Functionality is not an independent prognostic factor for pancreatic neuroendocrine tumors
2020-08-18HongYuChenYaLiangZhouYongHuaChenXingWangHaoZhangNengWenKeXuBaoLiuChunLuTan
Hong-Yu Chen, Ya-Liang Zhou, Yong-Hua Chen, Xing Wang, Hao Zhang, Neng-Wen Ke, Xu-Bao Liu, Chun-Lu Tan
Abstract
Key words: Neuroendocrine tumors; Pancreatic neoplasms; Prognosis; Paraneoplastic endocrine syndromes; Multivariate analysis; Neoplasm staging
INTRODUCTION
Pancreatic neuroendocrine neoplasms (pNENs) account for approximately 10% of primary pancreatic tumors and the incidence of pNENs has been increasing in recent decades[1]. Compared with pancreatic ductal adenocarcinoma, pNENs are generally considered a less aggressive tumor, which occur in relatively younger patients. Neuroendocrine neoplasms (NENs) include a heterogeneous group of neoplasms, and those which produce hormones leading to symptoms (e.g., Whipple triad, Zollinger-Ellison syndrome, and carcinoid syndrome) are classified as functional tumors[2], while others that produce a series of substances without hormone related symptoms are classified as nonfunctional tumors[2,3].
The traditional view is that functionality is a factor that affects the prognosis of pNEN patients. Patients with functional tumors had a longer survival than those with nonfunctional tumors[4-6]. Tumors that secrete insulin and cause endogenous hyperinsulinemic hypoglycemia, namely, insulinomas, are believed to have a better prognosis among functional tumors, especially in the early stage[7], while patients with somatostatinoma and vipoma have been reported to have a relatively shorter survival[8].
However, as the sample sizes of studies have increased, researches in recent years have proposed new viewpoints. Due to the lack of specific symptoms, the majority of pNEN cases are diagnosed at a relatively advanced stage[1]. Therefore, nonfunctional pNENs are more likely to present with aggressive clinical manifestations[9,10], such as large diameter, increased age, high mitotic count, presence of neural invasion, extrapancreatic organ invasion or metastases, and advanced stage, which may lead to a poor prognosis[10,11]. Because of the rarity of pNENs and the low proportion of functional tumors, few studies have performed multivariate Cox regression analysis to show the effect of functionality on survival.
地下水水质修复主要应考虑地下水污染成因、污染物成分、污染范围和经济技术条件等因素。可以优先考虑饮用水水源地的地下水水质修复,先易后难、先试点后推广,分清轻重缓急,逐步展开。
In the present study, we collected data from pNEN patients who underwent surgery at the primary site from the Surveillance, Epidemiology, and End Results (SEER) database and the West China Hospital database. The purpose of this study was to assess whether functionality is an independent factor to predict the prognosis of pNEN patients and explore the factors that influence the survival of these patients.
MATERIALS AND METHODS
SEER database
From January 2004 to December 2016, demographic, clinicopathological, and followup data of patients who underwent surgery for the treatment of pNENs were extracted from the SEER database using SEER*Stat software (version 8.3.5). The demographic data included age, race, and sex. The clinicopathological data included ICD-10 code, histology code, primary tumor location, tumor size, T, N, and M stages, pathologic grade, and surgery of the primary site. Survival data included survival months and vital status. Patients who underwent surgery other than pancreatectomy (local/partial resection, pancreaticoduodenectomy, or total pancreatectomy) were excluded.
West China Hospital database
Patients who underwent surgery with curative intent in West China Hospital between January 2004 and December 2016 with pathologically confirmed pNEN were included. Demographic, clinicopathological, and follow-up data of patients were retrospectively retrieved from the West China Hospital database. Patients with mixed neuroendocrine-non neuroendocrine neoplasms were not included. Patients were excluded if there was not enough information to determine the functionality of the tumor (n= 16). The follow-up deadline was August 2, 2019.
This study was approved by the West China Hospital Review Board under registration No. 2019 (124).
Pathologic grade and stage
The pathologic grade was evaluated using mitotic count and Ki-67 index according to the World Health Organization (WHO) 2017 classification[12]. The TNM stage of tumor was assessed following the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual[13]. Patients with pathologic grade unavailable and patients with G3 pancreatic neuroendocrine tumors (pNETs) or pancreatic neuroendocrine carcinomas (pNECs) (mitotic count > 20, Ki-67 index > 20%, and/or previously diagnosed G3 tumor) were excluded from further analysis.
Functionality
Functionality was assessed according to whether hormone-related symptoms existed, regardless of the immunohistochemistry features. In addition to the nonfunctional pNEN group (N), functional pNENs were divided into two groups for further analysis: Insulinomas (I) and other functional pNENs (O).
Statistical analysis
SPSS version 23.0 (IBM Corporation, Chicago, IL) was employed to perform statistical analyses, andP< 0.05 was considered statistically significant. Continuous variables are reported in the form of the mean ± SE and were compared using the Student'sttest. Nominal data (race, primary site of tumor, sex,etc.) are presented as frequencies and percentages and were compared usingχ2tests or Fisher’s exact tests. The primary end point of this study was overall survival, which was measured from the date of tumor diagnosis to the date of last follow-up or death. Patients with (1) primary tumor unevaluated (Tx), (2) grade unevaluated (Gx), and/or (3) mitotic count higher than 20 or Ki-67 index higher than 20% (NET G3 or NEC) were not involved in the subsequent statistical analysis. After verification of the proportional hazard assumption, Cox proportional hazard models were constructed to identify factors that predicted the prognosis. All variables with aPvalue < 0.1 in the univariate analysis were used as input variables for the multivariate analysis which was performed using a forward stepwise method.
RESULTS
Patient characteristics
From the SEER database, a total of 426 patients were enrolled in this study. The baseline data are shown in Table 1. The mean age was 56.74 ± 0.67 years, and the male:female ratio was 221:205. There were 100 functional tumors (23.5%), including 52 insulinomas, 32 gastrinomas, 12 glucagonomas, 2 vipomas, and 2 somatostatinomas.
From the West China Hospital database, the mean age of the 205 patients was 48.16 ± 0.93 years. There were 88 males (42.9%) and 117 females (57.1%). One hundred andfour (50.7%) patients had functional tumors including 85 insulinomas, 9 gastrinomas, 7 glucagonomas, 1 vipoma, 1 somatostatinoma, and 1 rare pNEN secreting adrenocorticotropic hormone (ACTH)[14]. Compared with the SEER database, patients in the West China Hospital database had fewer tumors of the pancreatic tail, were younger, had a lower T stage, fewer G2 tumors, and fewer distant metastases, and had more female patients (P< 0.05). Although the N stage was comparable in the two databases, the West China Hospital database had more patients with unevaluated N stage (Nx).

Table 1 Characteristics of patients in the two databases, n (%)

1Consisting of Asian and Pacific Islander patients. 2Consisting of Black and American Indian patients and patients whose races were unknown. 3Rows with the title Tx, Nx, Mx, Gx, and other surgeries were not involved in the χ2 test. SEER: Surveillance, epidemiology, and end results; NC: Not comparable; pNET: Pancreatic neuroendocrine tumor; pNEC: Pancreatic neuroendocrine carcinoma; I: Insulinoma; N: Nonfunctional pNEN; O: Other functional pNEN.
When we compared the characteristics of patients of different races (Table 2), the difference between the White and Asian populations was similar to the difference between the two datasets. In the other races, the ratio of male patients was higher, and the proportion of G3 pNETs or pNECs was higher than the respective values in the White and Asian populations. Survival curves of patients of different races from the two databases are shown in Figure 1.
Prognostic factors
Contemporaneous data from the two databases were analyzed separately as two cohorts and then merged as the third cohort to create a larger sample that was suitable for the univariate and multivariate analyses. Patients of races other than White or Asian and Pacific Islander, patients with primary tumor not assessed (Tx) or pathologic grade unevaluated (Gx), and patients who had G3 pNETs or pNECs were not enrolled in the subsequent analysis due to the limitations of the Cox regression model.
The univariate and multivariate analyses of the SEER cohort and the West China Hospital cohort are shown in Table 3. The two cohorts displayed similarities in the hazard ratios (HRs) of age, sex, T stage, regional lymph node metastasis, and distant metastasis, but showed differences in the HRs of primary site, grade, and functionality. In the multivariate analysis, the results of the SEER cohort showed that age (HR = 2.203, 95%CI: 1.249-3.884,P= 0.006) and T stage (HR = 2.589, 95%CI: 1.533-4.371,P< 0.001) were independent risk factors for predicting prognosis. The results of the West China Hospital cohort showed that age (HR = 4.558, 95%CI: 1.122-18.521,P= 0.034), sex (HR = 5.707, 95%CI: 1.161-28.057,P= 0.032), and grade (HR = 9.039, 95%CI: 1.118-73.051,P= 0.039) were independent prognostic factors.
In the cohort consisting of the combined populations from the two databases, factors that affected prognosis in the univariate analysis included country (P= 0.002), race (P= 0.001), age (P< 0.001), sex (P= 0.005), T stage (P< 0.001), regional lymph node metastasis (N1,P= 0.036), distant metastasis (P< 0.001), and functionality (nonfunctional pNETs,P= 0.031; other functional pNETs,P= 0.012). The multivariate proportional hazard model contained only race (HR = 0.438, 95%CI: 0.225-0.851,P= 0.015), age (HR = 2.315, 95%CI: 1.362-3.935,P= 0.002), sex (HR = 1.744, 95%CI: 1.049-2.899,P= 0.032), and T stage (HR = 2.612, 95%CI: 1.603-4.254,P< 0.001).
Effect of functionality on survival
In the West China Hospital database and in the total population, nonfunctional pNETs (West China Hospital database: HR = 1.473, 95%CI: 0.268-8.092,P= 0.656; total population: HR = 2.544, 95%CI: 1.090-5.938,P= 0.031) and other functional pNETs (West China Hospital database: HR = 7.913, 95%CI: 1.314-47.670,P= 0.024; total population: HR = 3.925, 95%CI: 1.359-11.337,P= 0.012) tended to have poorer prognoses than insulinoma. However, as shown in the multivariate analysis, functionality was not associated with the survival time of patients with pNETs since it was not selected into the model.
偏执性人格障碍的特点是多疑敏感,不信任别人,易把别人的好意当成恶意,嫉妒心强,对别人的荣誉、成就等感到紧张不安,喜欢挑衅、指责和抱怨,常常产生攻击、报复的心理,对别人缺乏同情心和热情,很少开玩笑,警惕性很高,常怕被人欺骗,处处提防他人等。网游中青少年的高度孤独和厌倦倾向、社交焦虑及自我封闭使这种偏执性人格障碍有明显加重倾向。有这种倾向的青少年经常负面地理解他人和社会,习惯性地消极解释问题,常常对别人表现出愤恨情绪,无论在网上还是现实生活中都易产生带有敌意情绪的攻击。
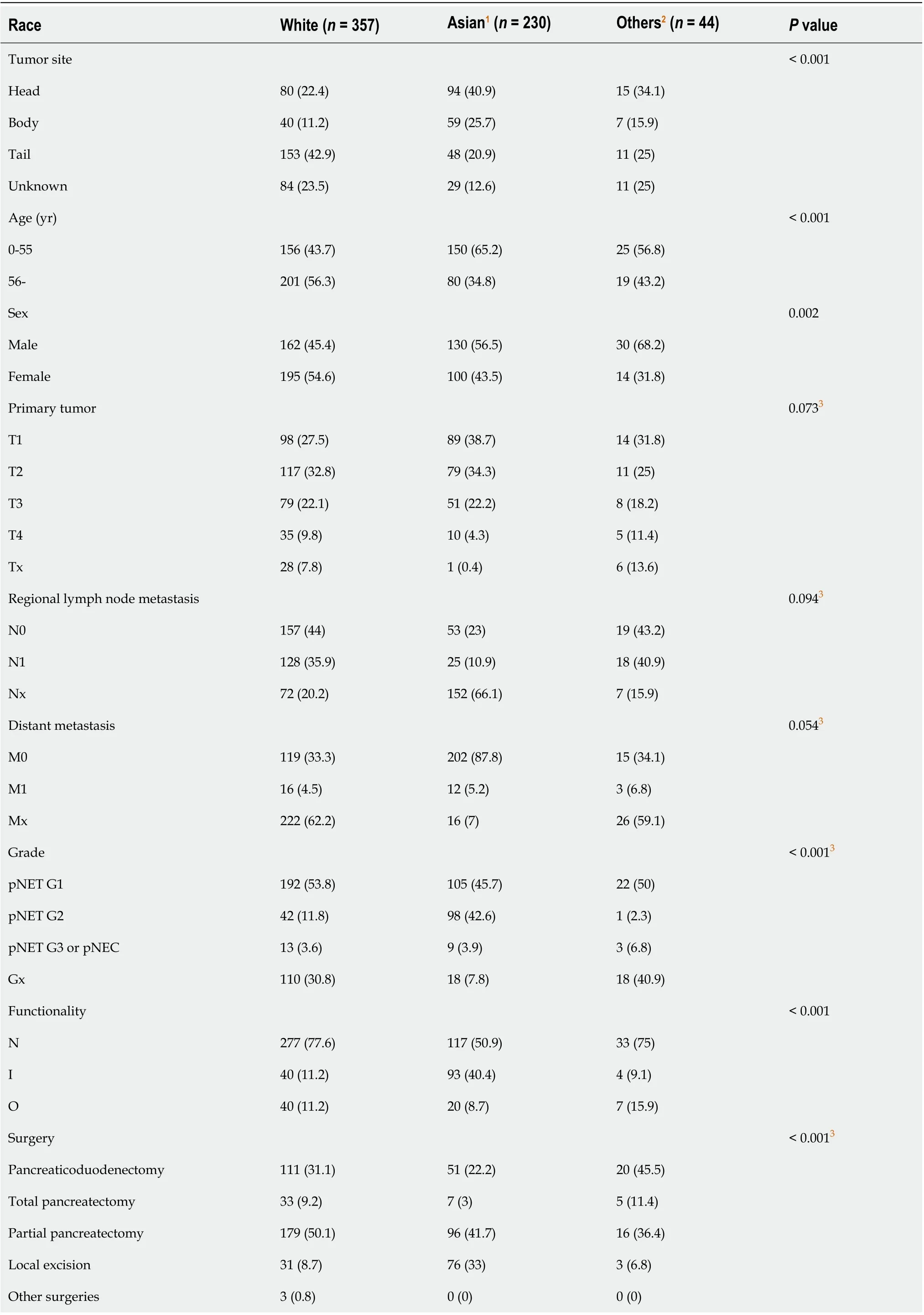
Table 2 Characteristics of patients of different races, n (%)
DISCUSSION
Compared with pancreatic ductal adenocarcinoma, pNETs are characterized by a lower incidence, younger age, and better prognosis[1]. According to morphological features, the WHO 2017 guidelines divide pNENs into biologically different groups, pNETs and pNECs. pNET cells have a fairly uniform, solid, trabecular, spiral or glandular patterned nucleus with pepper-salt chromatin and granular cytoplasm, while pNECs are similar to small or large cell neuroendocrine carcinomas of the lung[15]. Only pNETs can be divided into three different prognostic groups (G1, G2, and G3) according to mitotic count and Ki-67 index. Subsequently, the AJCC updated the staging system of pancreatic tumors[13]. PNET G1 and G2 are staged in a scheme that is similar to the European Neuroendocrine Tumor Society Consensus Guidelines staging system[16,17], while G3 pNETs and pNECs share the same staging system as pancreatic exocrine tumors.
pNENs were previously classified into several groups according to the existence and type of hormone related symptoms. The group, or rather, functionality was believed to be associated with the survival of patients with pNEN. Cienfuegoset al[4]performed a log-rank survival analysis on pNEN patients, and the results showed that the nonfunctioning tumor group had a relatively poor prognosis compared with the functioning tumor group (P= 0.052). Studies have indicated that functionality is positively related to the expression of somatostatin receptor 2[18]and negatively related to aurora kinase B[19], which may contribute to the improvement in survival. Wanget al[5]and Nannoet al[6]found that functionality is a prognostic factor affecting overall survival and disease-free survival in the results of univariate Cox regression analysis. However, the multivariate analysis was not carried out in the study by Wanget al[5](due to the small sample size) and did not include functionality as a factor in the model in the study by Nannoet al[6](only venous invasion and grade were used as input variables).
In recent years, studies have proposed new viewpoints. Studies[20,21]that included patients with NENs in almost all the locations suggested that functionality is not associated with progression-free survival13or disease-free survival[14]. However, there are differences in biological characteristics between NENs of lung origin and gastroenteropancreatic NENs: The majority of the functional NENs are carcinoid syndrome[20], while the functional tumors of gastroenteropancreatic NENs, especially pNENs, are mainly insulinomas[10].
Our results indicated that race, age, sex, and T stage were independent factors for predicting the survival of patients with pNETs. Although no significant differences were found in the effects of some factors on survival in the small sample cohorts, it does not mean that there is no relationship between these factors and survival. Only a sample that is large enough can reveal the real prognostic factor.
Functionality was correlated with survival in the univariate analysis, but was not associated with prognosis in the multivariate analysis. The prognosis of patients with nonfunctional tumors is generally considered to be poorer than that of patients with insulinoma. However, this is more likely related to the late diagnosis of patients with nonfunctional tumors, rather than the difference in biological properties between functional and nonfunctional tumors or the effect of hormones secreted by functional tumors. Hormone related syndrome is the only basis to distinguish between the nonfunctional neuroendocrine neoplasm and several types of functional neuroendocrine neoplasm. However, immunohistochemical staining also shows the expression of insulin/glucagon/gastrin/somatostatin in non-functional tumors. The reasonability of classification based on symptoms rather than gene expression needs to be further explored.
According to WHO guidelines, the assessment of grade depends on mitotic count and Ki-67 index, with a cutoff value of 2/10 high power fields and 3%, respectively. However, the cutoff values that make the most sense are still debatable. Some studies support that Ki-67 and mitotic count is correlated with prognosis[6,11], while there are also some studies that do not support this viewpoint[18]. In the Western China Hospital database, grade is an independent risk factor for prognosis. But in the SEER database, grade is not related to prognosis.

Table 3 Univariate and multivariate Cox proportional hazard regression analyses of predictors of survival in patients
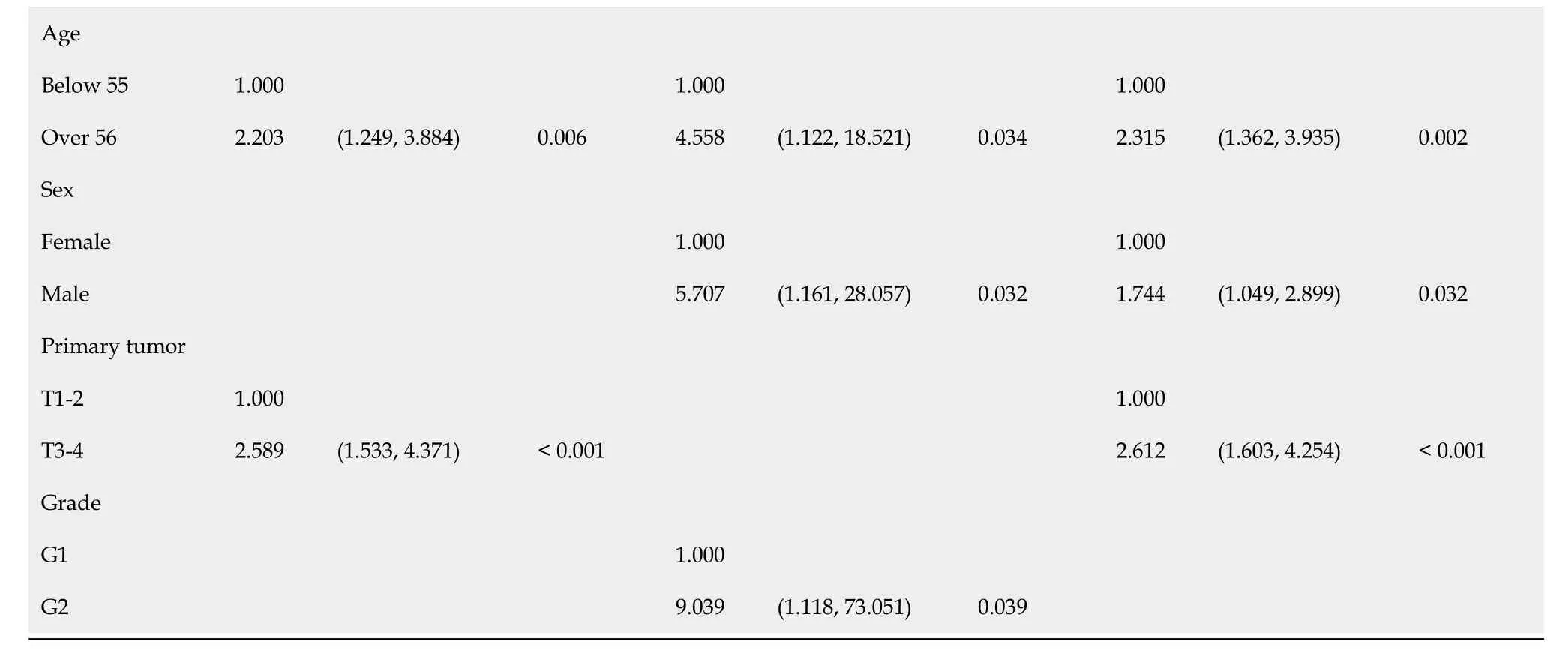
SEER: Surveillance, epidemiology, and end results; I: Insulinoma; N: Nonfunctional pNEN; O: Other functional pNEN.
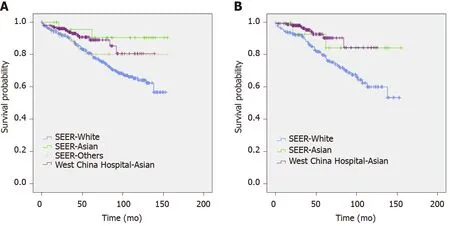
Figure 1 Survival functions of patients by database and race. A: Survival of patients enrolled; B: Survival of patients analyzed. SEER: Surveillance, epidemiology, and end results.
There was a trend of shorter survival time for patients with higher T stage in our small-sample cohort (n= 205), and T stage turned out to be an independent prognostic factor in large-sample cohorts (SEER,n= 426; total,n= 631), which is similar to the results of other studies[6,21]. On one hand, it indicated that T stage is indeed a factor that affects the prognoses of patients with pNETs; on the other hand, the results showed the importance of sample size in cohort study.
There were some limitations to this study: (1) Patients of the same time period were enrolled from the two databases, and the inclusion/exclusion criteria were the same. However, SEER is a multicenter database, while the West China Hospital database is a single center database. The baseline data of the two datasets had differences in the distributions of some variables. Most of the data were demographic data or objective clinical data (such as tumor size and lymph node metastasis). The tumor grade depends on the mitotic count and Ki-67 index whose implementation may vary in different centers. Cox regression of the combined dataset may not represent the relationship between grade and prognosis; and (2) This study collected data from 2004 to 2016 retrospectively, and there is not sufficient information to separate poorlydifferentiated pNECs from well-differentiated G3 pNETs in the SEER database. The TNM stages of G3 pNETs and pNECs may not have the same effect on survival as those of G1 and G2 tumors since they are completely different stage systems. Therefore, we excluded all tumors with mitotic counts higher than 20 or Ki-67 indexes higher than 20% (G3 pNETs and pNECs).
ARTICLE HIGHLIGHTS

猜你喜欢
杂志排行
World Journal of Gastroenterology的其它文章
- TBL1XR1 induces cell proliferation and inhibit cell apoptosis by the PI3K/AKT pathway in pancreatic ductal adenocarcinoma
- Development of innovative tools for investigation of nutrient-gut interaction
- Practical review for diagnosis and clinical management of perihilar cholangiocarcinoma
- Management of nonalcoholic fatty liver disease in the Middle East
- Quality of life in patients with gastroenteropancreatic tumours: A systematic literature review
- Risk factors associated with inflammatory bowel disease: A multicenter case-control study in Brazil
