, restored recognition, and improved learning and memory defects via attenuation of Aβ accumulation and tau phosphorylation,while transplanted human NSCs grafted into NSE/APPsw mice expressing human APP only increased synaptogenesis without curtailing the other deficits (Ager et al., 2015; Li et al., 2016c). Additionally, NGF nanoparticles may be used to release NGF to enhance the generation of cholinergic neurons in comparison with that achieved by NSC transplantation in AD rat models, providing new insights into the research and development of clinical applications (Chen et al., 2015b; Corrêa-Velloso et al., 2018).
Mesenchymal stem cells
As multi-potent stem cells, MSCs can generate diverse cell types in the bone marrow, adipose tissue, lungs, liver, and umbilical cord (Phinney and Prockop, 2007). Isolated MSCs can expand and differentiate into osteoblasts, adipocytes,and pancreatic islets (Dominici et al., 2006).
Several types of MSC show beneficial effects on neurological disorders via secretion of pro-inflammatory cytokines.The mechanism of treatment using placenta-derived MSCs and human umbilical cord-derived MSCs for hypox-ia-ischemia brain damage partially involves triggering inflammatory responses including tumor necrosis factor-α, interleukin(IL)-17, interferon-γ, IL-10, and IL-8 (Zhou et al., 2015;Ding et al., 2017). Bone marrow MSCs (BMMSCs) have been shown to be involved in immune system disorders and neurodegeneration disease mediated by immune factors, including IL-1β, IL-6, IL-17, and tumor necrosis factor-α (Liu et al., 2015, 2017; Ma et al., 2015; Cui et al., 2018).
In vitro, human MSCs are able to dramatically increase hippocampal neurogenesis and trigger the differentiation of NPCs into mature neurons via the Wnt signaling pathway(Oh et al., 2015). Further, human MSCs could decrease the levels of Aβ42by enhancing autophagy in vitro and in vivo(Shin et al., 2014). Moreover, transplantation of BMMSCs and human umbilical cord blood-derived MSCs into the lateral ventricles or hippocampi of AD mouse models improves memory and spatial learning by reducing Aβ42deposits and increasing neuronal survival (Salem et al., 2014; Kim et al.,2015; Matchynski-Franks et al., 2016; Oron and Oron, 2016).Similarly, autologous BMMSCs have been successfully transfused into the brains of patients with ischemic disorder, and the graft of human MSCs led to a reduced infarct size and functional improvement (Honmou et al., 2011).
Adipose-derived stem cells (ADSCs) isolated from rats can trigger differentiation into neurons or astrocyte-like cells in vitro. Transplantation of ADSCs can improve neural function, demonstrating that ADSCs can facilitate beneficial neural differentiation and induce functional improvement in rats (Chen et al., 2012a). When human ADSCs were in-travenously injected into AD mouse models, strong signals in the brain were found up to 12 days after injection using a Maestro Imaging System (Ha et al., 2014). Further,in rodent models, transplantation of ADSCs can ameliorate cognitive impairment and improve learning and memory via: (1) reduction of oxidative stress; (2) prevention of Akt activity, and activation glycogen synthase kinase-3β; or (3)decreasing Aβ levels, upregulating IL-10 and vascular endothelial growth factor levels, enhancing neurogenesis, and stabilizing synapses and dendrites (Chang et al., 2014; Yan et al., 2014; Yamazaki et al., 2015).
The use of MSCs has been reported to be a promising strategy for stem cell treatments in comparison with that of NSCs. However, many types of MSCs have various drawbacks in application. Extraction and culture of BMMSCs is difficult, which limits their use in clinical trials. Despite these limitations, in recent years, ADSCs and human umbilical cord blood-derived MSCs have been shown to be novel options for AD therapy (Wang et al., 2017).
Embryonic stem cells
As self-renewing, totipotent stem cells, ESCs innately differentiate into various neuronal phenotypes in vitro, including dopaminergic neurons (Martínez-Morales et al., 2013),spinal motor neurons (Hu and Zhang, 2009), and glial cells(Krencik et al., 2011), and are extracted from the inner cell mass of blastocysts (Glat and Offen, 2013; Tong et al., 2015).It has been reported that ESC-derived NSCs can be safely transplanted without tumor formation in AD animal models; however, these results need to be validated (Borlongan,2012; Kim et al., 2013; Tong et al., 2015).
As reported, ESCs can be induced into NPCs in vitro.When these cells are transplanted into AD animal models, a certain the MESPU35 ES cell line may show effective therapeutic activity under certain conditions. Escape latency in the Morris water maze test was found to be dramatically higher than that in control groups after ESC-derived NPC transplantation into an Aβ-injured rat model for 2 weeks.The mean time of NPC-treated group only is about 36 seconds, and control group is about 42 seconds. Sixteen weeks after transplantation, the escape latency had dramatically de-creased in comparison with that in the sham controls.Further, ESC-derived NPCs can also differentiate into astrocytic and neuron-like cells in vivo. These data demonstrate that ESC-derived NPC transplantation can improve memory impairment in AD models (Tang et al., 2008).
A few clinical trials involving grafting of human ESCs have already been initiated. The first US Food and Drug Ad-ministration-approved clinical trial using human ESC-derived cells was started in 2010 by Geron Corp. (Menlo Park, CA,USA), generating oligodendrocyte progenitors for spinal cord injury (Baker, 2011). In late 2011, because unex-pected results, and not apply for patents in Europe, Geron Corp.stopped this clinical trial. The San Francisco-based company Asterias Biotherapeutics/BioTime (Alameda, CA, USA) has purchased the shuttered stem cell program and may reinitiate the trials for the use of human ESC-to treat spinal cord injury in the next two years. Hopefully, more valuable information will be available for the treatment of patients with AD in the future (Martínez-Morales et al., 2013; Scott and Magnus, 2014).
Nevertheless, despite their many advantages, ESCs are associated with high risks of transplantation rejection and immune responses (Martínez-Morales et al., 2013; Wray and Fox, 2016). Unlike the use of NSCs and MSCs, direct transplantation of ESCs may be a concern because of the possibility of tumorigenesis and teratoma formation (Cui et al., 2013; Alonso-Alonso and Srivastava, 2015). There is insufficient information regarding the unique properties and culture conditions of ESCs, including the variability of donor cells, which have been reported for differently established ESC lines (Tong et al., 2015). Although the brain is thought to be immune-privileged, a very few donor cells will remain human leukocyte antigen haplotype-matched, necessitating a degree of immunosuppression in the recipients to avoid cell transplantation-induced immune rejection. Although a few exceptions exist, newer approaches are needed to improve donor cell and recipient compatibility and prevent immune rejection in the future (Hallett et al., 2014; Chen et al., 2015a).
Induced pluripotent stem cells
Typically, iPSCs are derived from mouse embryonic or adult fibroblasts through the introduction of reprogramming factors POU domain class 5 transcription factor 1 (POU5F1,also known as OCT3/4), sex determining region Y-box 2(SOX2), Krüppel-like factor 4 (KLF4) and myelocytomatosis oncogene (c-MYC) (together referred to as OSKM), which facilitate maintenance of pluripotency, and lead to differentiation into neural cells (Fan et al., 2014; Takahashi and Yamanaka, 2015). In 2016, Dr. Shinya Yamanaka’s group generated the first iPSCs upon induction of these transcription factors through retrovirus (Takahashi and Yamanaka, 2006).So far, many novel methods have significantly improved the reprogramming techniques to increase success rates. A potentially powerful tool in addressing the mechanism of AD,iPSCs show the most identical properties compared to ESCs,including morphology, the ability to differentiate into any cell type, and unlimited growth (Yamanaka, 2009; Imm et al., 2017).
Human-sourced iPSCs also provide a beneficial tool for cell-replacement therapy against AD. Peripheral blood mon-onuclear cells from patients with fAD or sAD, or early or late-onset AD can be converted into iPSCs (Israel et al.,2012; Lee et al., 2016a; Zhang et al., 2017). Furthermore,iPSCs can be generated from patients with AD carrying a mutation in PSEN1 or APP (Muratore et al., 2014; Li et al.,2016a, b; Tubsuwan et al., 2016; Yang et al., 2016). Human iPSCs could be a prospective model for detecting the synaptotoxic effect of Aβ, and may indicate Aβ-induced alterations in postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and tau protein phos-phorylation (Nieweg et al., 2015). In addition, the loss of forebrain cholinergic neurons plays a key role in AD. Thus, sAD-iPSC-derived FBCNs with a suitable APOE genotype (ε3/ε4) are more vulnerable to glutamate-mediated cell death following an increased Aβ42/Aβ40ratio than FBCNs derived from healthy people (Duan et al., 2014). Neurons generated from iPSCs are also used for testing small molecular chemical compounds in therapeutic drug screens (Xu et al., 2013;Robbins and Price, 2017). As reported, human pluripotent stem cells exhibit enhanced assembly of the T-complex protein 1-ring complex (TRiC)/chaperonin containing T-complex protein 1 (CCT) complex, which is re-quired for the striking ability of pluripotent stem cells to maintain proteostasis of the aggregation prone huntingtin in Huntington’s disease. Likely, increased expression of CCT8, a single subunit, extends Caenorhabditis elegans lifespan in a TRiC/CCT-dependent manner (Noormohammadi et al., 2016).
AD-iPSCs can be obtained via transcription activator-like effector nuclease or clustered regulatory interspaced short palindromic repeats technology, and repaired AD-iPSCs are now being utilized for cell transplantation to control the disease process (Mungenast et al., 2016; Yang et al., 2016). However, there are several challenges in the clinical use of iPSCs for patients with AD. A report showed that iPSCs could be derived from somatic cells via reprogramming factors, and the reprogramming process reset the somatic cell to a more youthful state, elongating telomeres, and rearranging the mitochondrial network, indicating that iPSCs could not mimic ESCs (Rohani et al., 2014).
Challenges and Promises
Considering the many advantages of stem cell therapy, more and more attention has been paid to it in scientific and medical research. In 2010, the global stem cell treatment market reached 10.9 billion US dollars. In 2017, the global stem cell treatment market share increased to 51.26 billion US dollars(Figure 3). The emergence of stem cells has brought new light to the disease. Stem cells used in cellular assays and animal models have achieved certain results, but there are still many problems to be solved before they can be extended to clinical applications (Table 1).
NSCs can self-replicate and differentiate into neurons in a certain micro-environment, and NSC grafts have obvious therapeutic effects on AD mouse models (Li et al., 2016c).However, direct transplantation of NSCs is not feasible because of their limited source and compulsive immunogenicity.
In addition to having self-renewing ability, multi-differentiating potential, rich source, and low immune rejection rates, MSCs are not susceptible to teratogenicity, and proliferate quickly in vitro. Thus, MSCs have their own advantages in clinical application. However, the differentiatingpotential of MSCs is less than that of ESCs, and they may have un-controllable factors upon transplantation, which influences the application of MSC in clinical treatment (Sugaya and Merchant, 2008).

Table 1 Advantages and disadvantages of stem cells
Similarly, there are many unsolved problems relating to the application of iPSC in AD clinical treatment (Yagi et al.,2012). The number of iPSCs required is not clear because inducing iPSC differentiation into neurons over an extended period may trigger PSEN1 or PSEN2 mutations, affecting the morphology and function of the neurons. In addition to the grafted stem cell types, the target transplantation region is another key factor. The pathogenesis of AD is unclear and AD-related damage has been observed in all parts of the brain, which makes it difficult to determine the optimum graft region.
Freeman et al. (2017) introduced a new molecular system that can regulate different bioactive signals via orthogonal DNA handles, and discovered that NSCs derived from the murine spinal cord organize themselves as neurospheres,and can disperse and migrate upon induction by an exogenous signal and then regroup into neurospheres when the signal is withdrawn, which are an attractive model to study the important role of cell-matrix interactions in the stem cell therapy and provides new avenues for developing rationally designed dynamic regenerative biomaterials in clinical application as a treatment for AD treatment.
Conclusion
An increasing amount of attention is being paid to stem cell therapy. Recent studies have suggested that it may play a key role in the treatment of neurodegenerative disorders,including AD, Parkinson’s disease, and amyotrophic lateral sclerosis. Although, there are many unsolved problems and challenges related to the use of stem cells, the data indicate that stem cell therapy is still a prospective method for AD treatment.
Author contributions:Conception of the work, initial draft and finalization of the manuscript: LLZ, FQZ, JLJ, XQF; literature search: LLZ, FQZ,JTZ, HN; editing of the manuscript: LLZ, XQF, FQZ, JTZ. All authors approved the final version of the paper.
Conflicts of interest:There are no conflicts of interest associated with this manuscript.
Financial support:The work was supported the National Natural Science Foundation of China, No. 81701076 (to LLZ) and No. 31670795(to XQF), 2017 Changbai Mountain Research Support Foundation, No.440050117010 (to XQF), Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences, No. YKFJ2-007 (to LLZ),grants from the Science and Technology Department of Jilin Province,China, No. 20190701037GH (to FQZ), 20180520138JH (to FQZ),20190701036GH (to LLZ). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Wipawan Thangnipon, Mahidol University,Thailand; Bruno Vincent, Mahidol University, Thailand.
Additional file:Open peer review reports 1 and 2.
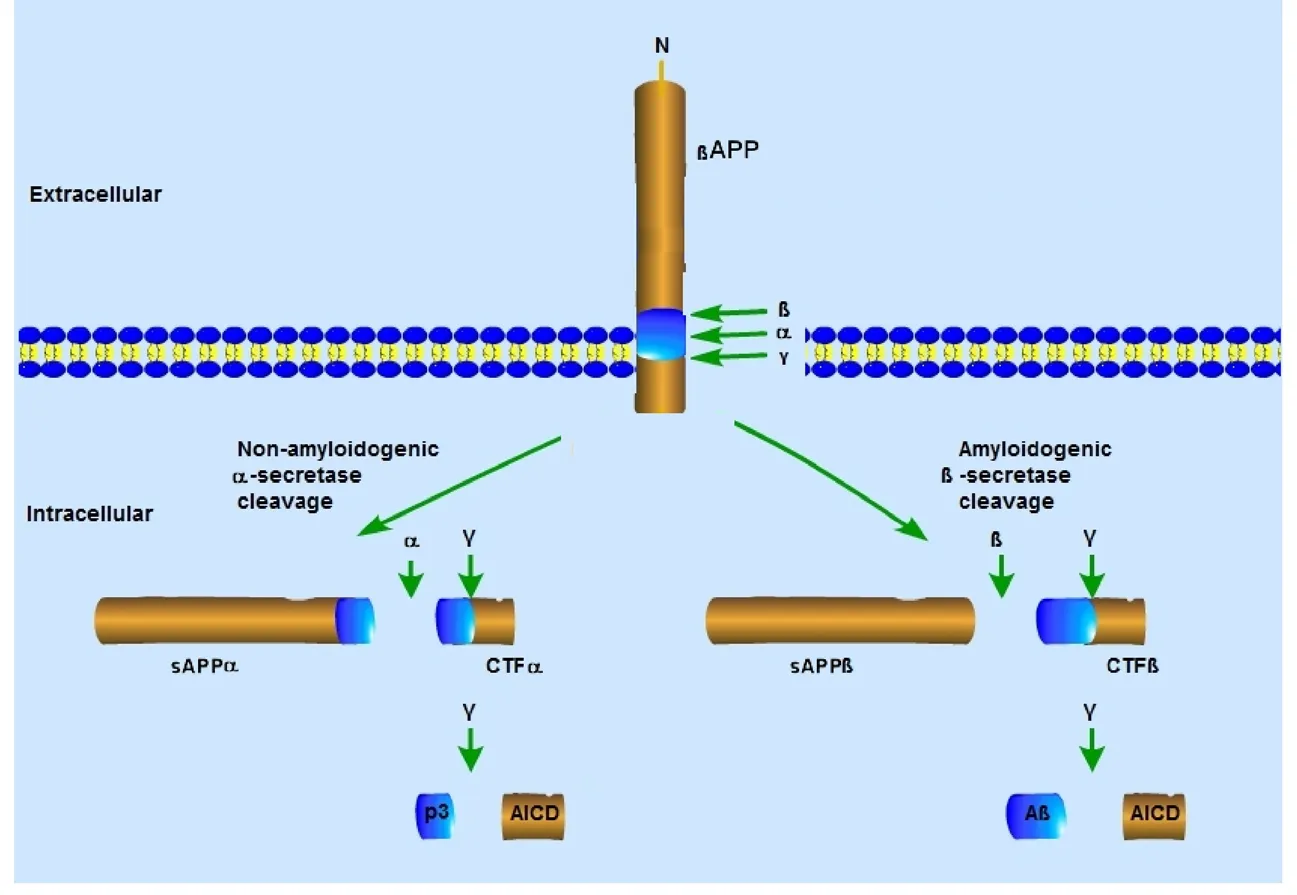
Figure 1 Secretase-mediated amyloid precursor protein processing pathways.
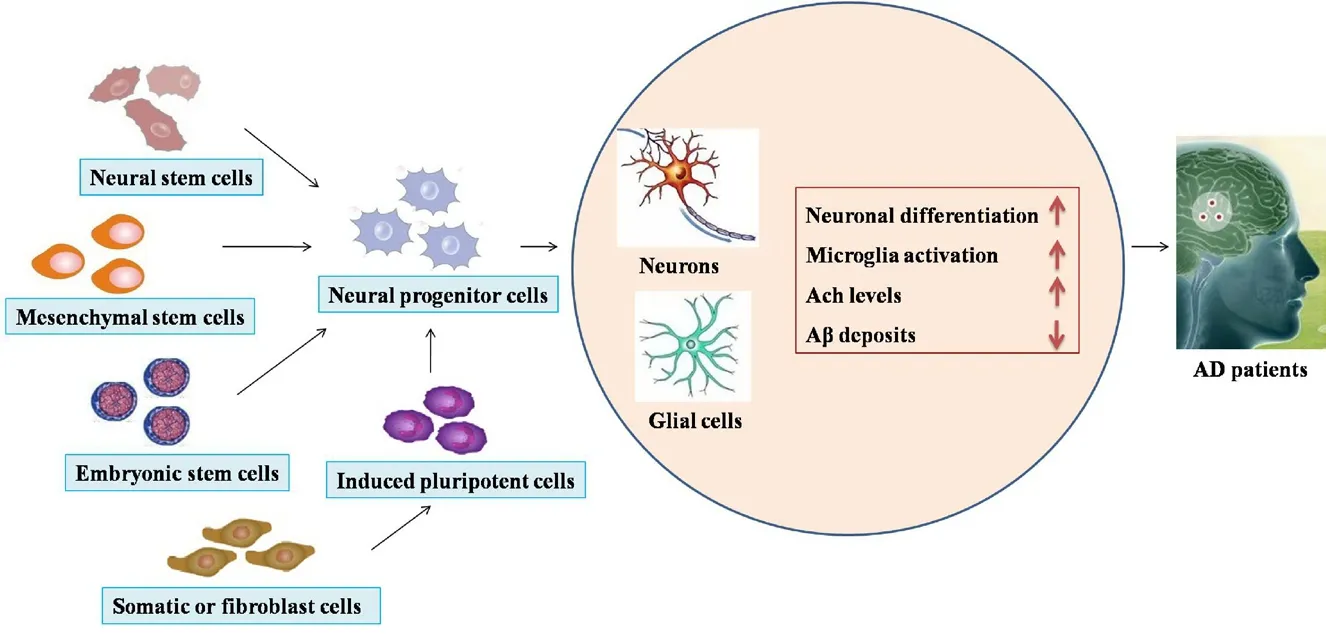
Figure 2 The application of stem cell therapy in Alzheimer's disease.

Figure 3 The global stem cell treatment market in 2010-2024.
杂志排行
中国神经再生研究(英文版)的其它文章
- Ethanol extract from Gynostemma pentaphyllum ameliorates dopaminergic neuronal cell death in transgenic mice expressing mutant A53T human alpha-synuclein
- Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration
- Genetic targeting of astrocytes to combat neurodegenerative disease
- Pathological significance of tRNA-derived small RNAs in neurological disorders
- Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalography
- Protective effect of hydrogen sulfide on oxidative stress-induced neurodegenerative diseases
