Quality of life in patients with gastroenteropancreatic tumours: A systematic literature review
2020-08-18CatherineWatsonCraigWilliamTallentireJohnRamageRajaventhanSrirajaskanthanOscarLeeuwenkampDonnaFountain
Catherine Watson, Craig William Tallentire, John K Ramage, Rajaventhan Srirajaskanthan, Oscar R Leeuwenkamp, Donna Fountain
Abstract
Key words: Gastroenteropancreatic neuroendocrine tumours; Health-related quality of life; Systematic literature review; Chemotherapy; Biological therapies; Peptide receptor radionuclide therapy
INTRODUCTION
Gastroenteropancreatic tumours (GEP-NETs) account for 2% of all gastrointestinal malignancies, with a United Kingdom age-standardised incidence rate of 8.6/100000/year[1]. GEP-NETs tend to be slow-growing cancers arising from diffuse endocrine cells in the gastrointestinal tract (GI-NETs) or pancreas (P-NETs). GEPNETs are categorised as “functioning” or “non-functioning”. Functioning tumours cause symptoms due to the hypersecretion of peptides and hormones[2,3], whilst nonfunctioning tumours have no hormone-related clinical features. Specific symptoms vary by primary tumour location[4,5]. Around 3 in 10 patients with GI-NET develop symptoms of diarrhoea, abdominal pain and flushing due to increased serotonin production[6], known as carcinoid syndrome (CS). P-NETs can produce a variety of hormones, the most common of which are insulin and gastrin. Other abnormal hormone production associated with GEP-NETs include glucagon, vasoactive intestinal peptide, adrenocorticotropic hormone, somatostatin, and parathyroid hormone-related protein[7].
Surgery is the only curative treatment for GEP-NETs and can be performed when tumours are localised and resectable. The majority of GEP-NET patients, however, are diagnosed with metastatic disease requiring systemic treatment[8,9], including chemotherapy, biological therapies, and peptide receptor radionuclide therapy (PRRT)[10]. These long-term therapeutic options provide symptomatic relief, and can slow down or stabilise disease progression, but are not curative. The 5-year survival rate for patients with metastatic GI-NETs is approximately 75% and the 5-year survival rate for patient with metastatic P-NETs is > 60%, whilst the prevalence of GEP-NETs has been on the rise in the recent decades[2,11]. For instance, the median overall time of survival from the date of diagnosis of distant metastatic disease was reported to be 103 mo (8.5 years) in patients with metastatic midgut GI-NETs[12]. It is therefore vital to assess the patient’s health-related quality of life (HRQoL) in general and related to the disease whilst taking treatments which can have a marked impact on well-being.
In oncology, an increased emphasis has been placed on HRQoL assessment as a secondary endpoint in clinical studies, using reliable and thoroughly validated patient-reported outcome (PRO) instruments for which the minimal important difference for improvement/worsening has been defined[13-17]. High-quality information on HRQoL serves a variety of purposes such as the development of targeted interventions, informed decision making about treatment options and allocation of healthcare resources[18]. Indeed, the United States Food and Drug Administration and European Medicines Agency have published guidance on the use of PRO measures in oncology studies emphasising the value of patient perspective when evaluating medicinal products[19,20].
To date, systematic assessments of HRQoL in patients with GEP-NETs have been performed to a limited extent. In the past, where the literature was systematically evaluated, the review focused on the methodological quality of the identified studies[18]. To our knowledge, there has been no systematic literature review published to provide a comprehensive overview of the different treatment options and associated impact on the patients’ HRQoL. The aim of our study was to perform a systematic review to assess HRQoL focusing on patients with inoperable metastatic GEP-NETs undergoing different treatments in order to uncover areas for future research.
MATERIALS AND METHODS
Search strategy and study selection
This systematic review adheres to the established international guidelines for conducting systematic reviews, such as those provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses report (PRISMA Statement[21]), and the Cochrane Handbook for Systematic Reviews of Interventions[22]. Methods of analysis and inclusion criteria were defined in advance and outlined in a designated protocol.
An extensive search of MEDLINE, Embase and the Cochrane library was performed by an experienced information specialist to identify relevant studies. We examined articles published in English between January 1985 and November 2019. The protocol outlined the electronic search strategy in full; searches were supplemented by manual searches of relevant review journals.
After de-duplication, article titles and abstracts were independently reviewed by two analysts, with discrepancies resolvedviadiscussion with a third reviewer. Fulltext articles were then obtained for records that met the inclusion criteria to re-evaluate eligibility.
Eligibility criteria
Eligibility for this systematic review was defined according to the PICOS[21]. Participants were adult patients (≥ 18 years) with inoperable GEP-NETs. Outcome measures included HRQoL, PROs, utility estimates, validity, and minimally important difference. The study design incorporated studies reporting utility estimates and HRQoL data, including economic evaluations, observational studies, and relevant randomised control trials. Cross-sectional studies were included, as they can provide useful HRQoL data at a specific point in time, although not providing longitudinal changes due to treatment over time. Studies were also required to report at least one relevant outcome for ≥ 15 patients. There were no limits defined in terms of intervention or comparator PICOS elements.
Articles were excluded if patients were diagnosed with neuroendocrine tumours that did not originate in the gastrointestinal tract or the pancreas, and when the HRQoL data for patients with GEP-NETs were not separated by sub-analysis (e.g., the population included patients with lung and/or thyroid neuroendocrine tumours). Studies indexed as case reports, case series, editorials and letters, conference abstracts, systematic reviews and non-human studies were also excluded. Finally, studies where the treatment applied did not target the malignancies specifically, such as psychotherapeutic interventions, were excluded.
Data extraction
A dedicated data extraction sheet was devised to capture all relevant data, including publication details, study design, country, intervention(s), comparator(s), eligibility, population description (including any specific sub-groups), sample size, age (average and range), sex ratio, tumour location, description of HRQoL measure (including valuation technique, method of elicitation, timepoints, follow-up duration, response rates), utility tool assessment (including disease state, timepoint, average utility) and HRQoL assessment (including scale domain where relevant, timepoint, average score, data spread). Disagreements were resolved by discussion between the two reviewers, with third reviewer involvement as required.
Quality assessment
The quality and relevance of HRQoL studies were assessed based on the key criteria described by Papaioannouet al[23]and Brazieret al[24], where applicable. Papers reporting insufficient information to assess study methods and populate the data extraction sheet were considered lower quality and were excluded on this basis.
RESULTS
Identified literature
A total of 42 studies published between 1985 and 2019 met the inclusion criteria of this study, of which 32 were found electronically and 10 were found by handsearching the reference lists of relevant articles (Figure 1). Of these studies, 6 can be classed as crosssectional studies. There was a combined total of 3552 participants in the 42 studies, excluding healthy participants used for comparisons and the duplicate patients taken from the same data, with study sample sizes ranging from 17 to 253 patients. The heterogeneous nature of the different study populations was evident; the percentage of female participants ranged between 30%-60%, whilst average age ranged from 53.8 to 67.0 years (Table 1). In terms of disease location, 8 studies focused on GI-NET patients only and 6 studies focused on P-NET patients; the remaining studies were either a combination of both populations or did not report the specific disease location.
Of the total studies, 14 included somatostatin analogues as the intervention, including octreotide (n= 7), lanreotide (n= 3), pasireotide (n= 1), and undefined (n= 3). Such treatments slow down the production of hormones, such as serotonin, and thus reduce the symptoms of CS. In two studies, somatostatin analogues were combined with immunotherapy drugs such as interferon[25,26]. Three studies compared somatostatin analogues with placebos, allocated within the context of a randomised controlled trial[16,27,28]. Five studies used somatostatin analogues as comparators for an alternative somatostatin therapy,177Lu-DOTATATE or octreotide combined with interferon alpha[17,29-32].
In PRRT, a radioactive substance can be combined with a relevant peptide (or its analogue), such as somatostatin, so that it preferentially binds with a high affinity to the tumour. Nine studies considered PRRT as the intervention:225Ac-DOTATATE (n= 1),177Lu-DOTATATE (n= 5),90Y-DOTATOC (n= 2) and/or177Lu-DOTATOC (n= 3) (Table 1).177Lu-DOTATATE was compared with octreotide administered at a high dose in one prospective study[17]. One retrospective study considered PRRT as the comparator[33], with somatostatin analogues or interferon therapy as the intervention.
Everolimus, sunitinib and erythropoietin were studied as interventions in three, three and one studies, respectively. Two studies compared sunitinib with a placebo within the context of randomised control trials[34,35]. Sunitinib was investigated as a comparator of everolimus in an additional study[36]. In some studies, interferon was used within the populations[25,31,33,37,38], but this was not applied to a specific subgroup.
Six studies used chemotherapy as the intervention: These combined bevacizumab with 5-FU/streptozocin (n= 2), capecitabine and streptozocin plus cisplatin (n= 1),and bevacizumab plus capecitabine (n= 1). Two studies followed patients with various ongoing treatments[37,39]. Three studies used chemotherapy treatments as the comparators to chemotherapeutic interventions; two studies combined capecitabine with the anti-angiogenic drug bevacizumab and used the same population data[40,41], whilst one study combined capecitabine and streptozocin as the comparator[42].
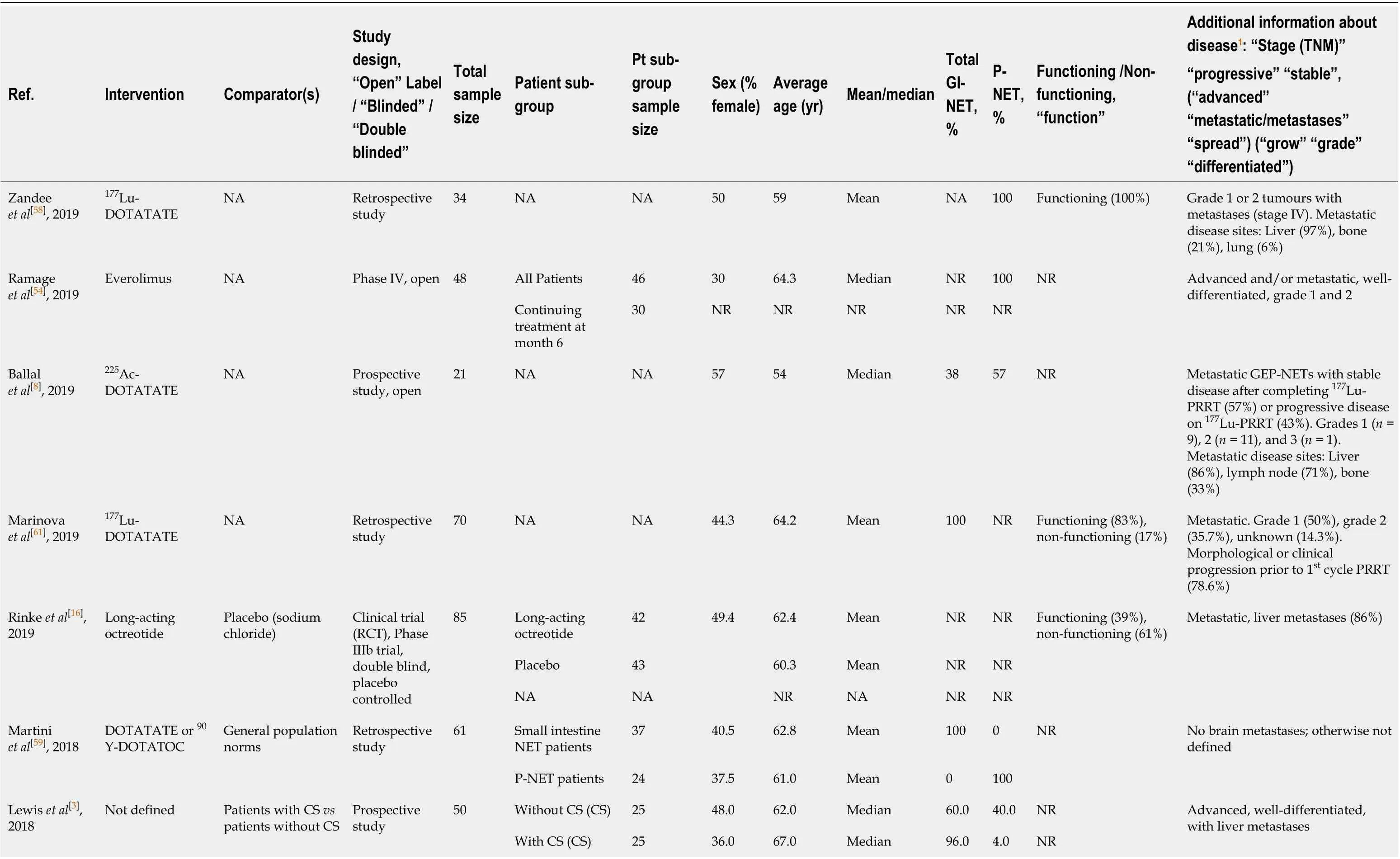
Table 1 Extracted literature
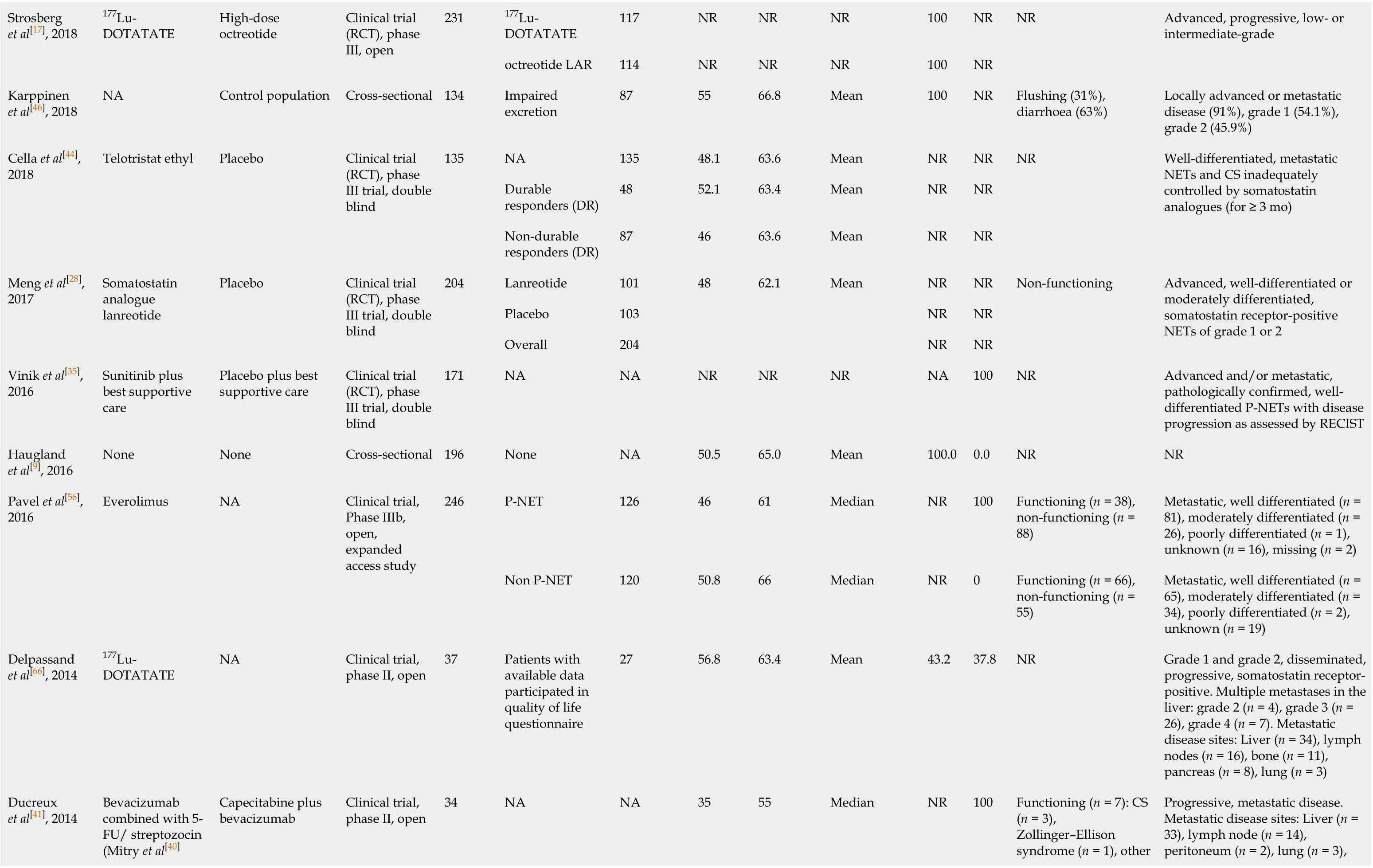
177Lu-DOTATATE 117 NR NR NR 100 NR Strosberg et al[17], 2018 177Lu-DOTATATE High-dose octreotide Clinical trial (RCT), phase III, open 231 octreotide LAR 114 NR NR NR 100 NR NR Advanced, progressive, low- or intermediate-grade Karppinen et al[46], 2018 NA Control population Cross-sectional 134 Impaired excretion 87 55 66.8 Mean 100 NR Flushing (31%), diarrhoea (63%)Locally advanced or metastatic disease (91%), grade 1 (54.1%), grade 2 (45.9%)NA 135 48.1 63.6 Mean NR NR Durable responders (DR)48 52.1 63.4 Mean NR NR Cella et al[44], 2018 Telotristat ethyl Placebo Clinical trial (RCT), phase III trial, double blind 135 Non-durable responders (DR)87 46 63.6 Mean NR NR NR Well-differentiated, metastatic NETs and CS inadequately controlled by somatostatin analogues (for ≥ 3 mo)Lanreotide 101 48 62.1 Mean NR NR Placebo 103 NR NR Meng et al[28], 2017 Somatostatin analogue lanreotide Placebo Clinical trial (RCT), phase III trial, double blind 204 Overall 204 NR NR Non-functioning Advanced, well-differentiated or moderately differentiated, somatostatin receptor-positive NETs of grade 1 or 2 Vinik et al[35], 2016 Sunitinib plus best supportive care Placebo plus best supportive care Clinical trial (RCT), phase III trial, double blind 171 NA NA NR NR NR NA 100 NR Advanced and/or metastatic, pathologically confirmed, welldifferentiated P-NETs with disease progression as assessed by RECIST Haugland et al[9], 2016 None None Cross-sectional 196 None NA 50.5 65.0 Mean 100.0 0.0 NR NR P-NET 126 46 61 Median NR 100 Functioning (n = 38), non-functioning (n = 88)Metastatic, well differentiated (n = 81), moderately differentiated (n = 26), poorly differentiated (n = 1), unknown (n = 16), missing (n = 2)Pavel et al[56], 2016 Everolimus NA Clinical trial, Phase IIIb, open, expanded access study 246 Non P-NET 120 50.8 66 Median NR 0 Functioning (n = 66), non-functioning (n = 55)Metastatic, well differentiated (n = 65), moderately differentiated (n = 34), poorly differentiated (n = 2), unknown (n = 19)Delpassand et al[66], 2014 177Lu-DOTATATE NA Clinical trial, phase II, open 37 Patients with available data participated in quality of life questionnaire 27 56.8 63.4 Mean 43.2 37.8 NR Grade 1 and grade 2, disseminated, progressive, somatostatin receptorpositive. Multiple metastases in the liver: grade 2 (n = 4), grade 3 (n = 26), grade 4 (n = 7). Metastatic disease sites: Liver (n = 34), lymph nodes (n = 16), bone (n = 11), pancreas (n = 8), lung (n = 3)Bevacizumab combined with 5-FU/ streptozocin (Mitry et al[40] Functioning (n = 7): CS (n = 3), Zollinger–Ellison syndrome (n = 1), other Progressive, metastatic disease. Metastatic disease sites: Liver (n = 33), lymph node (n = 14), peritoneum (n = 2), lung (n = 3), Ducreux et al[41], 2014 Capecitabine plus bevacizumab Clinical trial, phase II, open 34 NA NA 35 55 Median NR 100
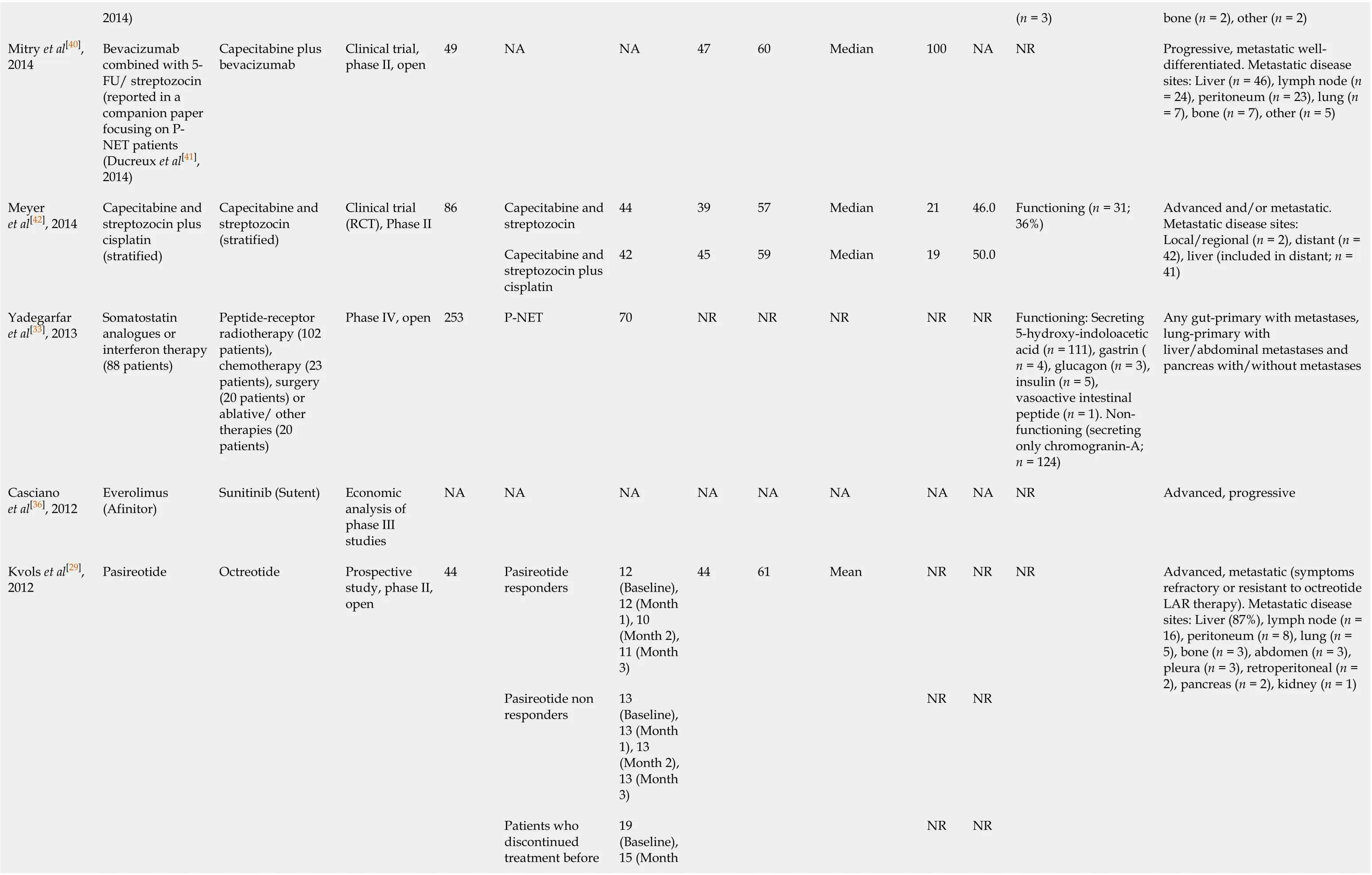
2014)(n = 3)bone (n = 2), other (n = 2)Mitry et al[40], 2014 Bevacizumab combined with 5-FU/ streptozocin (reported in a companion paper focusing on PNET patients (Ducreux et al[41], 2014)Capecitabine plus bevacizumab Clinical trial, phase II, open 49 NA NA 47 60 Median 100 NA NR Progressive, metastatic welldifferentiated. Metastatic disease sites: Liver (n = 46), lymph node (n = 24), peritoneum (n = 23), lung (n = 7), bone (n = 7), other (n = 5)Capecitabine and streptozocin 44 39 57 Median 21 46.0 Meyer et al[42], 2014 Capecitabine and streptozocin plus cisplatin (stratified)Capecitabine and streptozocin (stratified)Clinical trial (RCT), Phase II 86 Capecitabine and streptozocin plus cisplatin 42 45 59 Median 19 50.0 Functioning (n = 31; 36%)Advanced and/or metastatic. Metastatic disease sites: Local/regional (n = 2), distant (n = 42), liver (included in distant; n = 41)Yadegarfar et al[33], 2013 Somatostatin analogues or interferon therapy (88 patients)Peptide-receptor radiotherapy (102 patients), chemotherapy (23 patients), surgery (20 patients) or ablative/ other therapies (20 patients)Phase IV, open 253 P-NET 70 NR NR NR NR NR Functioning: Secreting 5-hydroxy-indoloacetic acid (n = 111), gastrin (n = 4), glucagon (n = 3), insulin (n = 5), vasoactive intestinal peptide (n = 1). Nonfunctioning (secreting only chromogranin-A; n = 124)Any gut-primary with metastases, lung-primary with liver/abdominal metastases and pancreas with/without metastases Casciano et al[36], 2012 Everolimus (Afinitor)Sunitinib (Sutent)Economic analysis of phase III studies NA NA NA NA NA NA NA NA NR Advanced, progressive Pasireotide responders 12 (Baseline), 12 (Month 1), 10 (Month 2), 11 (Month 3)44 61 Mean NR NR Pasireotide non responders 13 (Baseline), 13 (Month 1), 13 (Month 2), 13 (Month 3)NR NR Patients who discontinued treatment before 19 (Baseline), 15 (Month Kvols et al[29], 2012 Pasireotide Octreotide Prospective study, phase II, open 44 NR NR NR Advanced, metastatic (symptoms refractory or resistant to octreotide LAR therapy). Metastatic disease sites: Liver (87%), lymph node (n = 16), peritoneum (n = 8), lung (n = 5), bone (n = 3), abdomen (n = 3), pleura (n = 3), retroperitoneal (n = 2), pancreas (n = 2), kidney (n = 1)
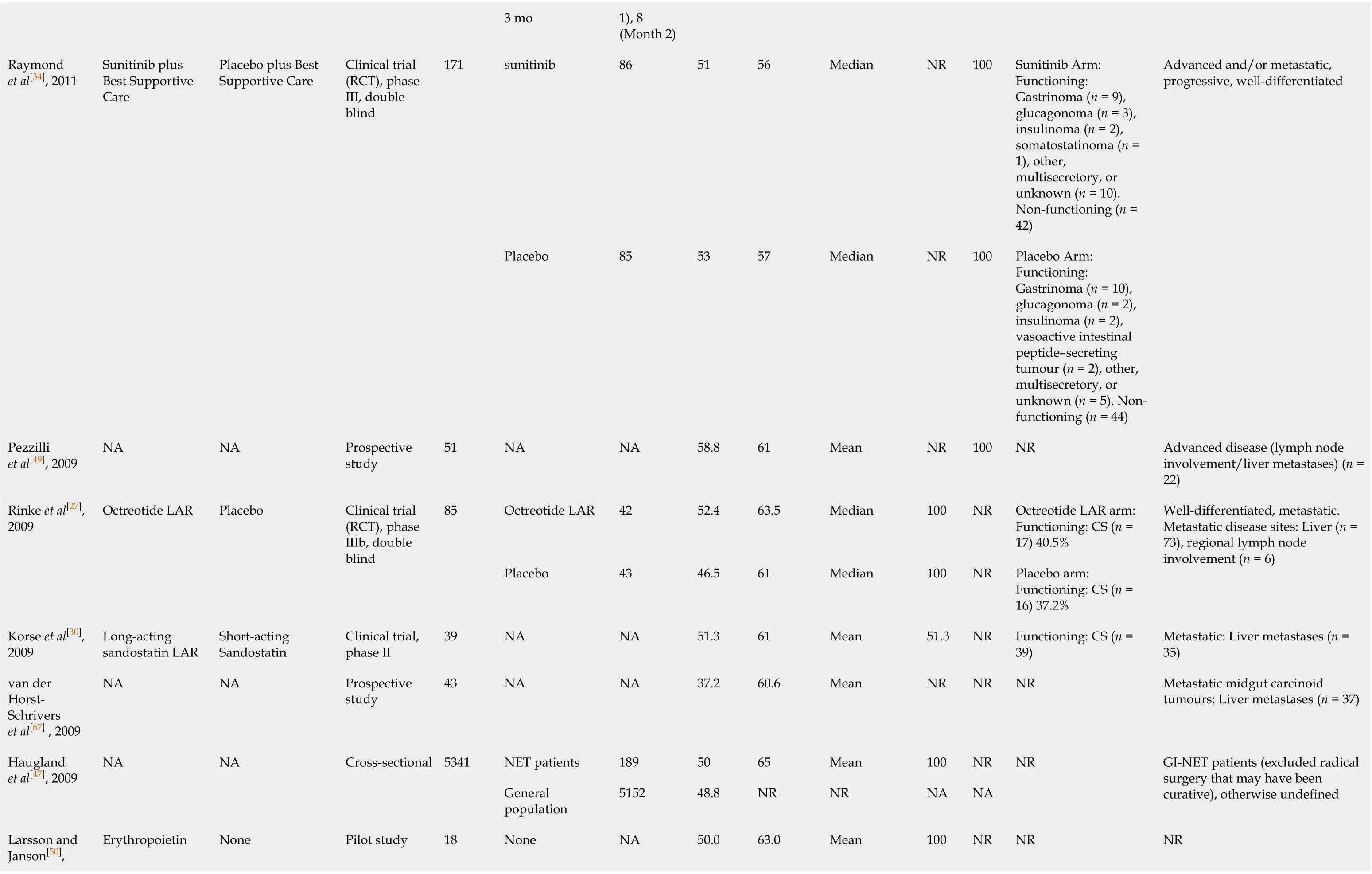
3 mo 1), 8 (Month 2)sunitinib 86 51 56 Median NR 100 Sunitinib Arm: Functioning: Gastrinoma (n = 9), glucagonoma (n = 3), insulinoma (n = 2), somatostatinoma (n = 1), other, multisecretory, or unknown (n = 10). Non-functioning (n = 42)Raymond et al[34], 2011 Sunitinib plus Best Supportive Care Placebo plus Best Supportive Care Clinical trial (RCT), phase III, double blind 171 Placebo 85 53 57 Median NR 100 Placebo Arm: Functioning: Gastrinoma (n = 10), glucagonoma (n = 2), insulinoma (n = 2), vasoactive intestinal peptide–secreting tumour (n = 2), other, multisecretory, or unknown (n = 5). Nonfunctioning (n = 44)Advanced and/or metastatic, progressive, well-differentiated Pezzilli et al[49], 2009 NA NA Prospective study 51 NA NA 58.8 61 Mean NR 100 NR Advanced disease (lymph node involvement/liver metastases) (n = 22)Octreotide LAR 42 52.4 63.5 Median 100 NR Octreotide LAR arm: Functioning: CS (n = 17) 40.5%Rinke et al[27], 2009 Octreotide LAR Placebo Clinical trial (RCT), phase IIIb, double blind 85 Placebo 43 46.5 61 Median 100 NR Placebo arm: Functioning: CS (n = 16) 37.2%Well-differentiated, metastatic. Metastatic disease sites: Liver (n = 73), regional lymph node involvement (n = 6)Korse et al[30], 2009 Long-acting sandostatin LAR Short-acting Sandostatin Clinical trial, phase II 39 NA NA 51.3 61 Mean 51.3 NR Functioning: CS (n = 39)Metastatic: Liver metastases (n = 35)van der Horst-Schrivers et al[67] , 2009 NA NA Prospective study 43 NA NA 37.2 60.6 Mean NR NR NR Metastatic midgut carcinoid tumours: Liver metastases (n = 37)NET patients 189 50 65 Mean 100 NR Haugland et al[47], 2009 NA NA Cross-sectional 5341 General population 5152 48.8 NR NR NA NA NR GI-NET patients (excluded radical surgery that may have been curative), otherwise undefined Larsson and Janson[50], Erythropoietin None Pilot study 18 None NA 50.0 63.0 Mean 100 NR NR NR

2008 Kulke et al[55], 2008 Sunitinib NA Clinical trial, phase II, open 107 NA NA 40.2 Carcinoid tumour = 58, PNET= 56 Median 37.3 61.7 P-NET: Functioning: Gastrinoma n = 5 (7.6%), insulinoma n = 3 (4.5%), VIPoma n = 2 (3.0%), glucagonoma n = 4 (6.1%), other n = 5 (7.6%). Nonfunctioning n = 46 (69.7%)Advanced carcinoid or P-NET Fröjd et al[37] , 2007 Various included None Longitudinal, prospective, comparative study 59 T1-T4 successful 36 47.0 60.0 Mean NR NR NR Metastatic (70% metastatic at start; 78% metastatic at end)Patients 35 42.9 60 Mean NR NR Davies et al[68], 2006 NA NA Crosssectional, open 50 Healthcare workers 15 NR NR NR NA NA NR Metastatic Frilling et al[48], 2006 90Y-DOTATOC then 177Lu-DOTATOC NA Prospective study 20 Excluding pt (no 3) with Paragangliomaneck 19 30 53.8 Median NR NR NR Advanced, progressive, and metastatic Octreotide monotherapy 51 47.1 58 Median NR NR Octreotide plus interferon alpha 54 44.4 57 Median NR NR Arnold et al[31], 2005 Octreotide plus interferon alpha Octreotide monotherapy Clinical trial (RCT), open 114 Nonrandomised 9 33.3 60 Median NR NR Octreotide: Functioning (n = 23), nonfunctioning (n = 28). Octreotide plus Interferon-alpha: functioning (n = 24), non-functioning (n = 30)Metastatic or locally advanced disease without curative therapeutic option Teunissen et al[62], 2004 177Lu-DOTATOC None Clinical trial 50 None NA 56.0 58.3 Mean NR 26.0 NR Metastatic I-MIBG 10 40 59 Mean 90 10 NR Progressive Pasieka et al[43], 2004 I-MIBG NA Clinical trial, open 19 Octreotide 9 55.6 55.6 Mean 66.6 33.3 Kwekkeboom et al[60], 2003 Somatostatin analogue 177Lu-DOTATOC NA Clinical trial, open 35 None NA 60 54 Mean NR NR NR Metastatic Larsson et al[25], 2001 Interferon, somatostatin analogue, interferon, and a somatostatin None Prospective study 24 None NA 42.0 62.0 Median 100 NR NR NR O'Toole Octreotide Lanreotide Clinical trial Patients received 33 16 50 63 NR 62.5 0
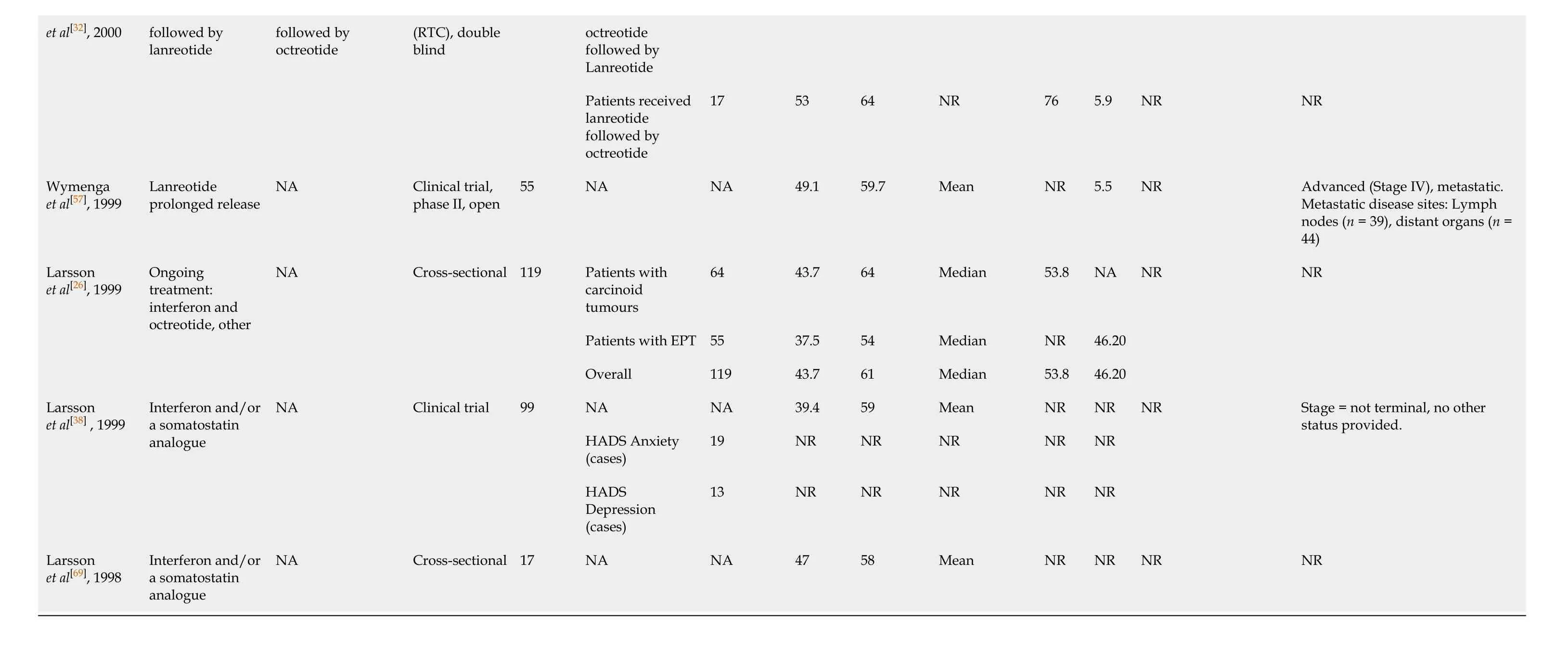
1Disease description provided in each reference. NA: Not available; P-NET: Pancreas-neuroendocrine tumours; CS: Carcinoid syndrome; NR: Not relevant; HADS: Hospital Anxiety and Depression Scale; PRRT: Peptide receptor radionuclide therapy.
Two additional studies identified within the scope of this review focused on metaiodobenzylguanidine (MIBG) therapy and telotristat ethyl[43,44], respectively. MIBG is a radiometabolic therapy with limited antitumor effect on GEP-NETs. Telotristat ethyl is used to inhibit L-tryptophan hydroxylases TPH-1 and TPH-2 and reduce the production of serotonin, thereby alleviating CS symptoms[44]; this was assessed in the context of a randomised controlled trial, randomised 1:1:1 to receive 250 mg TID, 500 mg TID, and placebo.

Figure 1 PRISMA diagram of included and excluded studies; the number of studies propagating to each stage of the process are included.
HRQoL assessment
In total, 30 studies employed a version of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ), in which linear transformation is used to standardise the raw score, so that scores range from 0 to 100. The majority of these studies utilised the EORTC QLQ-C30 HRQoL measure (n= 28). One study developed a disease-specific quality of life score questionnaire for patients with GI-NETs to supplement EORTC QLQ-C30. Six studies employed the GI-NET specific module QLQ-GI.NET21, of which one study apparently did not use the HRQoL measure in conjunction with EORTC QLQ-C30[8], thus will not show valid data. Others administered the EQ-5D or EQ-VAS to assess patient utilities and/or the Hospital Anxiety and Depression Scale (HADS) (Although HADS is not strictly a HRQoL measure, it provides insight into the anxiety and depressive symptoms of GEP-NET patients’ underlying HRQoL) in addition to the EORTC measures (n= 5)[45]. Larssonet al[25]reported mean baseline HADS anxiety and depression scores of 4.6 (SD: 4.0) and 3.2 (SD: 2.7), respectively. Four studies applied the Short Form-36 (SF-36) questionnaire[9,46-48], three of which were cross-sectional studies. Other measures used include the SF-12[49], the Functional Assessment of Cancer Therapy-Anaemia[50]and the Functional Assessment of Chronic Illness Therapy-Diarrhoea (FACIT-D). Pezzilliet al[49]reported no significant difference between P-NET patients and a sample of age- and sex-matched subjects in terms of physical domain (44.7 ± 11.0vs46.1 ± 9.9,P= 0.610), but a significantly lower mental score in the same patients compared to the normative population (42.4 ± 13.0vs48.2 ± 9.8,P= 0.036).
The average HRQoL global health score at baseline and at different timepoints during each trial, obtained by asking patients to rate their overall health, have been reported for the EORTC QLQ-C30 (Table 2). A higher score represents a higher (“better”) level of functioning, including the global health score. This particular tool became the primary focus of this manuscript, as EORTC QLQ-C30 was the single most commonly cited HRQoL dimension (n= 24), with global health score providing a single HRQoL dimension for comparison between studies. The average value for the baseline global health score had a range of 56–70. A higher score for symptoms represents a “worse” level of symptoms. Lowest average score ranges were observed for baseline EORTC QLQ-C30 scores in the following symptoms: nausea and vomiting (4.1–20), insomnia (11.0–38.6), appetite loss (9.2–27.8), constipation (0–24.4) and financial difficulties (6–23.6). Diarrhoea demonstrated a wide range of mean values (16.7–78.3), which can be attributed to the heterogeneity of disease symptoms in GINET and P-NET patients, indicating that diarrhoea in GEP-NET patients is multifactorial and very variable. Considering disease location, the average baseline global health scores were comparable, ranging from 56–68 for GI-NETs (4 studies) and56–67 for P-NETs (5 studies).

Table 2 Literature reporting scores for health-related quality of life average global health score for gastroenteropancreatic neuroendocrine tumours patients using European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30
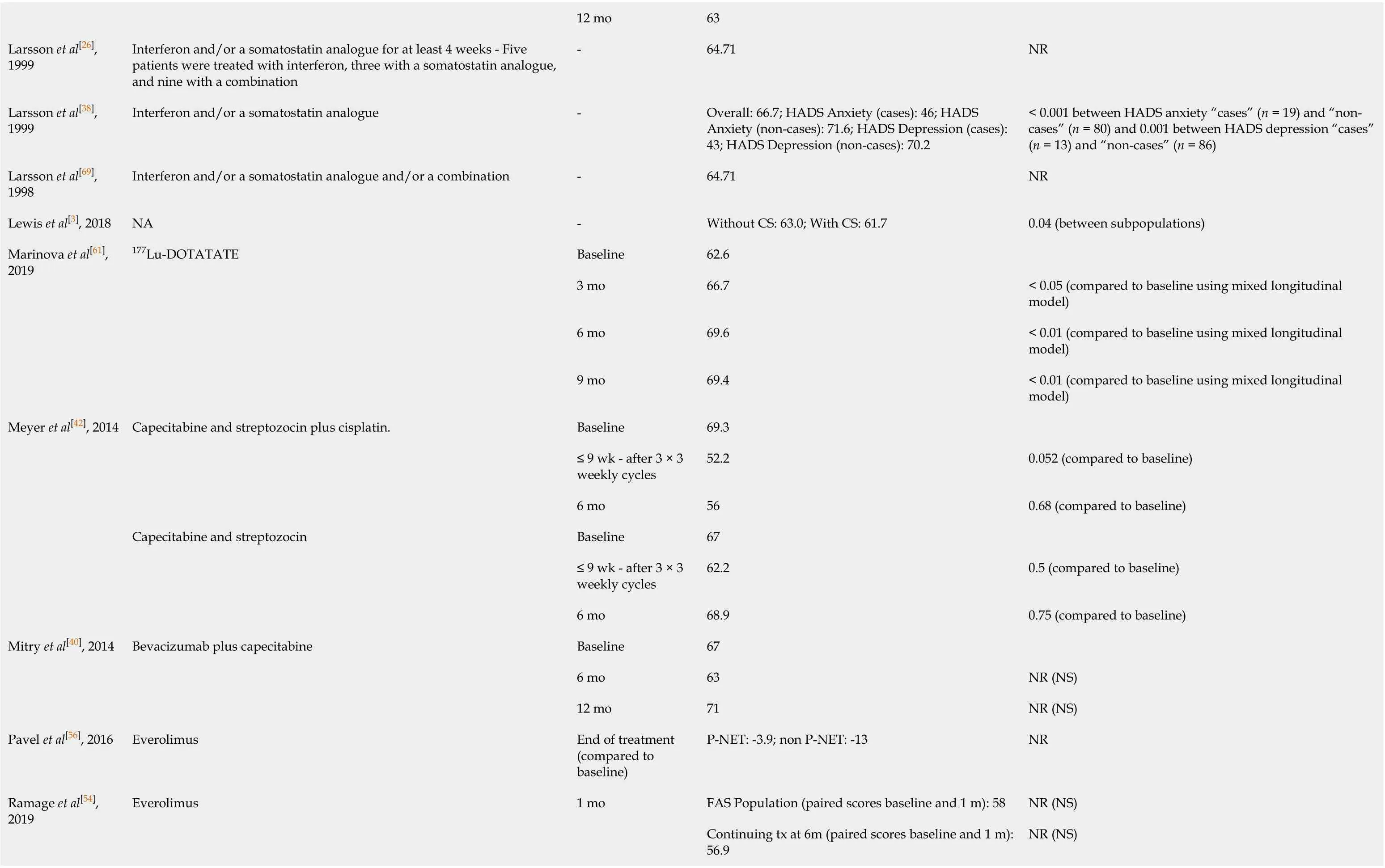
12 mo 63 Larsson et al[26], 1999 Interferon and/or a somatostatin analogue for at least 4 weeks - Five patients were treated with interferon, three with a somatostatin analogue, and nine with a combination-64.71 NR Larsson et al[38], 1999 Interferon and/or a somatostatin analogue -Overall: 66.7; HADS Anxiety (cases): 46; HADS Anxiety (non-cases): 71.6; HADS Depression (cases): 43; HADS Depression (non-cases): 70.2< 0.001 between HADS anxiety “cases” (n = 19) and “noncases” (n = 80) and 0.001 between HADS depression “cases” (n = 13) and “non-cases” (n = 86)Larsson et al[69], 1998 Interferon and/or a somatostatin analogue and/or a combination -64.71 NR Lewis et al[3], 2018 NA -Without CS: 63.0; With CS: 61.7 0.04 (between subpopulations)Marinova et al[61], 2019 177Lu-DOTATATE Baseline 62.6 3 mo 66.7< 0.05 (compared to baseline using mixed longitudinal model)6 mo 69.6< 0.01 (compared to baseline using mixed longitudinal model)9 mo 69.4< 0.01 (compared to baseline using mixed longitudinal model)Meyer et al[42], 2014 Capecitabine and streptozocin plus cisplatin.Baseline 69.3≤ 9 wk - after 3 × 3 weekly cycles 52.2 0.052 (compared to baseline)6 mo 56 0.68 (compared to baseline)Capecitabine and streptozocin Baseline 67≤ 9 wk - after 3 × 3 weekly cycles 62.2 0.5 (compared to baseline)6 mo 68.9 0.75 (compared to baseline)Mitry et al[40], 2014 Bevacizumab plus capecitabine Baseline 67 6 mo 63 NR (NS)12 mo 71 NR (NS)Pavel et al[56], 2016 Everolimus End of treatment (compared to baseline)P-NET: -3.9; non P-NET: -13 NR Ramage et al[54], 2019 Everolimus 1 mo FAS Population (paired scores baseline and 1 m): 58 NR (NS)Continuing tx at 6m (paired scores baseline and 1 m): 56.9 NR (NS)
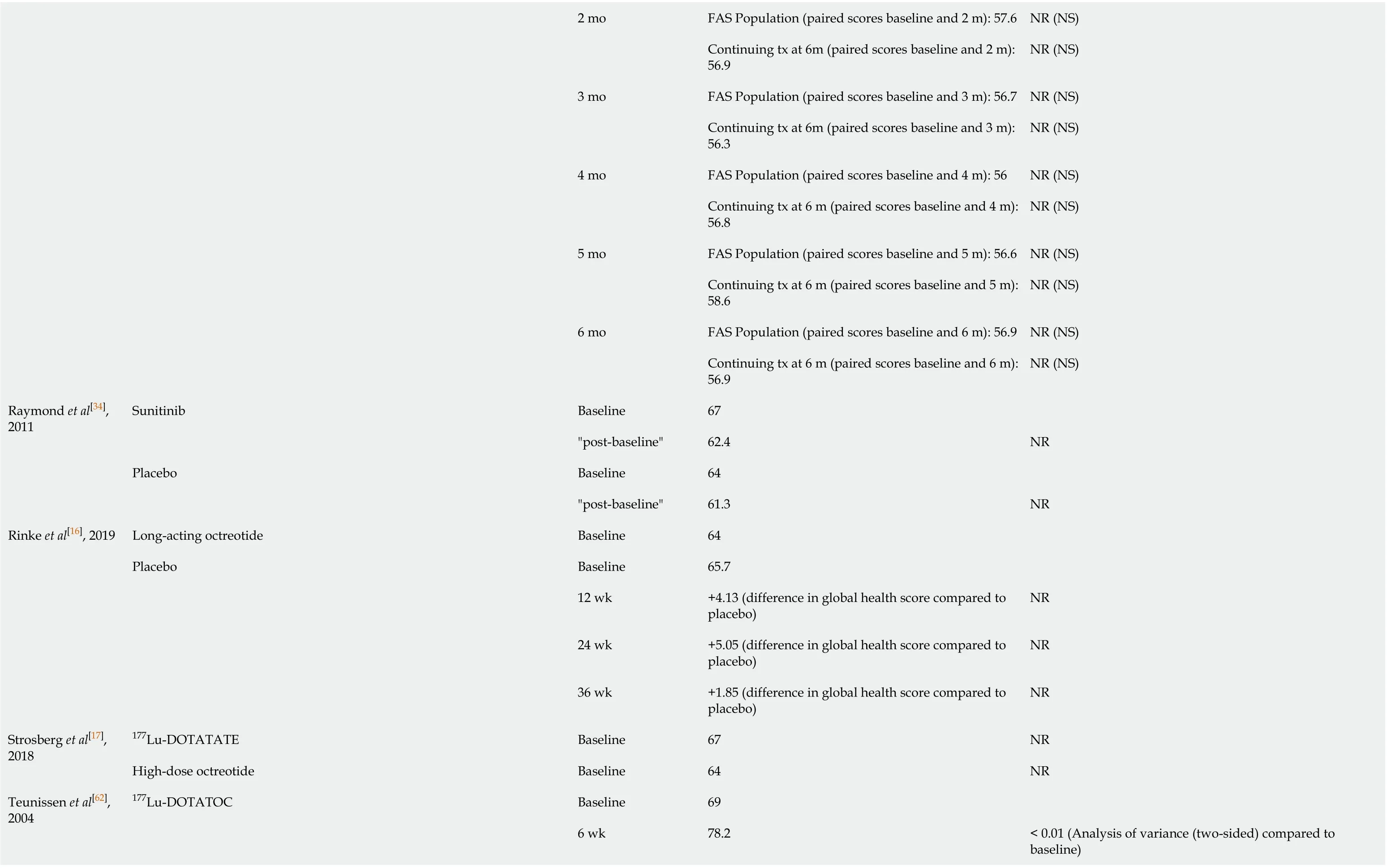
2 mo FAS Population (paired scores baseline and 2 m): 57.6 NR (NS)Continuing tx at 6m (paired scores baseline and 2 m): 56.9 NR (NS)3 mo FAS Population (paired scores baseline and 3 m): 56.7 NR (NS)Continuing tx at 6m (paired scores baseline and 3 m): 56.3 NR (NS)4 mo FAS Population (paired scores baseline and 4 m): 56 NR (NS)Continuing tx at 6 m (paired scores baseline and 4 m): 56.8 NR (NS)5 mo FAS Population (paired scores baseline and 5 m): 56.6 NR (NS)Continuing tx at 6 m (paired scores baseline and 5 m): 58.6 NR (NS)6 mo FAS Population (paired scores baseline and 6 m): 56.9 NR (NS)Continuing tx at 6 m (paired scores baseline and 6 m): 56.9 NR (NS)Raymond et al[34], 2011 Sunitinib Baseline 67"post-baseline"62.4 NR Placebo Baseline 64"post-baseline"61.3 NR Rinke et al[16], 2019 Long-acting octreotide Baseline 64 Placebo Baseline 65.7 12 wk +4.13 (difference in global health score compared to placebo)NR 24 wk +5.05 (difference in global health score compared to placebo)NR 36 wk +1.85 (difference in global health score compared to placebo)NR Strosberg et al[17], 2018 177Lu-DOTATATE Baseline 67 NR High-dose octreotide Baseline 64 NR Teunissen et al[62], 2004 177Lu-DOTATOC Baseline 69 6 wk 78.2< 0.01 (Analysis of variance (two-sided) compared to baseline)

NA: Not available; NET: Neuroendocrine tumours; CS: Carcinoid syndrome; NR: Not relevant; PRRT: Peptide receptor radionuclide therapy; NS: Not significant.
Whilst the most popular HRQoL measure was EORTC QLQ-C30, studies that employed the QLQ-GI.NET21 module provided domain scores specific to the disease that is the focus of this report, including: endocrine systems, gastrointestinal systems, disease-related worries, bone/muscle painetc. Table 3 presents the scores given for these domains. The average HRQoL score ranges for body image (13.3–29.1), weight gain (9-27.8) information/communication (1.1–16.0) and treatment-related symptoms (6.5–26.24) showed that these were only minor problems experienced by patients with GI-NETS. Other domains displayed a wide range of scores,e.g.sexual function (28.2–68.4), social function (30.0–72.9) and disease-related worries (37.6–84.2), making these some of the most important problems experienced by many patients. The QLQGI.NET21 module has been validated as a responsive tool for assessing HRQoL of patients with GEP-NETs[33], although it is accepted that numbers were too small for a proper validation in patients with P-NETs alone. This is of relevance, as P-NET patients often present with a more aggressive disease and concomitant treatments may be used.
In addition, a 10-point change in an EORTC domain score is frequently considered a minimal clinically important difference and, using Kaplan-Meier methodology, time to
deterioration (TTD) can be measured using a 10-point decline in analogy with time to event curves[51,52]. Two studies identified in the literature search applied the use of minimal important difference in the change in HRQoL domains as a means of assessing the impact of interventions using TTD, and time until definitive deterioration (TUDD),i.e. ≥ 10 point worsening of a HRQoL domain score from baseline without further improvement[16,17].
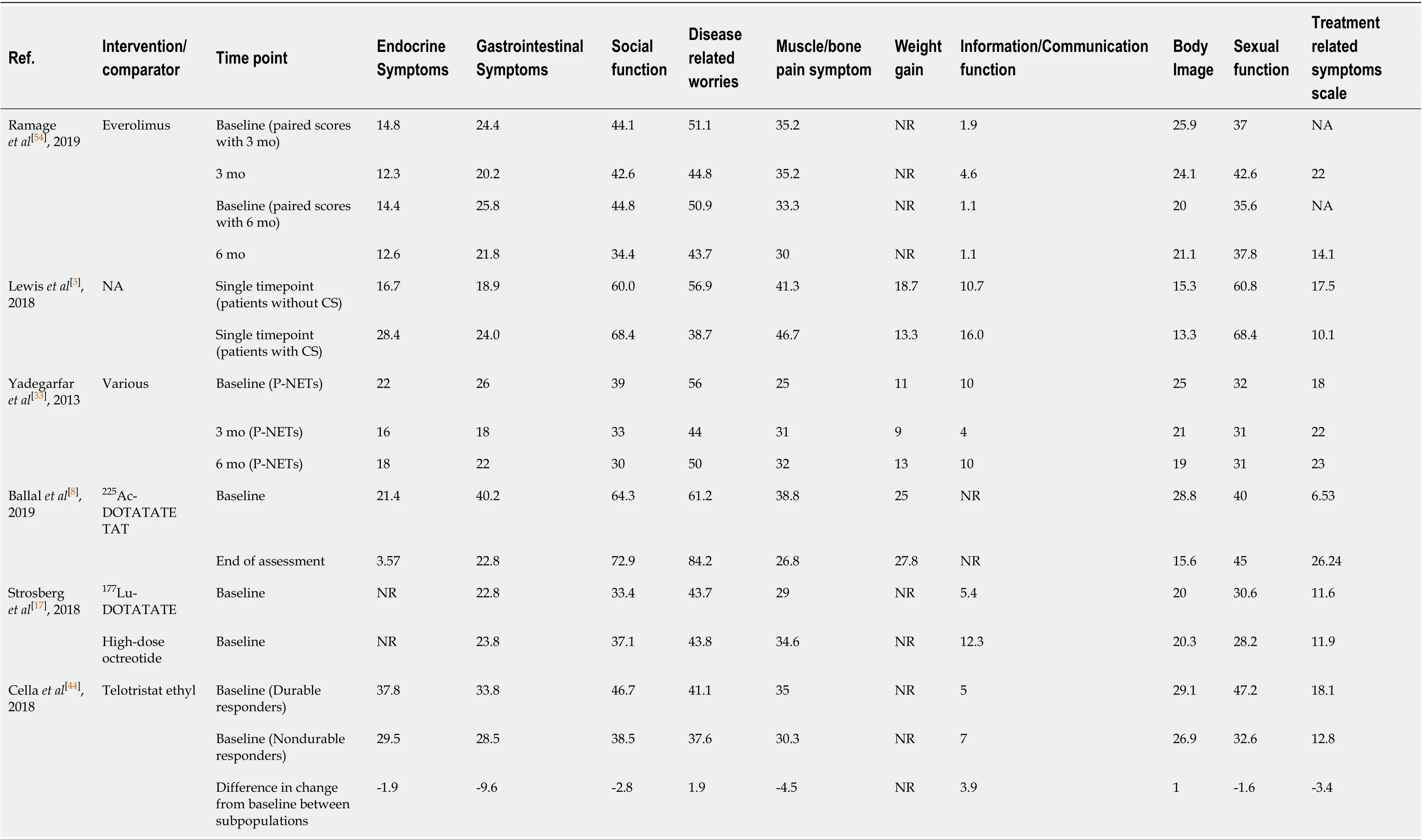
Table 3 The specific domain scores reported in the literature for gastroenteropancreatic neuroendocrine tumours patients using QLQ-GI.NET21
The SF-36 measures eight dimensions of HRQoL, where item scores are linearly transformed into scales ranging from 0 to 100, with higher scores indicating better HRQoL. In cross-sectional studies, HRQoL has been shown to be significantly reduced in patients with GEP-NETs compared to the general population in every SF-36 dimension[46,47]: 77.9vs70.0 for physical functioning, 65.0vs40.2 for role physical, 69.1vs64.3 for bodily pain, 74.3vs54.8 for general health, 60.9vs49.5 for vitality, 83.9vs75.5 for social functioning, 77.4vs64.4 for role emotional and 80.6vs74.9 for mental health. Mean scores for each scale were consistently worse in a sub-population of patients with impaired bowel/bladder function, which was strongly correlated with high scores for diarrhoea[46], a symptom of CS which reduces patient HRQoL. Elsewhere, Hauglandet al[9]reported only the physical component (39.6) and the mental component (45.9) scores; these were similar to baseline scores previously reported[53]. Frillinget al[48]reported improved HRQoL (assessed using SF-36) in 50% of patients in their prospective study of90Y-DOTATOC therapy followed by177Lu-DOTATOC, but sample size was low (n= 20).
Various studies used other HRQoL tools to evaluate HRQoL in GEP-NET patients; direct comparisons between these studies were limited by the small number of studies using each tool (n≤ 2) and the heterogenous population and treatment interventions. Pezzilliet al[49]was the only identified publication to use SF-12 in a population of PNET patients, reporting a good physical HRQoL that was not significantly different to the normative population (44.7 ± 11.0vs46.1 ± 9.9,P= 0.610), but a significantly impaired mental component (42.4 ± 13.0vs48.2 ± 9.8,P= 0.036). Pezzilliet al[49]was also the only study to employ the Beck Depression Inventory and State Anxiety Inventory Y-1; 8 patients (18.2%) identified with moderate depression, 9 patients (20.5%) with mild moderate depression, and 27 patients (61.4%) with no depression. Anxiety results were similar in the patients and in the normative population. Ramageet al[54]used the EQ-5D-5L (in addition to EORTC QLQ-C30 and GINET21), with no significant difference for the composite health index or self-rated (global) health score noted between baseline and 6-mo treatment with everolimus. The EQ-5D VAS was employed in two studies: Kulkeet al[55]examined patients receiving repeated 6-weekly cycles of oral sunitinib, noting no significant difference in VAS scores over the study period, whilst Pavelet al[56]reported a reduced mean VAS from baseline to end of treatment with oral everolimus (10 mg daily, in 28-d cycles) in non-P-NET patients (63.9 ± 19.0vs55.3 ± 23.0, respectively) compared to P-NET patients (68.8 ± 19.9vs66.5 ± 20.6, respectively). The FACIT-D measure is a symptom-specific measure used by Kvolset al[29]in a Phase II, open-label, multicentre prospective study of selfadministered pasireotide. Clinical responders were noted to have improved mean scores at the end of treatment compared to baseline. Kulkeet al[55]reported that the mean FACIT-fatigue score remained stable for each treatment cycle of sunitinib with a “modest” score increase during the dosing period. Elsewhere, Larssonet al[50]used the Functional Assessment of Cancer Therapy-Anaemia measure to assess the HRQoL of GEP-NET patients with anaemia being treated with erythropoietin; no significant changes were observed over the study period. Finally, O’Tooleet al[32]showed no significant difference in HRQoL scores between lanreotide and octreotide using the Index de Santé Perceptuel de Nottingham measure. These studies provide a basis for future randomised control trials to test whether interventions improve HRQoL for patients with GEP-NETs.
All studies included in this review reported the HRQoL of patients with inoperable tumours; the majority of which described the disease as metastatic and/or advanced (Table 1), and thus the disease could be categorised as stage IV. Out of these studies, only two provided explicit information regarding disease stage for the entire study population[57,58]. Notably, no studies investigated how disease stage and tumour functionality affect HRQoL in GEP-NET patients, with HRQoL data most often presented according to treatment comparator.
Treatment impact on HRQoL
Few studies reported a significant difference (P< 0.05) in the patients’ global health score between baseline and after a period of treatment (Table 2).
HRQoL was maintained with few deteriorations in patients receiving long-acting octreotide compared to the deterioration observed in patients who received a placebo[16,27]. Octreotide improved HRQoL scores for the clinically important GEP-NET symptoms such as fatigue, insomnia, diarrhoea and pain; whereas significantly earlier TUDD was observed in the placebo arm for fatigue, pain and insomnia in patients with GI-NETs than those treated with long-acting octreotide (P< 0.05)[16]. An improvement in the EORTC global health score was shown after 3 mo on octreotide but not when octreotide was combined with interferon alpha[31]. Flushing was shown to be reduced significantly following 12 mo of treatment with long-acting octreotide[30]. Further studies are deemed necessary to assess the HRQoL of GEP-NET patients treated with pasireotide, who are refractory or resistant to octreotide; symptom-specific scores and total FACIT-D assessments showed some variability and no clear trends but sample size was small (n= 30)[29].
Another somatostatin analogue shown to bring about significant improvement in the global health score of GEP-NET patients was prolonged-release lanreotide[57], possibly driven by improvements in symptom scales or single-term measures such as fatigue, pain, nausea/vomiting, sleeping problems, appetite, constipation, diarrhoea and financial problems; although dyspnoea became more severe during the 6-mo treatment period. O’Tooleet al[32]found no significant HRQoL differences between lanreotide and octreotide, although most patients prefer lanreotide due to its simplified mode of administration. However, examining evidence from the CLARINET study, and mapping EORTC QLQ-C30 to EQ-5D-based utility values, no significant differences in HRQoL were noted between the lanreotide and placebo arms[28].
PRRT treatment for GEP-NETs has been shown to result in stable HRQoL scores during and after a 3-mo treatment period, compared to baseline levels using the EORTC QLQ-C30 measure[59,60]. In more recent studies, treatment with177Lu-DOTATATE was shown to be a safe and effective therapy in the treatment of functioning P-NETs[58]leading to a significant increase in the global health score observed at follow-up compared to baseline (79.5vs61.7,P= 0.002). Furthermore, significant improvements in the physical functioning, role functioning, emotional functioning, and social functioning were reported. Of all symptom scales, only “fatigue” was found to have significantly decreased (27.3vs17.2,P= 0.02). These results are consistent with a recent retrospective analysis study conducted by Marinova[61]; HRQoL was significantly improved in GI-NET patients receiving177Lu-DOTATATE compared to baseline, revealing an increased global health score (3 mo after 1st, 2nd, and 3rdtreatment cycleP= 0.049,P= 0.004, andP= 0.041, respectively).
In the Phase III NETTER-1 study, 231 patients were recruited with 117 randomised to the177Lu-DOTATATE arm (200 mCi every 8 wk for four treatments) and 114 to the high-dose octreotide long-acting repeatable arm (60 mg every 4 wk)[17]. HRQoL analysis, conducted on an intention-to-treat basis, demonstrated that177Lu-DOTATATE provides a significant and clinically robust HRQoL benefit for patients with progressive GI-NETs (compared to high-dose octreotide). TTD, defined as the time from randomisation to first HRQoL deterioration of ≥ 10 points from baseline score, was significantly longer in the177Lu-DOTATATE arm (P< 0.001); the hazard ratio was 0.406 for global health status, 0.518 for physical functioning, 0.580 for role functioning, 0.621 for fatigue, 0.566 for pain, 0.473 for diarrhoea, 0.425 for body image and 0.572 for disease-related worries. Differences in median TTD were clinically significant in several domains: 28.8 movs6.1 mo for global health status, and 25.2 movs11.5 mo for physical functioning. Significant improvements in TUDD were also observed for the global health score (P< 0.001), as well as for physical functioning (P= 0.002), role functioning (P< 0.001), emotional functioning (P= 0.020), social functioning (P= 0.001), pain (P= 0.002), insomnia (P= 0.026), appetite loss (P= 0.009), constipation (P= 0.042), diarrhoea (P= 0.001), GI scale (P= 0.005), treatment scale (P= 0.002), social function scale (P= 0.011), disease-related worries scale (P= 0.001), and body image (P= 0.002).
Other PRRT interventions included the use of177Lu-DOTATOC[62],90Y-DOTATOC[59]and225Ac-DOTATATE[8].177Lu-DOTATOC treatment improved functional scales and most single item scores in GEP-NET patients, whilst symptom scale scores for fatigue and pain decreased significantly from 31.9 to 22.8 (P< 0.01) and 23.3 to 15.0 (P< 0.05), respectively[62]. Martiniet al[59]examined HRQoL following 4–6 cycles of177Lu-DOTATATE or90Y-DOTATOC. The most significant domain change for GI-NETs was noted for diarrhoea (-16.3 points,P= 0.008). Compared to90Y-DOTATOC,177Lu-DOTATATE demonstrated a reduced score for fatigue (-27.7 points,P= 0.020) and better physical functioning (+22.4 points,P= 0.050), cognitive functioning (+23.1 points,P= 0.003) and global health score (+17.3 points,P= 0.029) in P-NET patients.225Ac-DOTATATE therapy has the potential to overcome the resistance of GEP-NET to177Lu-DOTATATE beta therapy and promote remission with minimal or transient toxicity, whilst reducing endocrine (P= 0.001) and gastrointestinal symptoms (P< 0.001), although treatment-related symptoms were worsened by the end of the assessment (P= 0.001)[8].
A significant difference in the EORTC QLQ-C30 physical functioning scale was noted by Ramageet al[54]following 6-mo treatment with everolimus, but no other significant differences were noted during this treatment period; in line with what was also shown by analysis conducted in conjunction with the RADIANT trial[56]. Likewise, sunitinib has no significant impact on global health score of EORTC QLQ-C30, but it has been shown to improve the HRQoL symptom scores of insomnia and diarrhoea compared to placebo in P-NETs[34,35]. In another study[55], there was no significant difference in EQ-VAS scores over 6 treatment cycles of sunitinib. There was no significant impact on the HRQoL of patients with GI-NETs when treated with erythropoietin for the anaemia associated with interferon therapy[50].
Chemotherapy in the BETTER trial was chosen based on NET location; GI-NETs were treated with capecitabine and bevacizumab, whilst P-NETs patients received 5-fluorouracil/streptozocin with bevacizumab. Differences in the EORTC QLQ-C30 global health score between baseline, 3-mo, 6-mo and 12-mo follow-up assessments were not significant[40,41]. The NET01 trial suggested that the addition of cisplatin to the combined capecitabine and streptozocin regimen worsened HRQoL[42]. After three cycles, the mean global health score was 67.0vs62.2 (P= 0.5) in the capecitabine and streptozocin arm and 69.3vs52.2 (P= 0.052) in the capecitabine and streptozocin plus cisplatin arm. After 6 cycles, mean global health score remained virtually unchanged in the capecitabine and streptozocin arm from 66.7vs68.9 (P= 0.75) and 59.8vs56.0 (P= 0.68) in the capecitabine and streptozocin plus cisplatin arm. All three studies were based on small HRQoL sample sizes (less than 100).
Of the remaining treatments identified within the scope of this study, the HRQoL was improved or remained the same in all GEP-NET patients treated with MIBG therapy. Three studies used more than one treatment in the populations studied without breaking down HRQoL results into treatment categories[26,37,38]. Using HADS, Larssonet al[25]noted a lower anxiety rating at 12 mo (P< 0.05) following treatment with interferon, somatostatin analogue or a combination of both, whilst the depression score was significantly higher at 9 mo (P< 0.01) when compared to baseline. The study by Fröjdet al[37]suggested a slight increase in HADS anxiety over time (timepoints unspecified) in a longitudinal study of octreotide and/or interferon or chemotherapy or no treatment, but sample size was small and significance was not reported. Finally, telotristat ethyl therapy was not shown to be associated with any measurable shortterm HRQoL benefits when assessed using the EORTC QLQ-30 measure, beyond diarrhoea[44].
DISCUSSION
Given the indolent nature and slow progression of disease in most GEP-NET patients, assessment of HRQoL is vital for monitoring impact of treatment. The lack of conclusive data on outcomes in GEP-NETs can be attributed, in part, to the small sample size of clinical trials involving GEP-NETs that makes it difficult to capture clinically meaningful changes or a significant benefit in terms of HRQoL[6]. Moreover, the work by Martiniet al[18]showed that a transfer of HRQoL results into clinical practice is hindered by the scarcity of robust studies and the quality of HRQoL assessment[18]. To our knowledge, our study is the first to report a systematic review investigating the impact of treatments on the HRQoL of GEP-NET patients and the development status of instruments used in terms of validation and minimal important difference.
Martiniet al[18]performed a systematic review examining methodological robustness of HRQoL studies of patients with GEP-NETs. They concluded that heterogeneity and limited methodological quality of considered studies did not permit them to draw conclusions regarding the impact of HRQoL in clinical practice. In agreement, our review confirmed heterogeneity of studies and patient characteristics, including variety in intervention or comparator treatment, disease severity and location of primary tumour, age, gender and other symptoms or comorbidities. Sample size for studies considered eligible for inclusion ranged from 17 to 253 patients, with a median of 57, highlighting the usual small number of study participants. A similar median value of 51 (range 9–663 patients) was reported by Martiniet al[18]. Further, where comparator groups were used, HRQoL scores were sometimes reported for the entire study population and not for the respective treatment groups in the study[29,33,40,41,44]. Due to the wide range of comparators identified and the significant heterogeneity across the studies included in this review, which would violate the assumptions of meta-analysis, HRQoL related to various GEP-NET treatments was assessed in a qualitative manner rather than quantitative (e.g.meta-analysis).
In line with Martiniet al[18], we identified the well-validated EORTC QLQ-C30 as the most commonly used HRQoL instrument in GEP-NET patients. The EORTC instrument has been used in 30 out of 43 published studies; however, validation and definition of minimal important difference have not been reported to date specifically for GEP-NET patients. The EORTC QLQ-GINET21 module (n= 6) and the SF-12/36 tools (n= 5) have occasionally been used, with other instruments used in two or less publications.
Where change in the EORTC QLQ-C30 global health score could be calculated from baseline scores (Table 2), six studies demonstrated a trend towards an improved score, three showed a decline, with the remaining studies presenting mixed or inconsistent responses depending on intervention and/or follow-up time point. However, results should be interpreted with caution for the reasons given above. The mixed HRQoL trends are comparable to those reported by Jimenez-Fonsecaet al[63], who highlighted the existing evidence for improved treatment efficacy, safety or toxicity for a range of therapies, including somatostatin analogues, sunitinib and everolimus, but with mixed HRQoL response. Jimenez-Fonsecaet al[63]suggested the generic EORTC QLQ-C30 has not been developed specifically for GEP-NET patients and, therefore, may lack the sensitivity to detect subtle HRQoL changes in association with disease items that are relevant to GEP-NET patients.
Given the potentially long treatment duration in GEP-NET patients, one challenge of HRQoL analysis is taking the potential occurrence of a response shift into consideration. This can occur as self-assessment of HRQoL is inherently subjective; patients can adapt to their disease and the treatment toxicities resulting in changing standards of HRQoL over time[51]. Thus, the choice of the reference score to qualify a change such as deterioration is a major concern. With this in mind, it is difficult to determine the change in HRQoL over time in most studies identified in this systematic review. One approach that deals with response shift effect as a concept is evaluation of TTD, making use of minimal important difference as a criterion for signalling deterioration. In the Phase III trial NETTER-1, it was shown that adjusting for significant covariates (HRQoL baseline values, OctreoScan maximum tumour uptake score, and/or age), the impact of treatment remained statistically significant for global health score, physical functioning, diarrhoea, and body image domains[17]. Osobaet al[52]rated patients’ perceived change in EORTC QLQ-C30 in breast cancer or smallcell lung cancer patients, concluding a “moderate” change equated to a 10–20 change in mean scores. Use of a minimal important difference of at least 10 points as a criterion for deterioration yielded TTD curves with a time course similar to that of the curves for centrally reviewed radiological progression[17]. It should be noted, however, that the “minimal important difference” was not defined specifically for patients with midgut NETs participating in the NETTER-1 study. Moreover, the TTD and TUDD criterion of a drop in ≥ 10 points in a domain score was used to show more than half of the EORTC QLQ-30 domains improved for patients treated with177Lu-DOTATATE compared to a control arm; the same TUDD criterion was also applied by Rinkeet al[16], who showed long-acting octreotide significantly extended TUDD for fatigue, pain and insomnia HRQoL domains compared to a placebo.
Treatment of GEP-NETs with177Lu-DOTATATE consistently improved global health score compared to baseline[58,61,62]and increased TTD. The Phase III NETTER-1 study provides the best information available regarding HRQoL in patients with advanced progressive GEP-NETs not only in terms of benefit but also in terms of TTD[17]. However, the minimal important difference (and clinically meaningful thresholds) for improvement/worsening has not been defined yet for the EORTC QLQ-C30 specifically in GEP-NET patients, although it has for a number of other common cancers[64,65]. It is conceivable that the abbreviated version of the EORTC QLQC30 (possibly combined with GINET21 and computer adaptive testing) can be developed especially for use in clinical practice. Thus, there is scope for further research in this area and exploration of HRQoL as a potential tool for monitoring disease progression. Longitudinal studies with a long-term follow-up and involving clinical practice patients may provide further insight into the potential role of HRQoL in patient monitoring and management.
In conclusion, study and patient heterogeneity, small sample sizes and limited methodological quality in terms of assessing HRQoL has impaired definitive statements being made regarding the impact of the various treatment options for GEPNETs in terms of HRQoL[66-69]. A number of randomised trials demonstrated only small or no HRQoL changes between active treatment and placebo arms. The Phase III NETTER-1 is the only study to have shown more significant and robust differences between active and placebo groups. It demonstrated that PRRT can delay the time to worsening of HRQoL in GEP-NET patients. However, validation of QLQ-C30 and GINET21 specifically for GI-NET and P-NET patients, and definition of minimal important difference and clinical thresholds, are still lacking for these patients. As such, further research is necessary in order to offer a benchmark for interpreting the clinical importance of differences in HRQoL domain scores between groups or changes in these scores over time in conjunction with different treatment modalities.
ARTICLE HIGHLIGHTS


Research results
A total of 42 studies met the inclusion criteria of the study. The EORTC QLQ-C30 and gastrointestinal GI-NET module (QLQ-GI.NET21) were the most used HRQoL instruments, exhibiting a range of outcomes compared to baseline for the specific interventions.
The worst HRQoL observed at baseline were related to the following symptoms: Nausea and vomiting (4.1–20.0), insomnia (11.0–38.6), appetite loss (9.2–27.8), constipation (0–24.4) and financial difficulties (6.0–23.6). Diarrhoea demonstrated a wide range of mean values (16.7–78.3), which can be attributed to the heterogeneity of involved GEP-NET patients.
The average value for the baseline global health score had a range of 56–70. Where change in the EORTC QLQ-C30 global health score could be calculated from baseline scores, five studies demonstrated a significant trend towards an improved score. Treatment of GEP-NETs with177Lu-DOTATATE consistently improved global health score compared to baseline and increased time-to-deterioration,i.e. time until HRQoL worsens by at least 10 points for each domain (e.g.global health, physical functioning). The Phase III NETTER-1 study resulted in time-to-deterioration curves consistent with the curves for progression free survival for177Lu-DOTATATE. However, the EORTC QLQ-C30 and QLQ-GINET21 have not been validated, and the minimal important differences have not yet been defined, specifically for GI-NET and P-NET patients.
Research conclusions
Study and patient heterogeneity, small sample sizes and limited methodological quality in terms of assessing HRQoL has impaired definitive statements being made regarding the impact of the various treatment options for GEP-NETs in terms of HRQoL. A notable finding was that PRRT can delay the time to worsening of HRQoL in GEP-NET patients. However, validation of EORTC QLQ-C30 and GINET21 specifically for GI-NET and P-NET patients and the definitions of minimal important difference and clinical thresholds are still lacking for these patients. As such, further research is necessary in order to offer a benchmark for interpreting the clinical importance of differences in HRQoL domain scores between treatment groups or changes in these scores over time in conjunction with different treatment modalities.
Research perspectives
Further research is necessary in order to offer a benchmark for interpreting the clinical importance of differences in HRQoL domain scores over time and between treatment groups.
ACKNOWLEDGEMENTS
We would like to acknowledge Dr Persefoni Ioannou for providing editorial support and guiding the research from the outset of this project.
杂志排行
World Journal of Gastroenterology的其它文章
- TBL1XR1 induces cell proliferation and inhibit cell apoptosis by the PI3K/AKT pathway in pancreatic ductal adenocarcinoma
- Development of innovative tools for investigation of nutrient-gut interaction
- Practical review for diagnosis and clinical management of perihilar cholangiocarcinoma
- Management of nonalcoholic fatty liver disease in the Middle East
- Functionality is not an independent prognostic factor for pancreatic neuroendocrine tumors
- Risk factors associated with inflammatory bowel disease: A multicenter case-control study in Brazil
