Intratumoral heterogeneity of hepatocellular carcinoma: From single-cell to population-based studies
2020-08-17QiZhangYuLouXueLiBaiTingBoLiang
Qi Zhang, Yu Lou, Xue-Li Bai, Ting-Bo Liang
Abstract Hepatocellular carcinoma (HCC) is characterized by high heterogeneity in both intratumoral and interpatient manners. While interpatient heterogeneity is related to personalized therapy, intratumoral heterogeneity (ITH) largely influences the efficacy of therapies in individuals. ITH contributes to tumor growth, metastasis, recurrence, and drug resistance and consequently limits the prognosis of patients with HCC. There is an urgent need to understand the causes, characteristics, and consequences of tumor heterogeneity in HCC for the purposes of guiding clinical practice and improving survival. Here, we summarize the studies and technologies that describe ITH in HCC to gain insight into the origin and evolutionary process of heterogeneity. In parallel, evidence is collected to delineate the dynamic relationship between ITH and the tumor ecosystem. We suggest that conducting comprehensive studies of ITH using single-cell approaches in temporal and spatial dimensions, combined with population-based clinical trials, will help to clarify the clinical implications of ITH, develop novel intervention strategies, and improve patient prognosis.
Key words: Hepatocellular carcinoma; Tumor heterogeneity; Tumor microenvironment; Single-cell analysis; Local immunity on different terms, provided the original work is properly cited and the use is non-commercial. See: htt p://creativecommons.org/licenses/by-nc/4.0/
INTRODUCTION
Liver cancer is one of the leading causes of cancer-related death worldwide, and approximately 90% of these deaths are attributed to hepatocellular carcinoma (HCC)[1]. With several non-surgical therapeutic options (e.g., transcatheter arterial chemoembolization, radiofrequency ablation, stereotactic body radiation therapy, and systemic therapy) available, liver resection and liver transplantation remain the mainstay of cure of HCC. However, less than 30% of patients are eligible for surgery, and a considerable number of them will succumb to rapid tumor recurrence or metastasis[2]. Furthermore, HCC can demonstrate primary or secondary resistance to other interventions, including chemotherapy, targeted therapy, and immune checkpoint blockade. However, despite many cellular and molecular mechanisms revealed, the refractory nature of HCC to treatments is still far from understood. Thus, new perspectives are warranted to decode this intractable issue.
Intratumoral heterogeneity (ITH), first described in the 1830s by German physiologists Johannes Muller and Rudolf Virchow[3], is now found to be ubiquitous in all types of tumors and accounts for nearly all aspects of tumor progression[4]. Currently, people have realized that heterogeneity is not only a distinct morphological profile but also implicates genetics, epigenetics, transcriptomics, proteomics, posttranslational modification (PTM)-omics, metabolomics, and diverse tumor microenvironments (TMEs). Systematic ITH causes a wide functional divergence, providing a platform to perform natural selection in the particular TME and promoting the malignant phenotypes of HCC. Fortunately, the rapid development of next-generation sequencing (NGS) and mass spectrometry techniques has made elegant analysis of ITH a reality in recent years. Several advances in HCC have been achieved to help us gain in-depth insight into the evolutionary mode of tumorigenesis and development, the regulatory pattern of the signaling network, and the interaction between tumor cells and their microenvironment. Nevertheless, how to integrate the discoveries and implications of ITH into clinical practice is rarely discussed.
In this review, we provide an overview of ITH in HCC from microscopic to macrocosmic approaches to explore the clinical implications of ITH. Based on a systemic search of relevant studies from single-cell to population-based designs, we summarize the common patterns of ITH in HCC and propose a practicable way to guide the clinical management of this deadly disease.
CELLULAR HETEROGENEITY AND CANCER STEM CELL
Although heterogeneity at the DNA, RNA, and protein levels is frequently investigated, cells are actually the fundamental element of tumor heterogeneity that may impact tumor progression. Heterogeneity itself does not have a malignancyspecific implication since all healthy, transformed, and cancerous cells sustain heterogeneity to some extent. In the liver, hepatocytes are organized from the central vein to the portal node, showing a strong regularity of the gene expression profile[5,6]. For example, the expression of Alb, Glul, Cyp2e1, Cyp2f2, and other hepatocyte markers in different locations can be extremely different. Such cellular heterogeneity presented by highly regulated gene expression and exquisite cell spatial distribution is required for the normal physiological function of the liver. When hepatic cells transform, precancerous nodules show heterogeneous genetic and epigenetic patterns as well[7].
For cancer, pathologists first used microscopes to identify the diversity among tumor cells. Due to the limitation of observational technologies, it took more than a century to describe and explore cellular heterogeneity through pathological sections and immunohistological staining[3], by which ITH cannot be quantitatively evaluated. Thanks to the emergence of single-cell analytic approaches (e.g., single-cell sequencing, single-cell immunoblotting, single-cell mass spectrometry, and single-cell multiomic techniques) in the last decade, it is currently possible to establish a clear panorama of cellular heterogeneity[8-11]. Houet al[12]developed scTrio-seq, a single-cell triple omics sequencing technique, and provided the first piece of evidence regarding the genetic heterogeneity in HCC at a single-cell level. Two subpopulations with distinct biological functions were observed by unsupervised hierarchical clustering based on genomic copy-number variations (CNVs), although the cells in the same subset were also not identical[12]. Since then, cellular heterogeneity has been repeatedly corroborated in HCC from genomic, transcriptomic, and other dimensions[13-16](Table 1).
Since cellular heterogeneity varies greatly among individuals[14,17], it is of great value to find the key subpopulations of cancer cells with greater influence on ITH for further studies. Hence, a subset of cancer cells with stemness features, also known as cancer stem cells (CSCs), has attracted much attention[18-21]. Hepatic CSCs can be identified by various cell surface markers. For instance, EpCAM[22-25], CD90 (THY-1)[25-27], CD24[14,25,28], CD133 (Prominin-1)[14,25,28,29], CD13 (ANPEP)[29,30], CD44[16,25,30], CD47[30,31], and others have been identified in HCC in independent studies using single-cell sequencing[14,16]. More importantly, single-cell transcriptomic profiling indicated that stemness gene expression seemed to be consistent with the cancer diversity score based on principal component analysis and was significantly related to clinical outcomes[16,17,26]. These findings coincide with the “cancer stem cell model”, which is favored to explain the origin of ITH recently[32,33](Figure 1). Predominantly, this model demonstrates that only CSCs possess the capability of self-renewal, differentiation, and maintenance of tumor growth[34-36]. Similar to the normal tissue hierarchy, the hierarchical organization in cancer results from the punctuated proliferation of CSCs and consequently shapes.
Accumulating evidence likely reflects the fact that, even as an extremely tiny subset, CSCs can still be divided into various subpopulations with distinct cell membrane markers, molecular makeups, and functional phenotypes. Recently, elegant studies by Zhenget al[14]and Maet al[17]provided direct evidence of hepatic CSC heterogeneity and its clinical implications at a single-cell resolution. CSC sorting by CD24, CD133, and/or EpCAM proliferated divergently in both normoxia and hypoxia and showed a distinctive spatial distribution within HCC[14]. These findings demonstrated the uneven distribution of functionally diverse CSC subpopulations across different regions, and the current understanding of CSCs may only reflect the features of specific subgroups[26,37-42]. Moreover, CSC identity is not immutable[14,19]. Under the influence of genetic stochastic mutation and recombination, epigenetic modifications, and field effects in the TME, reversible transformation can occur between CSCs and non-CSCs, which establishes new hierarchical CSC clones to enrich ITH tremendously[32,43,44]. This ability to reconstruct heterogeneity cautions against therapies targeting CSC-related membrane epitopes and increases the difficulty of ITH management.
However, CSCs are not an undruggable subset. Growing evidence suggests that plenty of environmental factors, such as hypoxia, inflammation, and DNA damage, play crucial roles in CSC phenotypes mediating in various cancers[45,46]. As an illustration, hypoxia has received considerable attention in recent years[47]. Similar to oxygen gradients between arteries and veins in liver lobules, abnormal vascular perfusion in HCC induces differential activities of the HIF signaling pathway in an oxygen-dependent manner, and consequently stemness. Hence, it is a promising therapeutic strategy for treatment of CSCs and heterogeneity.
Further identification of CSC subpopulations and the regulatory mechanism of the CSC transformation process is of paramount importance to clarify the roles of CSCs in the development and management of ITH. An advanced understanding of CSCs may create new opportunities for overcoming cellular heterogeneity.
CLONAL HETEROGENEITY REVEALS TUMOR EVOLUTION
In addition to single-cell technologies, multiregional sampling, the method to sample biopsies from multiple regions within a single tumor, is the most commoninvestigational strategy to determine the extent of ITH[48-50]. This strategy is based on the “clonal evolution model”, which was first proposed by Nowell[51]in 1976, and became the most accepted hypothesis to depict the process of ITH in addition to the “CSC model”. In this theory, carcinogenesis is considered to be a stochastic process. Numerous random genetic alterations created by genomic instability and continuous stimulation of carcinogens results in the emergence of subclones. The subclones with a survival advantage in a spatiotemporally specific microenvironment will establish dominance, while the non-adaptive subclones will be eliminated. Upon the selective pressure of the TME, the complex architecture of ITH is preserved and dynamically reorganized[52,53](Figure 2).
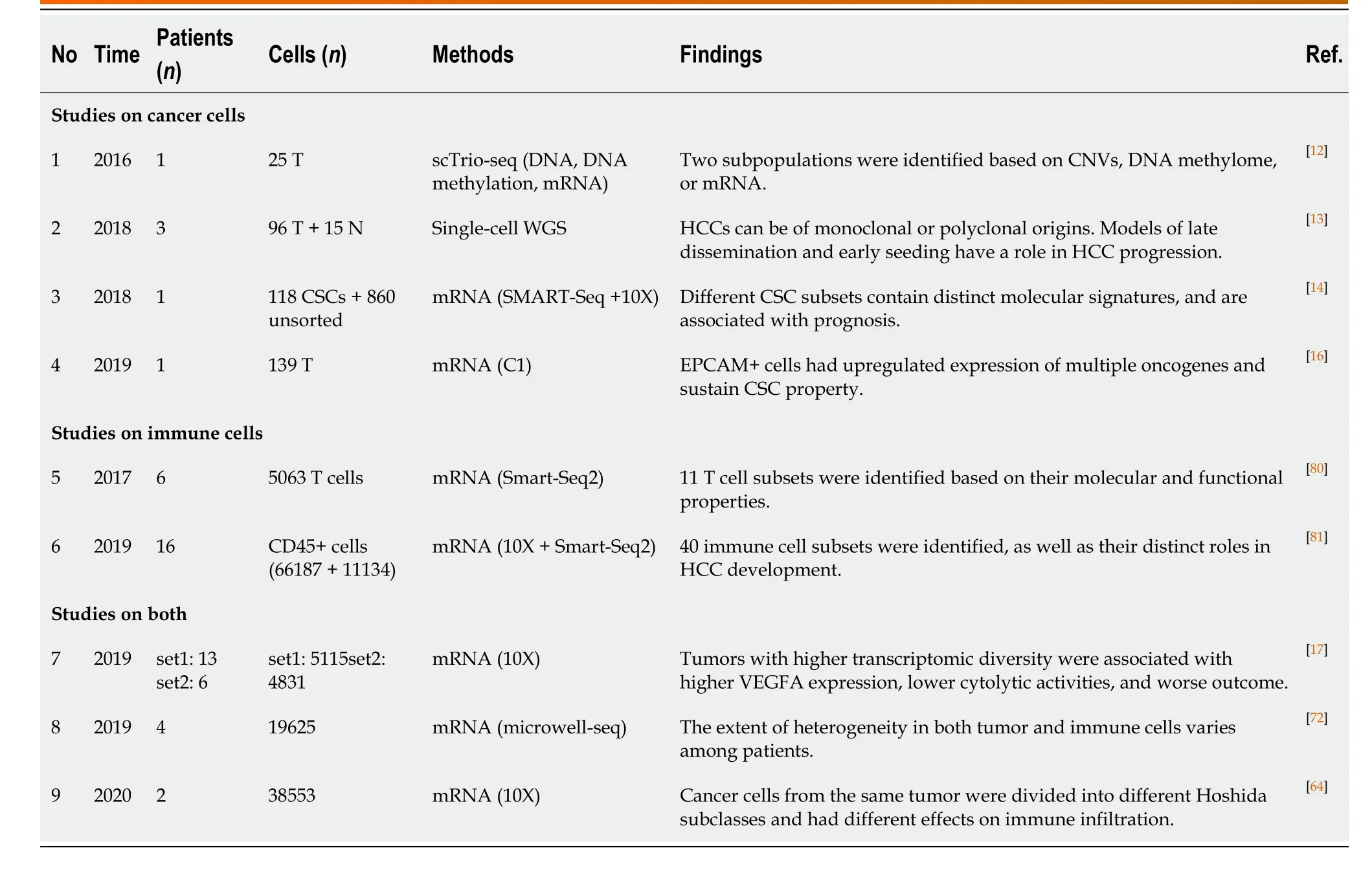
Table 1 Summary of studies on intra-tumoral heterogeneity in hepatocellular carcinoma using single-cell sequencing
Using a multiregional sampling strategy, spatially neighboring clones that are supposed to be genetically more similar can be pooled and compared with bulk samples from other regions. In early studies, ITH in morphology and genomics was poorly described[54-56]. Subsequently, bulk whole-genome sequencing (WGS)[57-59], whole-exome sequencing (WES)[59-62], targeted sequencing[63], single-cell se- quencing[13,64], and DNA methylation profiling[61,65]were introduced into heterogeneity studies to quantify genetic alterations and provide a more comprehensive blueprint of hepatic ITH. Public, shared, and private genetic changes were defined to construct an evolutionary tree of tumor subclones[66]. As expected, the variable extent of ITH and the branch evolutionary model are well recapitulated[13,57-59], and subclonal populations that are spatially closer tend to be genetically more similar in both planar and solid models[59,65], which suggests the existence of a common ancestral clone and a continuous clonal expansion pattern with the accumulation of mutations. In the meantime, the time when potential driver mutations formed can be estimated. Similar to the mutation profile of HCC, the most common alterations, such as key driver mutations (e.g.,TP53,CTNNB1, andTERT) and CNVs (e.g., amplification in 1q and deletions in 4q and 16q), are mostly identified in the trunks[57-59,61,63], revealing that they may represent an early event in HCC progression. However, an uneven distribution of driver mutations inTP53andCTNNB1across different regions was also observed in a minority of patients[58,62,63,65,67,68]. These findings indicated that this heterogeneous disease can be either monoclonal or polyclonal in origin. Additional mutations inAXIN1,RB1,KIT1,FAT4, and other HCC-related genes involved in clonal evolution could dramatically change marker expression (e.g., CK7, β-catenin, and GS) and functional phenotypes[60-62,65,67,68]. It is the functional diversity that promotes tumor development and, more importantly, worsens clinical outcome[60]. Unlike genetic alterations, ITH of genomic methylation appears prior to tumor initiation and forms clonal expansions in nonmalignant tissues[7,63]. In regard to cancer, regulation of DNAmethylated heterogeneity acts in a conservative manner, largely driven by the tumor itself and significantly related to tumor progression[13].
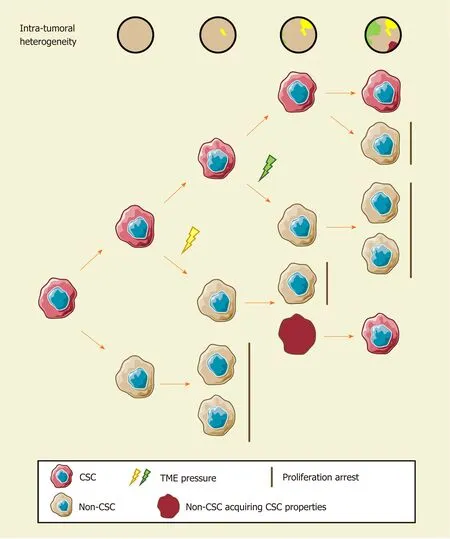
Figure 1 The cancer stem cell model and intratumoral heterogeneity. The cancer stem cell (CSC) model assumes that only CSCs possess robust selfrenewal characteristics and the ability to differentiate. Different stimuli of CSCs induce distinct functional phenotypes to adapt to certain environmental stresses. Non-CSCs can spontaneously convert to a stem-like state and establish a new hierarchical CSC clone under certain circumstances. Consequently, the extent of ITH in hepatocellular carcinoma increase gradually. CSC: Cancer stem cell.
Clearly, clonal diversity changes dynamically with tumor evolution, but ITH is not necessarily correlated with positive changes. In support of this hypothesis, the findings of several studies have suggested that various genetic alterations in the single key gene across regions (e.g., loss-of-function and stop-gain mutations inCTNNB1) embody convergent evolution of HCC under microenvironmental stress[57,65]. However, one size does not fit all in regard to clonal evolution. A study collected 286 samples on a plane of a single HCC and drew the most elaborate clonal map of HCC to date[69]. More than 100 million mutations are defined, illustrating astonishing genetic heterogeneity and a non-Darwinian mode during HCC development. In addition, another work using single-cell genome sequencing likely demonstrated the punctuated pattern[13], which is characterized by the early occurrence of numerous genetic alterations[70].
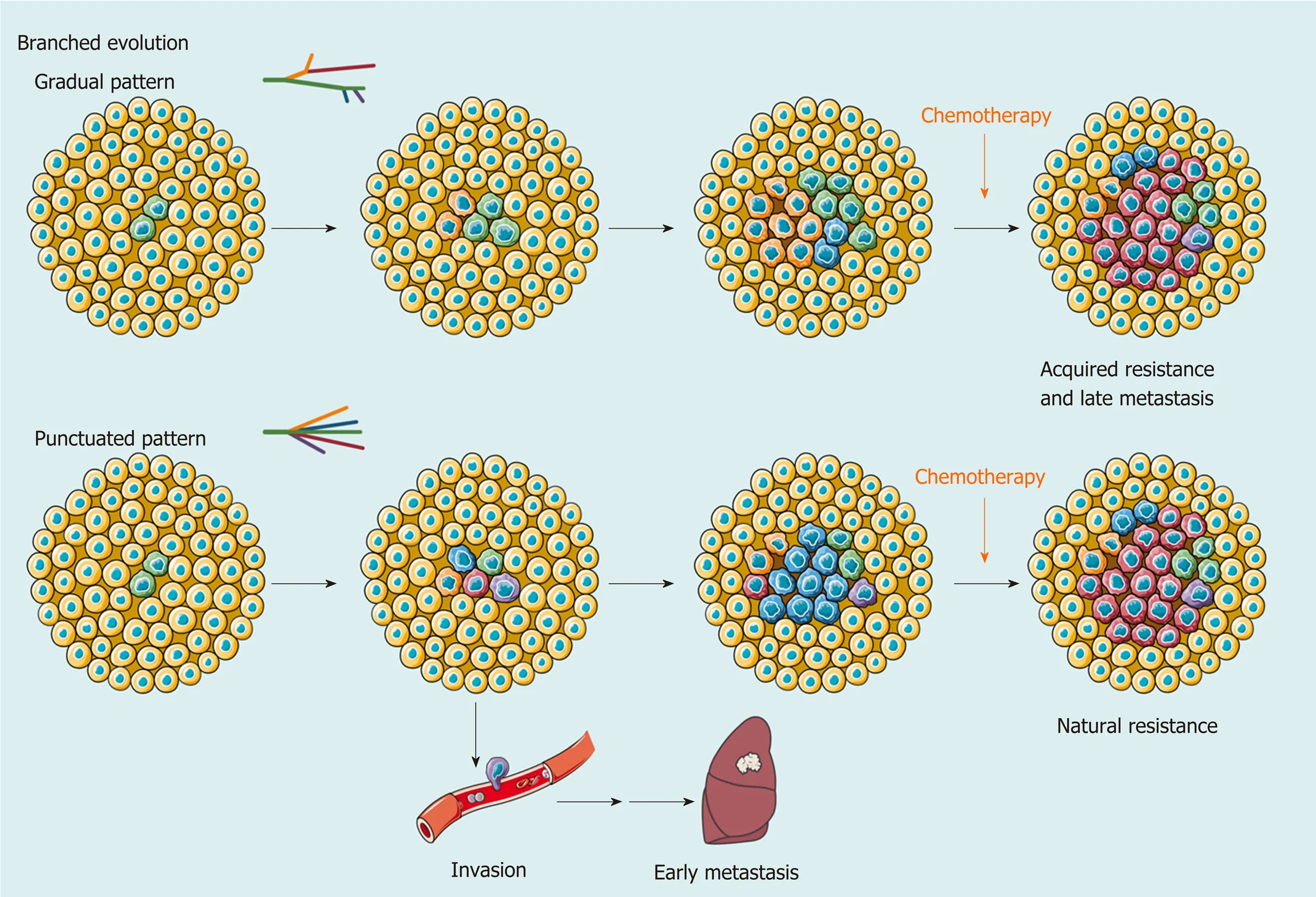
Figure 2 Branched evolution shapes intratumoral heterogeneity. As a result of branched evolution, the genetic heterogeneous subclones may be generated through two different trajectories. In the gradual pattern, subclones increase gradually due to genomic instability or microenvironment changes, such as acquired drug resistance and metastasis caused by systemic therapy. However, the punctuated pattern, also known as big bang mode, supposes that all subclones already exist in early development of hepatocellular carcinoma (HCC). Different subclones obtain a survival advantage in spatiotemporally specific microenvironment and change the extent of intratumoral heterogeneity at a clonal level. This model provides an explanation for early metastasis and natural resistance of HCC in clinic.
Another focus in ITH studies based on multiregional sampling is tracking the origins of different nodules to clarify the significance of ITH in invasion and metastasis. Comparing the primary lesions with intrahepatic metastases, tumor thrombi, and satellite nodules found that migration is likely to occur either at the early or late stage of HCC development, while satellite nodules appear at the late stage[57,59,65]. Multicentral lesions, regarded as a unique subtype of HCC, share few common driver mutations, which implies a parallel evolutionary pattern[57,62,63]. These findings provide a theoretical basis for accurate differentiation between intrahepatic metastases and multicentric lesions in clinical practice[58,63].
Generally, a multiregional sampling strategy improves the ability to explore the symbiotic relationship between clonal heterogeneity and tumor evolution and enriches the value of ITH investigations in precision medicine. However, it can be impractical to obtain multiregional samples from an advanced tumor in clinical practice due to the high risk of bleeding after biopsy. A clinically friendly approach for ITH investigation is urgently needed.
FUNCTIONAL HETEROGENEITY ORCHESTRATES THE TUMOR ECOSYSTEM
Although studies of genetic and epigenetic heterogeneity reflect the life history of tumors, functional diversity is the factor affecting clinical outcome directly. The concept of “functional heterogeneity” was proposed in 2018 to integrate information on genomics, nongenomics, stemness, and TME heterogeneity[71]. Here, we adopt this concept to summarize the functional and microenvironmental characteristics of ITH and discuss its significance in tumor progression and treatment.
Transcriptomic[58,62,72]and proteomic profilings[72,73]take the lead in describing functional heterogeneity. Unsurprisingly, ITH at the RNA and protein levels is apparent. However, the gene expression profile does not seem to strictly follow the law of genetic alterations[58,62,72,73]and has poor relevance to tumor geometry[73]. Due to the distinct gene expression profiles across regions, neither the origin of metastatic clones[58]nor the potential targets for clinical intervention[72,73]can be distinguished; however, the pivotal role of TME in functional heterogeneity is still reflected[62,72,73].
Owing to the breakthrough of immunotherapy, local immunity in HCC has attracted growing attention. Multiplex immunohistological staining was first used to decipher tumor infiltrating immune cells (TICs) in solid tumors[74]. Apparently, ITH of the microenvironment is neatly illustrated by the density, distribution, and composition of TICs and is likely correlated with tumor differentiation[74], which reveals the coordination of the tumoral functional phenotype and immune infiltration. To deeply explore the interaction between TICs and ITH, various algorithms have been developed to quantify the composition of immune infiltration using sequencing data[75-78]and expand the effectiveness of immune function assessment enormously[62,79]. Coincidentally, the latest two studies integrated DNA-seq, RNA-seq, T-cell receptor (TCR)-seq, neoantigen analysis, and other methods to uncover the role of functional heterogeneity in immune evasion[64,79]. Cancer cells across different regions present different immunogenicity and escape immune surveillance in distinct ways, such as a decrease in immune cell infiltration, loss of heterozygosity of human leukocyte antigen, low TCR clonality, and immunoediting[64]. This finding suggested that the coevolution of tumor and local immunity is quite ubiquitous in HCC and could partially explain the low response rate of immune checkpoint blockade monotherapy in HCC.
Similar to previous studies on clonal evolution[58,63], ITH in intrahepatic metastases and multicentral lesions was compared at an immunological resolution to evaluate the potential effect of immunotherapy[79]. Uneven composition of immune cell infiltration was observed, such as abundant TAM infiltration in intrahepatic metastases and rich CD8+ T-cell infiltration with a higher level of inhibitory immune checkpoint expression in multicentral lesions, which suggested that patients with multicentric HCC could be more likely to benefit from immune checkpoint blockade[79].
In addition, single-cell sequencing is also applied to TME research for a better understanding of immunological heterogeneity. Systematic works completed by Zemin Zhang’s group provided a dynamic atlas of the immune landscape of HCC[80,81]. When integrating tumoral single-cell transcriptome data, the HCC cells of different Hoshida subclasses in a single tumor induced regional variance in the antitumoral immune response[64]. More importantly, the extent of ITH was responsible for hypoxia adaptation, VEGF expression, and drug resistance[17], which may be considered in the choice of transarterial (chemo)embolization, sorafenib, lenvatinib, or other antivascular therapies.
Our group also made some efforts to provide a comprehensive landscape of ITH of HCC in multiple dimensions[72]. The research integrating genomic, transcriptomic, proteomic, metabolomic, and immunomic (mass cytometry) data together demonstrated a heterogeneous interactive network of HCC cells, metabolites, and TICs and proposed a novel clinically friendly immunophenotypic classification to improve the clinical management of patients with HCC. We found that tumor cell heterogeneity and microenvironmental heterogeneity, which included diverse TICs, were closely related to each other and showed coevolution. Thus, the evaluation of the functional heterogeneity of HCC requires comprehensive investigation and currently lacks accurate and practical biomarkers.
ITH FROM THE PERSPECTIVE OF POPULATION
The significance of heterogeneity has been demonstrated in accumulating studies, but large-scale population-based studies are still needed to verify the effectiveness of ITH investigation in clinical practice (Table 2). A well-known prospective cohort study on non–small cell lung cancer (NSCLC) included 100 patients with NSCLC and validated the feasibility of predicting clinical outcome and recurrence with ITH[82]. ITH may also play a prognostic role in HCC since genome instability is a common feature of cancer[59]. A clinical trial aiming to investigate the clinical trajectories of HCC during its progression (NCT03267641) is critical to understand the dynamic change and clinical relevance of ITH. However, it is still ongoing with limited information to disclose. Current limited evidence reveals that heterogeneity evaluation of recurrent and primary tumors may serve as a predictor of clinical outcome[65,83].
To clarify the clinical implication of heterogeneity, researchers have developed different methods to quantify ITH with single bulk sequencing data from public databases to predict survival and recurrence. With single-region bulk DNA data, the heterogeneity score (or diversity score) calculated by mutant allele tumorheterogeneity and clonality approaches significantly distinguished patients with a higher ITH signature and poor prognosis[61,64]. It is remarkable that tumor clonality outperforms mutational burden in predicting prognosis in the TCGA-HCC dataset[64], indicating the crucial value of ITH in HCC. Other methods, such as the mITH method for DNA methylation data[61], the ITH gene signature algorithm for RNA data[17,64], and ITH-related models of CSCs and immune evasion[79], shared similar results that a higher level of ITH was associated with limited survival, which is also observed in many other types of cancer[84-86]. Moreover, evidence across a wide range of cancer types illustrated that ITH fostered resistance to various treatments[82,87-89]. In HCC, sorafenib-targeted genetic alterations have been constantly validated as a subclonal change[62], which may explain the low objective response rate to sorafenib in the clinic[90,91]. Furthermore, the response to immunotherapy is also manifested by functional heterogeneity. Tumors with higher ITH tend to secrete more VEGFA to promote immune suppression and limit the clinical efficacy of immune checkpoint blockade[17]. Similar phenomena were also observed in other tumors[92,93].
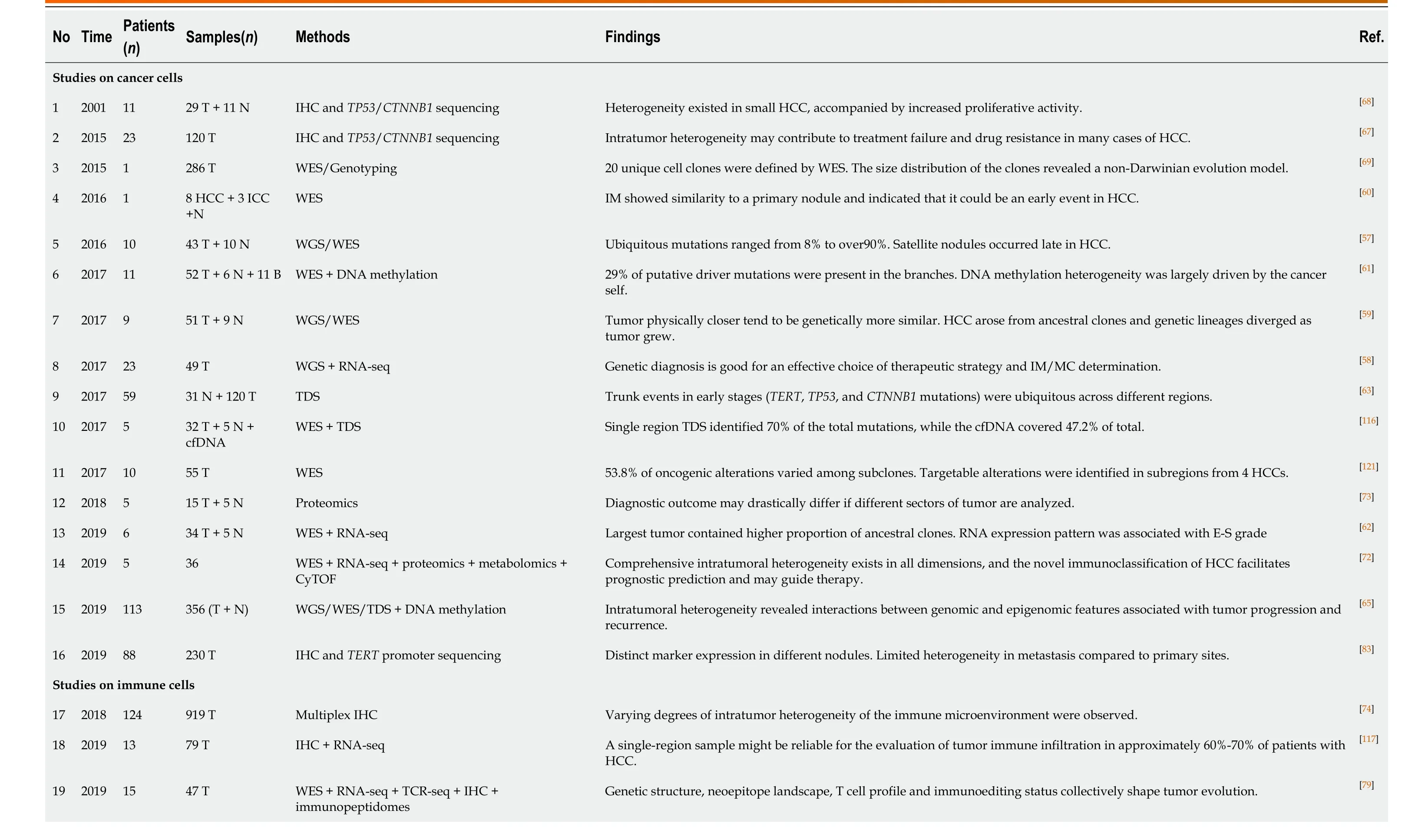
Table 2 Summary of studies on intra-tumoral heterogeneity in hepatocellular carcinoma using multiregional sampling

HCC: Hepatocellular carcinoma; T: Tumor tissues; N: Adjacent tissues; WGS: Whole-genome sequencing; WES: Whole-exome sequencing; cfDNA: Cell-free DNA; IHC: Immunohistochemistry; TDS: Targeted deep sequencing.
In the future, more stable, practicable, and cost-effective techniques should be introduced to large-scale population-based studies to broaden the scope of the clinical application of ITH. Based on current evidence, liquid biopsy and advanced imaging are promising candidates. Cell-free DNA (cfDNA) is one of the most notable minimally invasive examinations for cancer in recent years and is used to evaluate tumor load, tumor mutational burden, tumor recurrence, drug resistance, and patient survival[94-96]. There is evidence suggesting that cfDNA can reflect ITH in HCC and offer some insight into tumor evolution[97,98]. Using cfDNA sequencing, practical genetic heterogeneity assessment in advanced HCCs with a large size or multiple lesions becomes possible, although many technical and clinical confirmations are warranted before it can be accepted by physicians. cfDNA sequencing also creates the possibility for dynamic observation of ITH either combined with or even substitute sequential sampling[99]. By tracking the dynamic evolution of HCC, people can make more precise interventions for this heterogeneous disease and may obtain better efficacy. In addition, multiparametric magnetic resonance imaging and computed tomography have been proven effective in reflecting the histologic and genomic features and prognosis of HCC[100,101]. In lung cancer, breast cancer, and esophageal cancer, a quantitative ultrasound system and positron emission tomography/computed tomography are qualified to image ITH[102-105]. Combined with liverspecific contrast agents and radiomic analysis, the digital imaging system may bring ITH assessment into a new chapter[106-109]. However, more clinical trials are expected to test the potential role of these approaches in benefit-gain for HCC patients.
MANAGEMENT OF ITH IN HCC
Here, we take ITH into clinical consideration and discuss how to embody the implication of functional heterogeneity in HCC patient management. At present, only a few traditional indicators are well accepted and widely adopted by clinicians in HCC. Tumoral macrovascular invasion (MVI)[110-112]and TNM classification[113,114]systems, for example, are normally used for disease staging, therapy decision making, and prediction of prognosis. However, the demand for precision medicine forces clinicians to biopsy every tumor to obtain a molecular makeup for individual treatment. How to ensure the representativeness of biopsy samples under the influence of heterogeneity becomes an important and unavoidable problem. A study on renal cancer proposed that at least three distinct regions of the tumor should be sampled to detect five key genes with an accuracy of 90% or above[115]. In HCC, researchers carried out WES, targeted deep sequencing, and cfDNA sequencing to identify the effects of sampling strategy on genetic mutation detection[116]. The results indicated that targeted sequencing with single sampling could identify approximately 70% of the total genetic mutations, while cfDNA had merely 47.2%. Another study revealed that 60%-70% of patients with HCC obtained reliable results of immune cell infiltration with a single sample[117]. Altogether, these results suggested that ITH did affect clinical examination at least to some extent, and multiregional sampling might diminish or even eliminate such influence. Considering the clinical risk of the sampling strategy, it is of great value to find more effective methods for patients with advanced HCC.
ITH may also act as a novel measure combined with other indicators to establish a more comprehensive and effective system for several clinical scenarios. ITH quantification can be performedin silico, as mentioned above, using high-throughput sequencing data and radiomic data. Acquiring data from cfDNA or imaging can not only provide information on the entire tumor but is also minimally invasive or noninvasive, cost-effective, and temporally dynamic, which brings infinite possibility to clinical practice. In support of this strategy, the findings of a previous study highlighted the positive correlation between VEGFA expression and ITH, which leads to severe immune exhaustion in more heterogeneous tumors. This phenomenon indeed provides an explanation for the combination therapy of immune checkpoint inhibitors and anti-VEGF molecules to improve therapeutic efficacies (NCT03434379, NCT03006926, NCT03418922)[118,119], suggesting that ITH investigation may screen out HCC patients who benefit from anti-VEGF therapies.
The use of ITH to propose effective intervention strategies is the most attractive aim of the translational study of ITH. Pan-cancer therapeutic strategies targeting CSCs or clonal evolution have been fully described in previous reviews. The former includes inhibition of CSC signaling pathways, CSC ablation using antibody-drug conjugates, and epigenetic therapies[43]; the latter includes clonal events targeting therapies, attenuating or exploiting genome instability, and exploiting evolutionary constraints[66]. To provide a model for drug sensitivity assessment of heterogeneous clones, Nuciforoet al[120]established an organoid system with needle biopsies and succeeded in preserving the heterogeneity of original cancers. In another study, WES of 55 samples from multiple regions of ten HCC patients indicated that 40% of patients could be targeted by existing pharmacologic agents, and the significance was verifiedin vitro[121]. Our group developed a potential intervention strategy based on a novel immunoclassification according to ITH; however, the validity still needs to be verified in clinical trials[72]. Indeed, there are few treatments against ITH currently published regarding HCC and other types of liver cancer, and more research is thus urgently needed to explore the feasible strategy for HCC treatment and determine the roles of ITH in HCC management (Figure 3).
CONCLUSION
In general, ITH is a crucial issue that cannot be avoided in HCC progression and management. Although great efforts have been made to obtain a more comprehensive understanding of heterogeneity, the field of hepatic ITH-targeting therapeutics is still in its infancy. Heterogeneous clones and relevant ecosystems are spatially distinct and dynamically change over time, which poses many challenges to clinicians and scientists. Therefore, particular attention should be paid to several aspects in further research. First, integrative analysis of multi-omics data should be performed to establish a systematic relationship between genetic evolution and immune evasion in
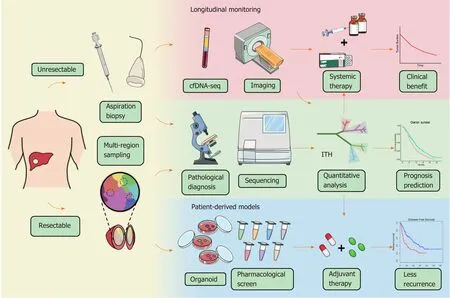
Figure 3 Schematic diagram of potential workflow for intratumoral heterogeneity clinical applications. For the patients with resectable liver cancer, intratumoral heterogeneity (ITH) can be evaluated by multiregional sampling of surgical samples, and in the meantime, patient-derived organoid model can be constructed for drug screening. The individualized treatment strategy will be determined according to the heterogeneity evaluation and the pharmacological results. For the patients with advanced hepatocellular carcinoma, biopsies will be used for both pathological diagnosis and ITH investigation. Moreover, ITH evaluation based on cell-free DNA and advanced imaging should be carried out to monitor ITH dynamically and guide medication adjustment. ITH: Intratumoral heterogeneity; cfDNA: Cell-free DNA.
HCC. Second, longitudinal monitoring of ITH alterations should be conducted for a high-resolution trajectory of clonal evolution. Finally, prospective population-based studies on HCC should be performed to accelerate the translation of ITH assessment into clinical practice. With an improved understanding of ITH, the evolutionary trajectory of HCC is likely to be predictable in the near future. Through the entire process of tracking tumor development, people may rewrite the clinical management of HCC and ultimately find solutions to completely change the outcome of HCC patients.
杂志排行
World Journal of Gastroenterology的其它文章
- Nomogram for predicting transmural bowel infarction in patients with acute superior mesenteric venous thrombosis
- Functional gastrointestinal disorders in inflammatory bowel disease: Time for a paradigm shift?
- Helicobacter pylori-induced inflammation masks the underlying presence of low-grade dysplasia on gastric lesions
- Adipose-derived mesenchymal stem cells alleviate TNBS-induced colitis in rats by influencing intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity
- Combining protein arginine methyltransferase inhibitor and anti-programmed death-ligand-1 inhibits pancreatic cancer progression
- Expression of Notch pathway components (Numb, Itch, and Siah-1) in colorectal tumors: A clinicopathological study
