Lipopolysaccharide-binding Protein Involved in Process of Mouse Embryo Implantation and Decidualization of Endometrial Stromal Cells
2020-07-15CuiYunfengXingYuanyuanRenJieYuHaonanandNiHua
Cui Yun-feng, Xing Yuan-yuan, Ren Jie, Yu Hao-nan, and Ni Hua
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
Abstract: Lipopolysaccharide-binding protein (LBP) functions as an acute phase protein and plays a role in the innate immune response to bacterial challenge. To investigate the uterine expression of LBP during peri-implantation in mice, in situ hybridization and immunohistochemical staining were used to detect the mRNA and protein expression of LBP in mouse uteri in the early pregnancy,pseudopregnancy, arti ficial decidualization and hormone-treated mice. The results showed that LBP was expressed in uterine luminal epithelium (LE) and glandular epithelium (GE) during days 1-4 of pregnancy. During days 5-8, LBP was weakly expressed in the decidual cells around the embryo on the 5th day of pregnancy (implantation occurred), then gradually increased, LBP was strongly expressed in the decidual zone on the 8th day of pregnancy. The expression of LBP in pseudopregnancy was similar with pregnancy on days 1-4. In arti ficial decidualization mice, LBP was observed in uterine LE and GE in the control horn, whereas LBP expression was significantly higher in decidua of mouse uteri under arti ficial decidualization. In hormone-treated mice, the expression of LBP was up-regulated by 17β-estradiol (E2) and progesterone (P4). In addition, the cultured mouse endometrial stromal cells (mESCs) were induced for in vitro decidualization with 10 nmol · L-1 E2 and 1 μmol · L-1 P4. Real-time PCR results showed that LBP mRNA expression was highly induced in mESCs after decidual stimulus. In vivo and in vitro experiments showed that LBP was expressed in the decidual cells, indicating that LBP involved in decidualization of mouse uteri.
Key words: mouse, LBP, uterus, embryo implantation
Introduction
Lipopolysaccharide-binding protein (LBP) is an acute phase protein (APP), primarily produced in hepatocytes (Bidneet al., 2018), it presents in the serum of normal humans and animals and plays a key role in the innate immune response to bacterial challenge. LBP binds lipopolysaccharide (LPS) and initiates the immune response by presenting LPS to cluster of differentiation CD14, which in turn interacts with toll-like receptor (TLR) 4 on immune cells (Huanget al., 2017; Kim and Kim, 2017; Sakuraet al., 2017).
LBP has a dual role in infection and inflammation which depends on its concentration (Gutsmannet al.,2001). At a low concentration, LBP enhances LPS-induced expression of proinflammatory cytokines,such as interleukin (IL)-1βand tumor necrosis factor(TNF)-αin membrane-bound CD14 (mCD14)-positive cells or activates the expression of proinflammatory cytokines, such as IL-6 and IL-8 in mCD14-negative cells. At a high concentration, LBP neutralizes the effect of bacterial endotoxin, and thus down-regulates the expression of proinflammatory cytokines, such as TNF-α(Ding and Jin, 2014). Furthermore, LBP has been found to mediate LPS transfer to high density lipoprotein (HDL) and low density lipoprotein (LDL),attenuating the stimulatory effects of LPS (Van Lentenet al., 1986; Wurfelet al., 1994). Above all, LBP may have opposing biological activities that may be critical for the consequences of infection and inflammatory diseases.
Interleukin (IL)-6, IL-1β(Taddonioet al., 2015)and dexamethasone (Schumannet al., 1996) stimulate hepatic LBP production. Other sources of LBP synthesis have been identified, such as skin (Kleinet al.,2000), renal cells (Stasiet al., 2017), lung (Denteneret al., 2000; Taddonioet al., 2015), spleen (Tsaiet al.,2014), intestine (Weberet al., 2003) and human gingival tissues (Renet al., 2005). The expression of LBP is so extensive that it is predicted that LBP has other functions besides mediating LPS. Several studies showed that LBP as an adipocytokine involved in adipocyte differentiation. Down-regulation of LBP can lead up-regulation of genes associated with adipocyte differentiation and metabolism. It has been reported that LBP mRNA and protein levels are overexpression in adipose tissues with metabolic disorders (Moreno-Navarreteet al., 2013).
There is an interesting interaction between the fetus and the maternal uteri during mammalian pregnancy.For maternal immune system, fetus is recognized as semiallograft. However, the maternal immune cells do not attack and reject a fetus during pregnancy. The reason why immune tolerance is established in the maternal decidua is the specific area defined as the feto-maternal interface. Studies have shown that there are multiple immune mechanisms to maintain maternal pregnancy (Nakamura, 2009). The role of LBP in in fl ammatory responses has been extensively studied.However, there are few reports on the function of LBP in reproductive immunology. The objective of the present study was to investigate the expression and hormone regulation of LBP in mouse uteri utilizing various experimental mouse pregnancy models,and to explore the expression of LBP in the decidua and related mechanisms involved in the decidua. In addition, the cultured mouse endometrial stromal cells(mESCs) were induced forin vitrodecidualization with 10 nmol · L-1E2and 1 μmol · L-1P4to study the role of LBP in the decidualization of mouse endometrial stromal cells. This would be helpful to study the function of LBP and to reveal the role of LBP in embryo implantation and reproductive immunology.
Materials and Methods
Experimental animal
Sexually mature C57 mouse strains were used in the study. Mice were caged in a controlled environment with a 12 h light. All the animal procedures were approved by the Animal Care and Use Committee of Northeast Agricultural University. Three parallel mice were prepared for each model.
Animal models
Pregnancy mice
Pubescent female mice were mated with fertile male mice. The morning of vaginal plug observed was designated as day 1 of pregnancy (D1). The implantation sites on D5 were identi fied by intravenous injection of 0.1 mL 1% Chicago blue (Sigma). The uteri of the mice on D1-D8 were harvested and stored at -80℃.
Pseudopregnancy mice
Healthy adult male mice were used for the experiment of vasectomy, then followed a recovery period for 20 days. Female mice were housed with the vasectomised males, and the day of appearance of a vaginal plug was considered as day 1 of pseudopregnancy (PD1). The uteri of the mice on PD1-PD6 were collected.
Arti ficial decidualization mice
Artificial decidualization was induced by injecting 20 μL sesame oil (Sigma) into one side of the uterine horn on PD4 (9:00 a.m.), while the contralateral uninjected horn was as the control. The uteri were collected on PD8. Decidualization was confirmed by observing the difference in morphology and weighing.
Mice treated with ovarian steroid hormones
Adult female mice were ovariectomized (OVX) and caged for 20 days so as to consume their ovarian hormones. The OVX mice were given subcutaneous injections of vehicle (sesame oil, 0.1 mL · mouse-1,Sigma), 17β-estradiol (E2, 25 ng · 0.1 mL-1· mouse-1,Sigma), progesterone (P4, 1 mg · 0.1 mL-1· mouse-1,Sigma) and a combination of E2and P4. The uteri were collected at 24 h after injection of the hormones.
In situ hybridization
Primers were designed according to the mouseLBPgene sequence in GenBank, forward primer: 5'-ATCA GGAAATGTGGGGTGGC-3'; reverse primer: 5'-AA CTGGAGAGCGGTGATTCC-3' (GenBank Accession number NM_0084892). The total RNAs of mouse uteri on D8 were reverse-transcribed and amplified with the primers. The ampli fied fragment ofLBPgene was cloned into a plasmid and transformed into competent cells ofE.coliDH5αstrain. Plasmids were extracted and further verified by sequencing. The probe was labeled using a DIG RNA labeling kit (Roche).In situhybridization method was according to Dinget al(2018).
Immunohistochemistry
Mouse uterus tissues were embedded in paraffin.Sections (5 μm) were cut, deparaffinized, rehydrated and boiled in 0.01% citrate buffer for antigen retrieval.Sections were soaked in PBS, then incubated with a solution of 3% hydrogen peroxide in methanol for 10 min. Non-specific binding signal was blocked in 10% horse serum in PBS for 1 h at 37℃. The sections were incubated with primary antibody speci fic to LBP(1 : 300, 11836-1-AP, Proteintech) overnight at 4℃.After being rinsed in PBS, the sections were incubated with HRP-conjugated goat anti-rabbit antibody (1 : 200,ab6721, abcam) for 1.5 h at 37℃. Sections were rinsed and the signal was visualized with DAB solution.Slides were counterstained with hematoxylin, dehydrated, cleared and sealed with neutral gum.
Culture of endometrial stromal cells and in vitro decidualisation
Endometrial stromal cells of mice were isolated as previously described (Zhanget al., 2018). The isolated stromal cells were cultured in complete medium at 37℃ with 5% CO2. The cultured stromal cells were induced forin vitrodecidualization with 10 nmol · L-1E2and 1 μmol · L-1P4for 24, 48, 72 and 96 h, respectively. The cells were collected for quantitative real-time PCR. Mouse decidual prolactin-related protein (Dtprp) was used as a decidualization marker.
RNA isolation and quantitative real-time PCR
The total RNA was extracted from uterine tissues or using HiPure Total RNA Mini kit (mangen). The total RNA was reverse-transcribed into cDNA. Real-time PCR was run using SYBR Green qPCR Master Mix(TaKaRa) on the Amplied Biosystems®7500 Real-time PCR System. The primer sequences were designed as the followings: LBP, forward: 5'-GCATCCAGACAA GGCACAAG-3' and reverse: 5'-TCAGGGTAGAGG CTGTTGAGA-3'; GAPDH, forward: 5'-TAGCCGTA ACTTCTGTGCTGT-3' and reverse: 5'-TGATGGCA ACGATGTCCACTT-3', Dtprp, forward: 5'-AGCCA GAAATCACTGCCACT-3' and reverse: 5'-TGATCC ATGCACCCATAAAA-3'. The 2-ΔΔCtmethod was used to calculate the relative expression of LBP and Dtprp with GAPDH as an inner control. Three parallel mice were prepared for each group. Each sample was run in three independent experiments in triplicate.
Statistical analysis
Experiments were performed at least three times for each group. All the quantitative data were recorded as mean±SD. Comparison between the two groups was performed by unpairedt-test. Data were reported as mean±SEM. All the statistical tests were performed using Prism software (GraphPad). *p<0.05, **p<0.001 and ***p<0.0001 were considered statistically signi ficant.
Results
Expression of LBP mRNA and protein in mouse uteri during days 1-8 of pregnancy
During days 1-4 of pregnancy, the expression of LBP mRNA and protein presented within uterine LE and GE.On the 5th day of pregnancy (implantation occurred),LBP mRNA and protein were weakly expressed in the decidua around the embryo. On days 6-8 of pregnancy,the expression of LBP mRNA and protein in the decidual zone gradually increased with the progression of the decidualization (Fig. 1).
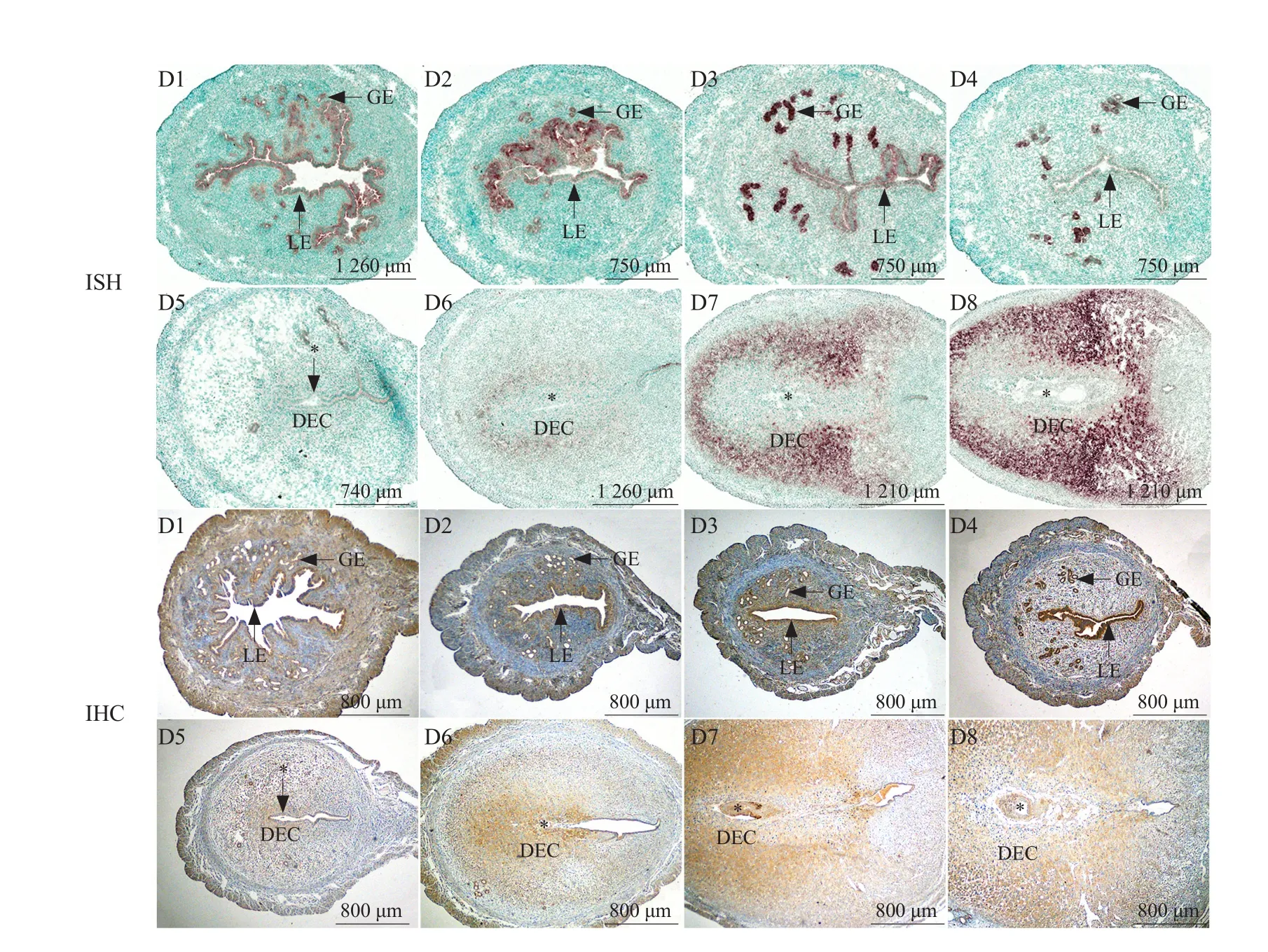
Fig. 1 Expression of LBP in mouse uteri during days 1-8 of pregnancy
Expression of LBP mRNA and protein in mouse uteri during days 1-5 of pseudopregnancy
LBP mRNA and protein were detected in the uterine LE and GE during days 1-4 of pseudopregnancy,which were similar to those of the normal pregnancy on days 1-4. LBP mRNA and protein also were detected in the uterine LE and GE, during days 5-6 of pseudopregnancy (Fig. 2).
Expression of LBP mRNA and protein in mouse uteri under arti ficial decidualization
To further demonstrate that LBP was involved in the decidualization in mice during embryo implantation,this study constructed the artificial decidualization models.
As shown in Fig. 3, LBP mRNA and protein were observed in the uterine LE and GE in the control horn, whereas LBP mRNA and protein expression were significantly higher in the uterine decidua under arti ficial decidualization.
Steroid hormones regulated expression of LBP mRNA and protein in mouse uteri
To investigate whether LBP was regulated by the ovarian steroid hormones, the OVX mice were subcutaneously injected with oil, E2, P4and a combination of E2and P4, respectively. In the oil treated mice, LBP mRNA and protein were weakly expressed in LE and GE; in P4treated mice, the expression of mRNA and protein weakly increased in LE and GE; in E2treated mice, the strong expression of mRNA and protein was induced in LE and GE and the stromal cells; in E2and P4co-treatment groups, the expression of mRNA and protein also increased in LE, GE and the stromal cells(Fig. 4).
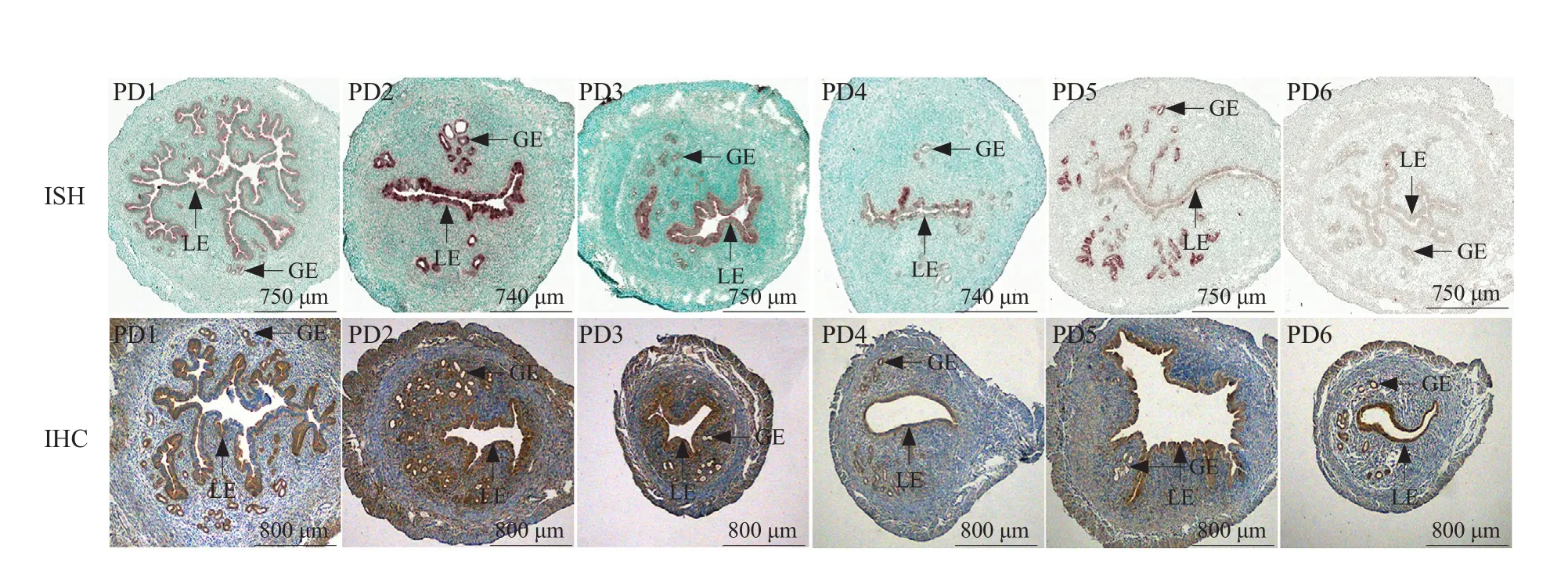
Fig. 2 Expression of LBP in mouse uteri on days 1-6 of pseudopregnancy
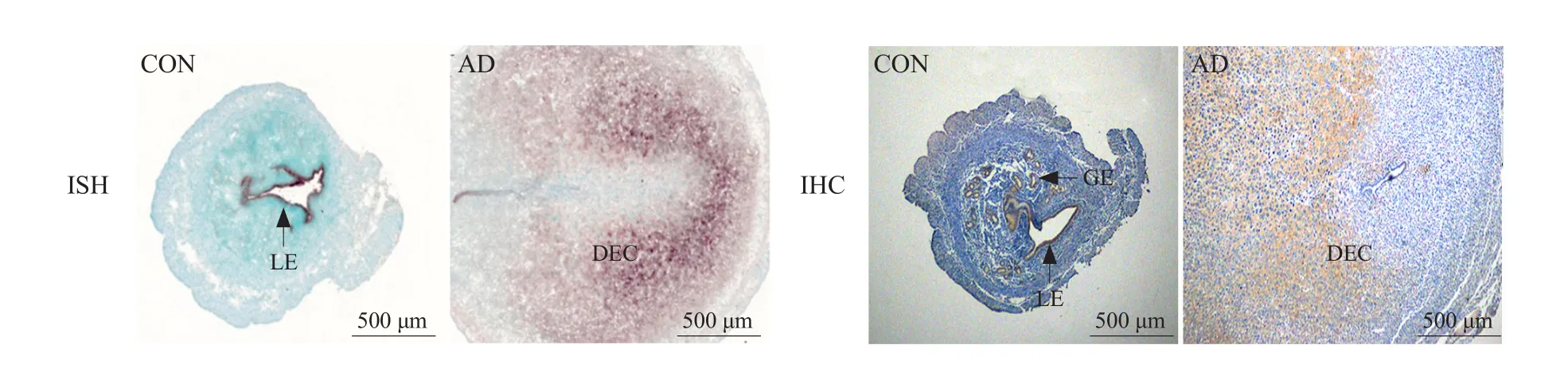
Fig. 3 Expression of LBP in mouse uteri under arti ficial decidualization

Fig. 4 Expression of LBP in steroid-treated mouse uteri
Expression of LBP mRNA in decidualized mESCs
In view of LBP was mainly localized in the decidual cells during the early pregnancy, real-time PCR was conducted to examine the expression levels of LBP mRNA in mESCs underin vitrodecidualization.Dtprp, a decidual marker, was significantly upregulated underin vitrodecidualization (Fig. 5A).LBP mRNA increased with the process of the decidualization in the stromal cells (Fig. 5B).
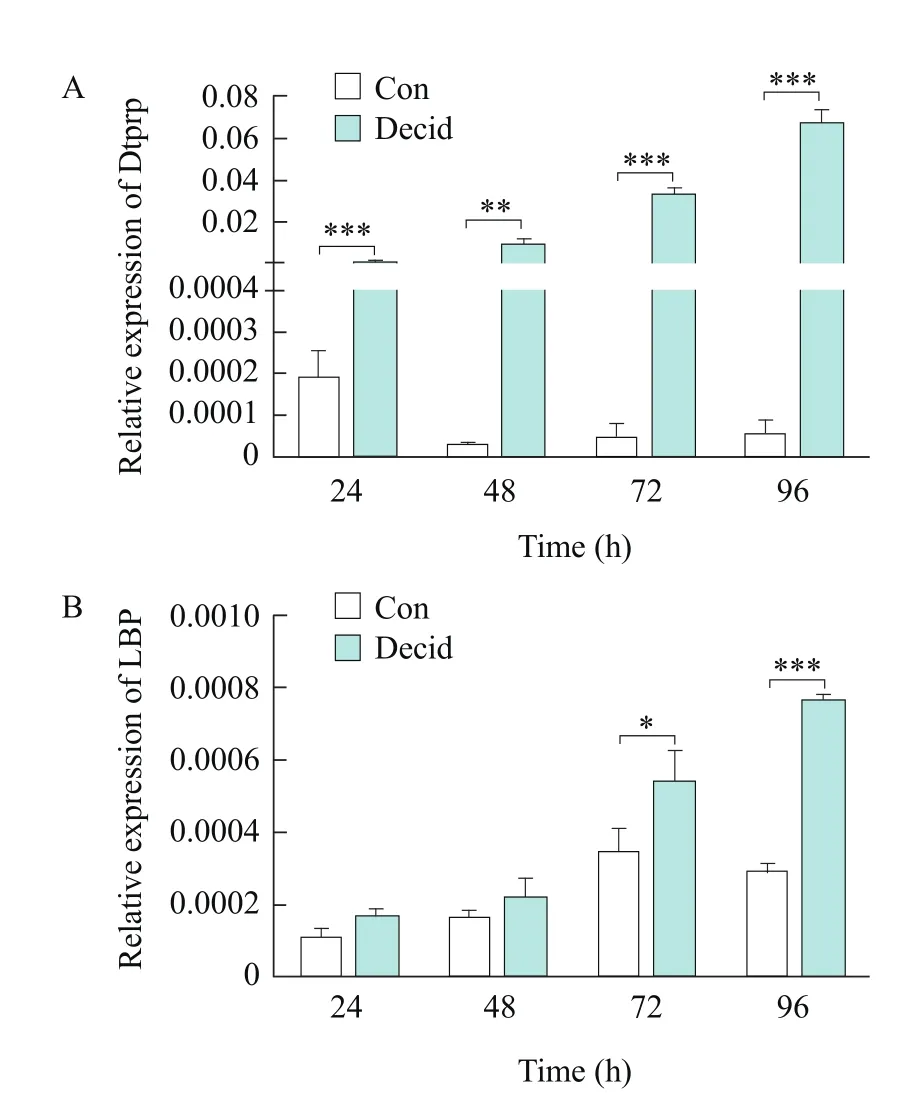
Fig. 5 Real-time PCR analysis of Dtprp and LBP mRNA expression in decidualized mESCs
Discussion
Previous studies indicated that LBP formation at extrahepatic cells might be crucial in containing microbialin situchallenge constantly (Ding and Jin,2014). The results of this study showed that LBP was expressed in the uterine LE on days 1-4 of pregnancy and days 1-4 of pseudopregnancy, it was speculated that LBP might play a role in resistance to bacterial invasion in the uteri before implantation. In addition,LBP was also expressed in the uterine GE, suggesting that LBP might have some unknown functions in GE.
Additionally, the results in this study showed that LBP was highly expressed in the uterine LE and GE on the 1st day of pregnancy and pseudopregnancy.Generally, the estrogen levels were higher during these two days. To know whether LBP was regulated by steroid hormones, the OVX mice were respectively injected with oil, E2, P4and a combination of E2and P4after they had been fed for 2 weeks. The results showed that the expression of LBP was up-regulated by E2and P4. Therefore, it was speculated that LBP might be an important effector molecule downstream of ovarian steroid hormones and involved in the regulation of ovarian steroid hormones on embryo implantation.
During the embryo implantation, the early decidual response is similar to the inflammatory response.With the embryo develops, the immune tolerance is established in the maternal deciduas in order to reduce the local immune response, which protects the fetus from maternal rejection. This will be helpful to maintain the immune balance and ensure the normal development of embryos. During the decidualization,maternal peripheral immune cells were recruited into the endometrium, proliferated and further became decidual immune cells (Fu and Wei, 2016).These decidual immune cells secreted cytokines that promoted vascular remodeling in the decidua,neovascularization and to maintain the immune balance in pregnancy. Studies had shown that LBP could bind to LPS and CD14 to form LPS-LBP-CD14 complex. LPS-LBP-CD14 complex was transduced by a series of recognition complexes, and then transmitted the signals to the nucleus, which regulated the expression and secretion of proinflammatory cytokines, such as TNF-α, IL-1 and IL-6 (Fanget al.,2014; Koppet al., 2016). IL-6 belongs to T-helper2 type (Th2) cytokines and TNF-αbelongs to T-helper1 type (Th1) cytokines. The imbalance of Th1/Th2 system in the decidual tissues may be associated with pregnancy failure and certain pregnancy complica-tions. Clinical evidences and experimental studies suggested that Th1-type (inflammatory) responses were weakened during pregnancy, while Th2 responses were augmented (Raghupathy and Kalinka,2008). The results in this study showed that LBP was weakly expressed in the decidual cells on the 5th day of pregnancy (implantation occurred), whereas its expression in the decidual zone gradually increased with the progression of the decidualization. LBP expression was also significantly higher in the decidua under arti ficial decidualization. It was speculated that LBP might be associated with an immune response in the decidua.
In vivoexperiments showed that LBP was expressed in the decidual cells. To analyze whether LBP was involved in the decidualization of mESCs, real-time PCR was conducted to examine the expression levels of LBP mRNA in mESCs underin vitrodecidualization. Dtprp, the decidual marker molecule, was significantly up-regulated, indicating that mESCs differentiated into the decidual cells, and the expression of LBP mRNA increased with the process of the decidualization in the stromal cells. These results suggested that LBP played a role in the decidualization of mESCs.
Conclusions
In conclusion, the results showed that LBP was expressed in mouse uterine epithelial cells and decidual cells and regulated by E2and P4. In addition,LBP was significantly up-regulated in decidualized mESCs, which was similar to the findings in mouse model.In vivoandin vitroexperiments showed that LBP was expressed in the decidual cells, indicating that LBP involved in the decidualization of mouse uteri. This study provided new highlights for studying the function of LBP in mouse embryo implantation.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Evidence-based Landscape Design of University Campus: Relationship Between Characteristics of Outdoor Environments and Actual Uses of Students
- Design and Experimental Evaluation of a Spiral Feeding Device Based on Friction Characteristics of Wheat Straw
- Study on Rice Yield Estimation Model Based on Quantile Regression
- Effect of Milbemycin oxime on Toxocara canis Eggs and Larvae
- AcrAB Efflux Pump in Fluoroquinolone Resistant Salmonella gallinarum Induced by Cipro fl oxacin Selective Pressure
- Localization of RanBP1 in Early Embryonic Development of Mice
