Helicobacter pylori-induced inflammation masks the underlying presence of low-grade dysplasia on gastric lesions
2020-08-17AlbaPanareseGiovanniGalatolaRaffaeleArmentanoPedroPimentelNunesEnzoIerardiMariaLuciaCarusoFrancescoPesceMarcoVincenzoLentiValeriaPalmitessaSergioColettaEndritShahini
Alba Panarese, Giovanni Galatola, Raffaele Armentano, Pedro Pimentel-Nunes, Enzo Ierardi, Maria Lucia Caruso, Francesco Pesce, Marco Vincenzo Lenti, Valeria Palmitessa, Sergio Coletta, Endrit Shahini
Abstract
Key words: Autoimmune gastritis; Dysplasia; Diagnosis; Malignancy; Gastric cancer; Symptoms; Signs
INTRODUCTION
Helicobacter pylori(H. pylori) infection has been associated with premalignant gastric conditions (PGC), such as chronic atrophic gastritis and intestinal metaplasia (IM), which are strongly associated with dysplasia and Lauren intestinal-type of gastric carcinoma (GC)[1-7]. Autoimmune atrophic gastritis (AAG) is responsible for progressive mucosal atrophy (antrum-sparing) with or without IM[8]. Many studies have attributed gastric carcinogenesis to both genetic predisposition andH. pyloriinduced gastric inflammation[9-13].H. pyloristrains possessing higher cytotoxicity that infect genetically predisposed subjects have been considered responsible for more severe degrees of inflammation and rapid progression to intestinal-type GC[14-20]. Nowadays, PGC detection and surveillance are considered a cost-effective strategy for the prevention of high-grade dysplasia and GC only in intermediate or high-risk populations[7]. Heterogeneous long-term endoscopic follow-up studies, with a duration of between 2-16 years, have shown conflicting results on the efficacy ofH. pylorieradication in reducing the prevalence and histological progression of advanced PGC, and in decreasing GC incidence[3,21-25]. The current European guidelines recommendH. pylorieradication in high-risk subjects[7,22,26,27]. Nevertheless, even afterH. pylorieradication, the risk for PGC may remain or even increase in patients undergoing long-term surveillance[3,22].
A high-quality upper endoscopy should include at least five non-targeted biopsies at the lesser and greater curvatures of the antrum-corpus and at theincisura angularisforH. pyloriinfection diagnosis and the optimal detection and staging of advanced PGC, which are randomly distributed across the stomach[7,28,29]. Additional targeted biopsies of any visible lesions are recommended, since low-grade dysplasia (LGD) and high-grade dysplasia (HGD) may appear as endoscopically evident, depressed, flat, or raised lesions[7,28,29].
The Sydney System, the Operative-Link on Gastritis-Assessment (OLGA) and the Operative-Link on Gastric-Intestinal Metaplasia (OLGIM) classifications are commonly used to evaluate the histological inflammation and activity due to mononuclear and polymorphonuclear cells infiltration, as well as atrophy and IM, whereas dysplasia is commonly graded according to the World Health Organization classification[30,31]. During white-light endoscopy (WLE), the Kimura-Takemoto modified classification has been used to assess the extension of atrophy[32]. Several studies showed that magnification chromoendoscopy (CE) and narrow-band imaging (NBI) with or without magnification performed by expert endoscopists could be more accurate than WLE alone in diagnosing PGC, although random biopsies may detect PGC otherwise undetectable by NBI targeted biopsies. Thus, a combination of both random-WLE and targeted-NBI biopsies is suggested as the most accurate[29,33-37]. More recent some investigators created and validated a simplified NBI classification, with magnification [(endoscopic grading of gastric intestinal metaplasia (EGGIM)], using reproducible NBI features (based on endoscopic mucosal and vascular patterns)[29]of the whole gastric mucosa, with an accuracy (resulting from a multicentre study) of 73%, 87% and 92%, forH. pylori-infection, IM and dysplasia diagnosis, respectively[37].
For patients with an indefinite diagnosis for dysplasia, or with dysplasia resulting from random biopsies without endoscopic evidence of visible lesions, the current guidelines suggest an “immediate” endoscopic reassessment with high-resolution endoscopy with NBI to exclude LGD or HGD on missed visible lesions[7]. This procedure revises what was indicated in previous guidelines[28], which advised, in such an event, a delayed endoscopic follow-up within one year after the diagnosis[7,28].
On these premises, supposing that dysplastic lesions might be missed on the background of an activeH. pylorirelated gastritis, we focused on the assessment of the combined diagnostic performance of high-resolution white-light endoscopy (HR-WLE) with NBI in detecting PGC, before and 6 mo afterH. pylorieradication (primary endpoint). As a secondary aim we considered some clinical factors, autoimmune laboratory markers, and histopathological features as potential co-factors for the development of dysplastic lesions.
MATERIALS AND METHODS
Study design, patient selection and endoscopic follow-up
This is an observational, prospective study performed at the tertiary care center of the National Institute of Gastroenterology “S De Bellis” (Castellana Grotte, Bari, Italy). Our research was carried out in compliance with the Declaration of Helsinki and with routine clinical practice (Clinical Trial Gov number NCT03917836). All the procedures received local ethics committee approval (Protocol 67/18/CT, 140/18/CE). All patients gave their written informed consent to take part in the study.
From June 2017 to April 2019, we enrolled 85 consecutive subjects, from a cohort study of 156 outpatients, who underwent high-resolution gastroscopies with/without NBI using the Olympus Evis Exera III processor®and Olympus GIF-HQ190®instruments (Olympus Tokyo, Japan). Figure 1 shows the inclusion and exclusion of enrolment steps.
For patients with suspected AAG, anti-parietal cell antibody (APCA) and antiintrinsic factor (AIF) were measured in serum using indirect immunofluorescence method and immunoenzimatic assay respectively. APCA antibodies were detected by NOVA Lite®Stomach Kit (Inova Diagnostics, San Diego, CA, United States) and AIF antibodies were detected by an automated assay using a Beckman Coulter Unicel DXI 800 (Beckman Coulter Inc., Fullerton, CA, United States).
The endoscopic procedures were performed by two expert endoscopists (more than 200 HRE-NBI per year), one of whom (AP) was present during all procedures as either operator or supervisor. The statistical review of this study was executed by an experienced biomedical statistician. Based on previously published data[38], we calculated the sample size for the analysis of the primary outcome using the function ss2x2 implemented in the package exact2x2. We examined that 48 patients (24 controls and 24 treated) would yield a power of 0.82 at a significance level of 0.05. Therefore, we aimed at enrolling at least 48 patients. The study was completed when the 85 enrolled subjects had been endoscopically reassessed with HR-WLE and NBI, and with gastric biopsy samples according to the Sydney System[28], 6 mo afterH. pylorieradication confirmed by13C-urea breath-test (13C-UBT), in order to identify the diagnostic yield of HR-WLE with NBI in detecting PGC, during this short surveillance time.
13C urea breath test was performed under the following conditions: An 8-h fast, mouth washing before dosing, administration of 75 mg13C urea in a water solution, collection of breath samples in two 10-mL glass-sample containers, (at baseline and 20 min) and subjects in a sitting position. The breath samples were analyzed by gaschromatography-mass spectrometry (GC-MS; ABCA Sercon Gateway, United Kingdom).H. pyloriinfection was considered present if the difference in13C/12C between baseline value and 20-min value exceeded 40/00.
Inclusion criteria were as follows: Patients ≥ 18 years undergoing upper gastrointestinal endoscopy forH. pylori-related chronic symptoms (i.e., recurrent abdominal pain, dyspepsia, or unexplained anemia), with positivity to13C-UBT and with a histological diagnosis ofH. pylori-related gastritis with/without PGC.
Exclusion criteria were as follows: Past GC or gastric related surgery, impossibility to perform at least five biopsies during endoscopy, relevant comorbidities (cardiac, respiratory, chronic renal insufficiency, chronic liver disease, and psychiatric conditions), anticoagulant therapy/coagulation disorders, non-steroidal antiinflammatory drugs and/or long-term proton pump inhibitors users, and active smoking habit. Autoimmune co-morbidities were not considered as exclusion criteria.
A history ofH. pyloriinfection was investigated on the basis of medical records or a face-to-face clinical examination. During each procedure the qualified endoscopists performed the biopsies according to the study protocol to ascertain or confirmH. pyloripresence and its related histological alterations. All visible gastric lesions were initially evaluated by HR-WLE with NBI. In detail, we performed at least five random biopsies using HR-WLE with NBI, followed by targeted biopsies on endoscopically evident lesions, suggestive of IM or dysplasia[28]. Furthermore, at least five different images were acquired during gastroscopy by a single expert observer, who was blind to the final histology, and who predicted the diagnosis of normal mucosa, atrophy, IM, or dysplasia using the EGGIM classification according to Pimentel-Nuneset al[29].
Histopathological characteristics
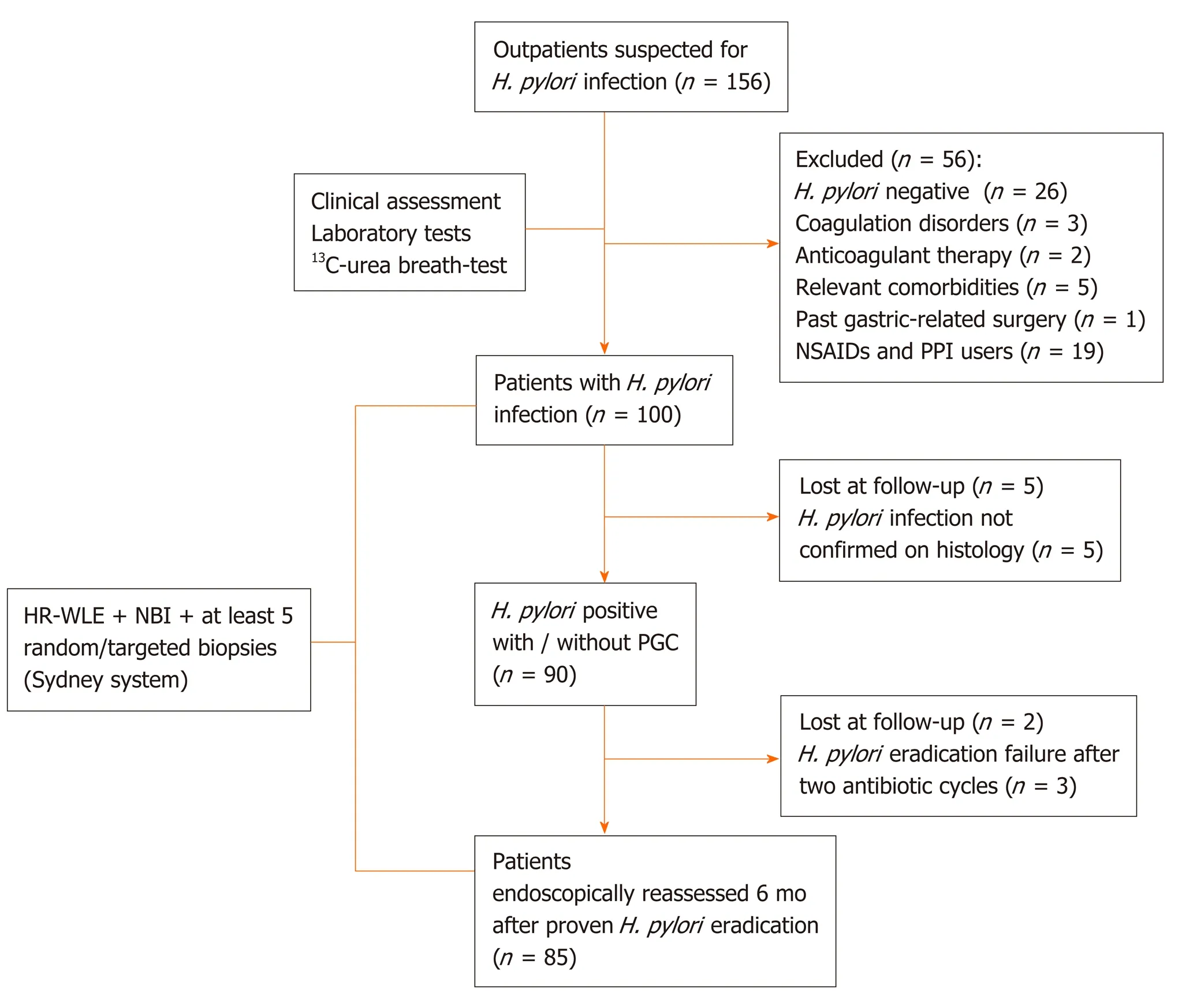
Figure 1 Flow diagram of patient’s selection. H. pylori: Helicobacter pylori; NSAIDs: Non-steroidal anti-inflammatory drugs; PPI: Proton pump inhibitor; HR-WLE: High resolution-white light endoscopy; NBI: Narrow band imaging; PGC: Precancerous gastric conditions.
The histological result was considered as the gold standard for the diagnosis of dysplasia and IM; all gastric biopsy specimens were independently assessed by two expert gastrointestinal pathologists (MLC and RA). The Sydney System, and the OLGA and OLGIM systems assessment were used for histological staging of gastritis[30,31,39]; the following diagnostic categories were considered for the evaluation of dysplasia according to the World Health Organization classification: Negative for intraepithelial neoplasia/dysplasia, indefinite for intraepithelial neoplasia/dysplasia, low-grade intraepithelial neoplasia/dysplasia, high-grade intraepithelial neoplasia/dysplasia and intra-mucosal invasive neoplasia/intra-mucosal car- cinoma[39]. In all patients,H. pylorieradication was assessed by a prior negative result of13C-UBT and confirmed by histology.
Statistical analysis
Normal distribution of continuous variables was assessed with the Shapiro-Wilk test and data were expressed as mean and ± SD. Categorical variables were reported as percentages and compared using theχ2test or Fisher’s exact test, when needed. The probability of detecting gastric dysplasia afterH. pylorieradication was evaluated using univariate and multivariate logistic regression analyses. The association between each explanatory variable and the outcome (detection of dysplasia) was tested using the likelihood ratio test. For each variable included in the multivariate model, we calculated both unadjusted and adjusted odds ratios (OR), with their 95% confidence intervals (CI), and the level of significance (using the likelihood ratio test). Statistical significance was set atP< 0.05. All statistical analyses were performed using SPSS 23.0 software (SPSS, Chicago, IL, United States) and R version 3.4.3 (http://www.Rproject.org/).
RESULTS
The clinical characteristics of the 85 enrolled patients are described in Table 1. At baseline, the majority of patients (71.8%) had an extensive endoscopic atrophic gastritis (pan-atrophy, corpus-predominant or antrum-predominant atrophy), and the minority (22.4%) had focal antrum atrophy (Table 2)[32]. The patients with a less advanced degree (0-2 and 3-4) of the EGGIM scale were 80%; histological gastritis wasmoderate/severe and mild in 77.6% and 22.4% of the patients respectively; 45.8% of patients also had mucosal associated lymphoid tissue (MALT) hyperplasia (Table 2). Fifteen patients (17.6%) had LGD on random biopsies. Among the 49 patients with
histological pan-atrophy or corpus-predominant atrophy, 11 (22.4%) tested positive for APCA and/or for AIF antibodies. In 100% of the cases the histological mononuclear and polymorphonuclear cell infiltration disappeared or decreased afterH. pylorieradication. Additionally, the proportion of patients with mild and moderate/severe grades of MALT hyperplasia significantly decreased afterH. pylorieradication (29.4%vs3.5%,P< 0.001 and 16.4%vs0%,P< 0.001, respectively; Table 2). Nevertheless, the proportion of OLGA, OLGIM stages, and that of patients with histological diagnosis of LGD on random biopsies did not significantly change afterH. pylorieradication (17.6%vs10.6%,P= 0.19) (Table 2 and Figure 2). The detection of LGD on visible lesions significantly increased afterH. pylorieradication (0%vs22.3%,P< 0.001; Table 2). Among the 9 patients who showed LGD on random biopsies afterH. pylorieradication, only 2 (22.2%) had already had a diagnosis of LGD on random biopsies beforeH. pylorieradication. Among the 6 patients with LGD on random and visible lesion biopsies afterH. pylorieradication, two (33.3%) were negative for LGD before eradication. Furthermore, the proportion of the 13 patients with a new diagnosis of LGD only on visible lesions was significantly higher afterH. pyloridisappearance (0%vs15.3%,P< 0.001) and in 6 of them (46.1%) LGD was not detected beforeH. pylorieradication.

Table 1 Baseline demographic and clinical characteristics of patients who underwent Helicobacter pylori eradication

Table 2 Endoscopic and histological characteristics of patients before and after Helicobacter pylori eradication
The characteristics of the 19 subjects in whom LGD was detected on visible lesions afterH. pylorieradication are shown in Supplementary Table 1. In details, 11 subjects (57.9%) were male, and in 8 patients (42.1%) LGD was missed beforeH. pylorieradication. Five patients (26.3%) had APCA and/or AIF positivity. Comparison of the patients with or without LGD (n= 28vs n= 57 respectively) afterH. pylorieradication is shown in Table 3. In patients with LGD, the age was older (60.9 ± 8.2vs53.7 ± 13.4 years,P= 0.01), there were more alcohol users or past smokers (53.6%vs21.1%,P= 0.002 and 35.7%vs3.5%,P< 0.001, respectively), there was a higher proportion of APCA and/or AIF antibody positivity (28.6%vs7%,P= 0.02), there were more familial cases of GC (14.3%vs0%,P< 0.001) and more advanced stages of OLGA and OLGIM, both at the baseline and afterH. pylorieradication (P< 0.001 for both). When we compared the histological characteristics of the 28 patients with LGD afterH. pylorieradication, we observed that the moderate/severe grades of gastritis and MALT hyperplasia significantly regressed during surveillance (P< 0.001 andP= 0.004, respectively), whereas OLGA stages were similar (P= 0.76; Supplementary Table 2). A higher prevalence of OLGIM stages 3-4 was observed afterH. pylorieradication, but it did not reach the level of statistical significance (57.1%vs75%,P= 0.16).
As shown in Supplementary Table 3, in patients without LGD afterH. pylorieradication only gastritis significantly improved at the follow-up endoscopy (P< 0.001). Table 4 shows the results of the linear regression analysis. According to the multivariate analysis adjusted for age, alcohol use, MALT hyperplasia regression/reduction and for APCA and/or AIF antibody presence, the probability for detecting gastric LGD, randomly or on visible lesions after eradication ofH. pylori, was significantly higher in patients with regression/reduction of MALT hyperplasia (OR = 3.10, 95%CI: 1.05-9.12,P= 0.04), alcohol consumption (OR = 3.88, 95%CI: 1.31-11.49,P= 0.01), and APCA and/or AIF antibody positivity (OR = 5.47, 95%CI: 1.33-22.39,P< 0.04).
DISCUSSION
Our study shows that HR-WLE in combination with NBI can diagnose gastric LGD on visible lesions with the highest accuracy after regression ofH. pylori-induced signs of active infection (Figure 3 and 4). This suggests that, in high-risk subjects without alarm features for malignancy, non-invasive tests should be used for priorH. pyloriidentification, and a high-quality upper endoscopy to identify dysplastic lesions should be postponed afterH. pylorieradication has been achieved. This strategy couldbe useful for yielding a higher dysplasia detection rate and for better defining cancer risk and surveillance time.
现有降维方法有PCA和Pareto占优机制算法等方法[17].这些方法均通过纯数学手段降维,降维后的数据都失去了原有的物理意义,不便于观察模型的训练过程.

Table 3 Demographic and clinical characteristics of patients with low-grade dysplasia detected on visible gastric lesions or randomly and without low-grade dysplasia, before and after Helicobacter pylori eradication

Data are expressed as number of patients and percentage (in parenthesis). 1Bismuth + Metronidazole + Tetracycline + Proton pump inhibitor. 2Amoxicillin + Levofloxacin + Proton pump inhibitor. 3Amoxicillin + Clarithromycin + Proton pump inhibitor. 4Amoxicillin + Clarithromycin + Proton pump inhibitor Amoxicillin (5 d), then Clarithromycin + Metronidazole (5 d) + Proton pump inhibitor. 5Before Helicobacter pylori eradication (by Sydney System). 6After Helicobacter pylori eradication. aBy t-test. bBy χ2 test. cBy Fisher’s exact test. BMI: Body mass index; SD: Standard deviation; APCA: Anti-parietal cell antibody; AIF: Anti-intrinsic factor; H. pylori: Helicobacter pylori; PPI: Proton pump inhibitor; OLGA: Operative Link on Gastritis Assessment; OLGIM: Operative Link on Gastric Intestinal Metaplasia; MALT: Mucosa-associated lymphoid tissue.
Despite conflicting evidence,H. pylorieradication is presently advised in high-risk subjects for its potential to reduce GC incidence and induce regression of inflammation and atrophic gastritis[7,22-27,40]. Originally, the use of WLE alone provided a weak association between endoscopic and histological findings in diagnosing PGC, probably due to the inefficient technology of endoscopy imaging[41-43]; recent studies have instead shown that the use of HR-WLE allows for a high concordance accuracy for this purpose[7,36]. There is strong evidence that virtual chromoendoscopy should be used for the diagnosis of PGC because of its better performance compared to HR-WLE[34-37,44]. Capelleet al[33]studied high-risk patients with IM or dysplasia undergoing endoscopic surveillance 2.0 years (range 0.8–21.1) and 1.9 years (range 0.2–5.2) respectively after the initial diagnosis and showed a slightly better diagnostic yield for the detection of advanced PGC using HRE with NBIvsHR-WLE[33]. Conventional CE with the application of dyes has been associated with the highest PGC detection accuracy as compared to virtual chromoendoscopy although procedural time is considerably longer[45-47]. As a result of such evidence, the updated version of the European guidelines suggests that a high-quality endoscopy requires the use of NBI[7].
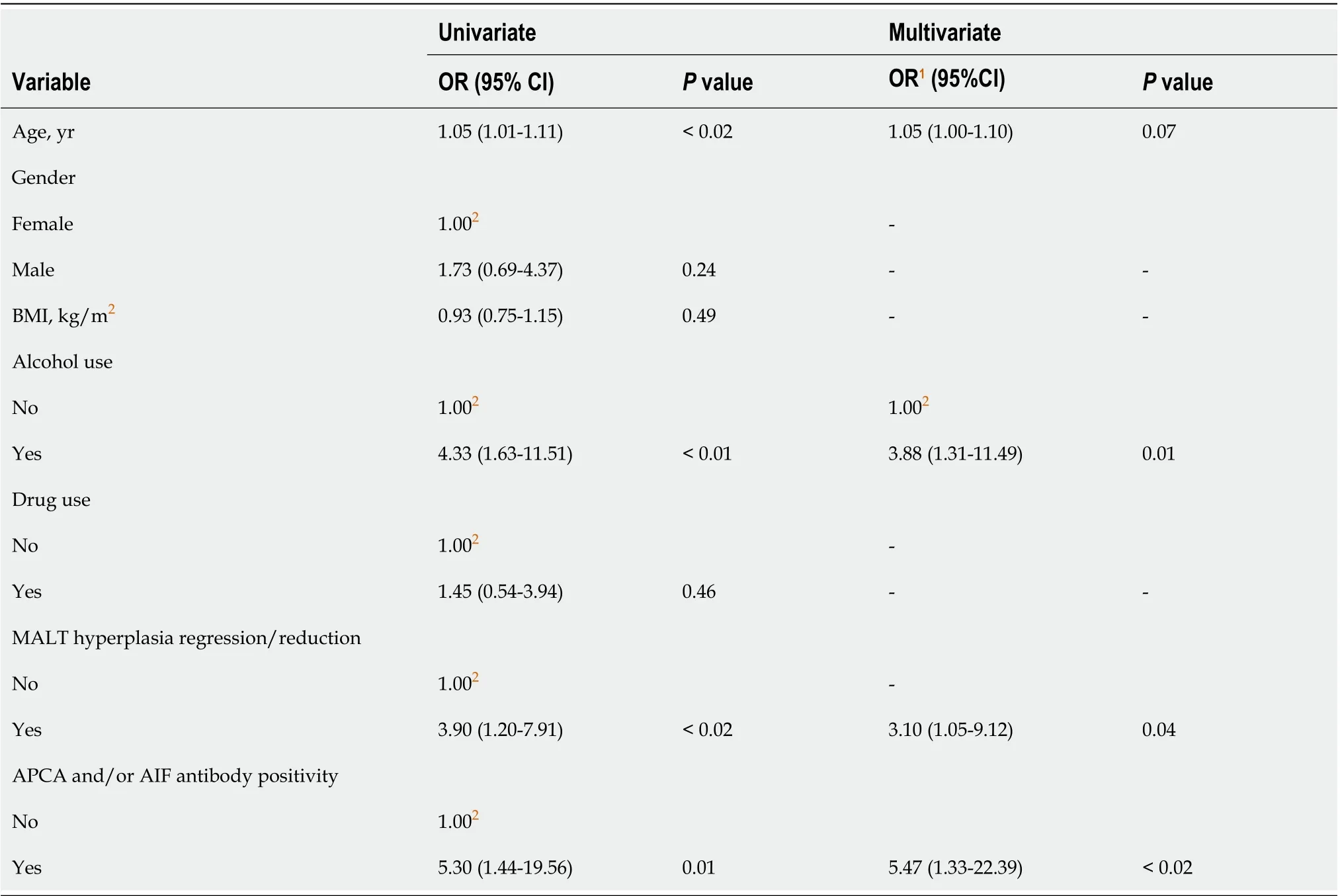
Table 4 Probability of detecting low-grade dysplasia randomly or on visible lesions after Helicobacter pylori eradication
In our study, the histological signs of active gastritis and MALT hyperplasia disappeared or decreased in 100% and 96.5% of patients with or without PGC afterH. pylorieradication, respectively. The overall prevalence of LGD on random biopsies at a 6-mo interval was similar (17.6%vs10.6%). Nevertheless, among the patients with newly diagnosed LGD on visible lesions, the percentage of endoscopically missed dysplasia was 42.1% beforeH. pylorieradication, when active gastritis was present. Unexpectedly, at the baseline 26.3% of such patients (5/19) had an overlapping AAG with APCA and/or AIF positivity. This prevalence rose to 28.6% (8/28 patients) when we considered the total patients with dysplasia, and the percentage of this positivity did not change afterH. pylorieradication (P= 1.0, data not shown). We observed an almost doubled prevalence of gastric LGD on biopsies performed randomly or on visible lesions before and afterH. pyloridisappearance (17.6%vs32.9% respectively). A similar prevalence was found in another study from two referral centers using NBI endoscopy, in which dysplasia was detected in 28/85 (33%) and 38/85H. pyloripositive patients (45%)[37].
Patients with LGD were older and showed more advanced OLGA and OLGIM stages as compared to those without LGD, at baseline as well as afterH. pylorieradication. The prevalence of OLGIM stages 3-4 showed a tendency to increase afterH. pylorieradication only in patients with dysplasia, from 57.1% to 75%.
The higher prevalence of LGD afterH. pylorieradication could depend on the presence of more severe and extensive mucosal atrophy and IM at baseline in our high-risk subgroup of patients rather than disease progression itself, considering the short interval of endoscopic surveillance of our study. In this scenario, the background of activeH. pyloriinflammation is likely to play a confounding role that may have hampered the accurate detection of gastric dysplasia. Nevertheless, the unexpected high baseline prevalence of dysplasia may be partially justified by the concomitant presence of AAG in a considerable number of our patients, in accordance with results from a recent study showing that in the presence of AAG the risk of developing more advanced stages on long-term follow-up is greater in patients with more severe gastric lesions[8]. The overlap between autoimmune andH. pylori-induced chronic gastritis may presumably be associated with a more severe gastric injury, especially in older subjects.
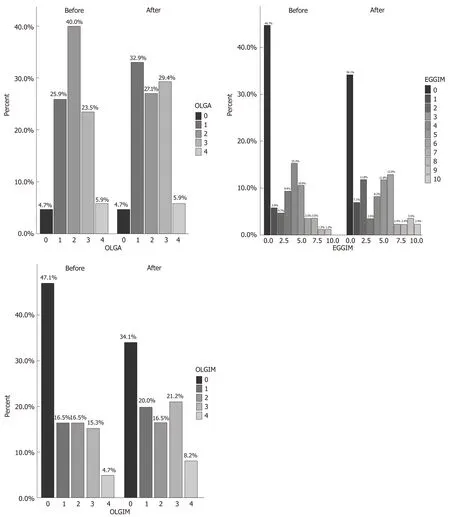
Figure 2 Prevalence of Operative Link on Gastritis Assessment, endoscopic grading of gastric intestinal metaplasia, and Operative Link on Gastric Intestinal Metaplasia scores, respectively in the 85 patients before and after Helicobacter pylori eradication. OLGA: Operative Link on Gastritis Assessment; EGGIM: Endoscopic grading of gastric intestinal metaplasia; OLGIM: Operative Link on Gastric Intestinal Metaplasia.
At a multivariate analysis, the probability for detecting gastric dysplasia after resolution ofH. pylori-related active inflammation was significantly higher in older patients with regression or reduction of MALT hyperplasia, greater alcohol consumption, and APCA and/or AIF positivity. Therefore, MALT hyperplasia with active gastritis, whether induced or not byH. pylori, could be an additional confounding factor influencing the detection of PGC, and, particularly, LGD in highrisk patients (Table 4).
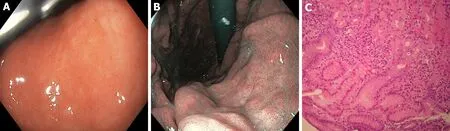
Figure 3 Gastric white-light endoscopy, narrow band imaging and histological evaluation before Helicobacter pylori eradication. A, B: Gastritis during white-light endoscopy and narrow band imaging assessment of corpus before Helicobacter pylori eradication; and C: Histological evaluation: moderately atrophic chronically active gastritis with lymphoplasmacellular infiltration of the lamina propria and foveolar epithelium hyperplasia (fundus before Helicobacter pylori eradication. Sections of 3 microns colored with Hematoxylin Eosin and Giemsa respectively. Magnifications: × 10).

Figure 4 Gastric narrow band imaging and histological evaluation after Helicobacter pylori eradication. A: Low-grade dysplasia on flat lesion during narrow band imaging assessment of corpus after Helicobacter pylori eradication; B: Histological appearance: inactive chronically mild atrophic gastritis with intestinal metaplasia and low-grade dysplasia on intestinal metaplastic epithelium (fundus after Helicobacter pylori eradication); C: Low-grade dysplasia aspect of antrum on visible lesion during narrow band imaging assessment after Helicobacter pylori eradication; and D: Histological appearance: inactive chronically moderate atrophic gastritis with intestinal metaplasia and low-grade dysplasia on intestinal metaplastic epithelium (antrum after Helicobacter pylori eradication). Hematoxylin Eosin. Sections of 3 microns. Magnification: × 10.
The prevalence of autoimmune diseases (24.7%) in our patients showed a tendency to increase among patients with LGD (35.7%vs17.5% in those without LGD,P= 0.06 byχ2test). Since autoimmune disorders are linked to the presence of DRB1 and DQB1 haplotypes our finding may suggest a reciprocal role between the genetics of immune response andH. pylorichronic infection.
Our results are in full accordance with the current European recommendations, which suggest to perform an endoscopic reassessment with HRE-NBI as soon as possible after dysplasia is diagnosed in the “apparent” absence of endoscopically visible lesions, to search for malignancy on misdiagnosed lesions[7,27,48]. Where the presence ofH. pyloriactive gastritis without alarming features for malignancy is suspected, considering the present lack of clinical recommendations on the diagnosis and surveillance of PGC[7], our study suggests that the best diagnostic workout in symptomatic patients should entail performing priorH. pylorinon-invasive tests, thus increasing the probability of detecting LGD on visible lesions at an endoscopy carried out soon afterH. pylorieradication has been achieved.
In conclusion, HR-WLE with NBI can be more accurate in diagnosing LGD on visible lesions afterH. pylorieradication has been achieved, probably due to the disappearance of the underlying confounding effects of inflammatory and mucosal lymphoproliferative changes induced byH. pylorichronic active infection. Elderly patients and those with autoimmune diseases could be at higher risk forH. pylorichronic infection. An effective and cost-effective strategy to diagnose LGD with the highest accuracy should entail a high-quality upper endoscopy performed soon after eradication ofH. pyloriinfection, detected by prior non-invasive tests.
ARTICLE HIGHLIGHTS

Research perspectives
Our findings will appeal to both clinical gastroenterologists and endoscopists, and stimulate the development of more accurate and cost-effective strategies for identifying patients withH. pyloriinfection who are at risk of gastric cancer. Subjects with an overlap between autoimmune andH. pylori-induced chronic gastritis should be considered to be at a higher risk of more severe gastric injury.
猜你喜欢
杂志排行
World Journal of Gastroenterology的其它文章
- Nomogram for predicting transmural bowel infarction in patients with acute superior mesenteric venous thrombosis
- Functional gastrointestinal disorders in inflammatory bowel disease: Time for a paradigm shift?
- Intratumoral heterogeneity of hepatocellular carcinoma: From single-cell to population-based studies
- Adipose-derived mesenchymal stem cells alleviate TNBS-induced colitis in rats by influencing intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity
- Combining protein arginine methyltransferase inhibitor and anti-programmed death-ligand-1 inhibits pancreatic cancer progression
- Expression of Notch pathway components (Numb, Itch, and Siah-1) in colorectal tumors: A clinicopathological study
