Increased syndecan-1 and glypican-3 predict poor perinatal outcome and treatment resistance in intrahepatic cholestasis
2020-07-07lerSibelzler
Bşk GümüşGüler , Sibel Özler
a Department of Health Sciences, Istinye University, Istanbul 34010, Turkey
b Department of Perinatology, Selcuk University Faculty of Medicine, Konya 42130, Turkey
Key words:Intrahepatic cholestasis of pregnancy Syndecan-1 Glypican-3 Adverse perinatal outcome Response to UDCA
A B S T R A C T Background: Intrahepatic cholestasis of pregnancy (ICP) increases the risk of adverse pregnancy outcomes.This study aimed to explore the association between serum syndecan-1 and glypican-3 levels and the adverse perinatal outcome as well as the responses to the treatment of ursodeoxycholic acid (UDCA).Methods: This prospective, case control study included 88 pregnant women (44 women with ICP and 44 healthy controls). The primary end points were the perinatal outcome and the response to UDCA therapy. A logistic regression model was used to identify the independent risk factors of adverse pregnancy outcomes and reduced response to UDCA therapy.Results: Women with ICP had significantly higher serum syndecan-1 (1.27 ± 0.36 ng/mL vs.0.98 ±0.50 ng/mL; P = 0.003), glypican-3 (1.78 ±0.13 ng/mL vs.1.69 ±0.16 ng/mL; P = 0.004), AST(128.59 ±1.44 vs. 13.29 ±1.32 U/L; P < 0.001), and ALT (129.84 ±1.53 vs. 8.00 ±3.67 U/L; P < 0.001)levels compared with the controls. The increased levels of syndecan-1 (OR = 4.715, 95% CI: 1.554-14.310;P = 0.006), glypican-3 (OR = 8.465, 95% CI: 3.372-21.248; P = 0.007), ALT (OR = 1.382, 95% CI: 1.131-1.690; P = 0.002), and postprandial bile acid (PBA) (OR = 3.392, 95% CI: 1.003-12.869; P = 0.026) were correlated to ICP. The adverse neonatal outcome was related to increased glypican-3 (OR = 4.275, 95% CI:2.726-5.635; P = 0.039), and PBA (OR = 3.026, 95% CI: 1.069-13.569; P = 0.037). Increases of syndecan-1 (OR = 7.464, 95% CI: 2.130-26.153, P = 0.017) and glypican-3 (OR = 6.194, 95% CI: 2.951-13.002;P = 0.025) were the risk factors of decreased response to UDCA treatment.Conclusion: Syndecan-1 and glypican-3 might be powerful determinants in predicting adverse perinatal outcome in patients with ICP, and they can be used to predict the response to the UDCA treatment.
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a specific disease in the gestational period and characterized by abnormal liver function tests (LFT), increased serum bile acids (SBA), and pruritus without skin eruptions [1 , 2] . Hormonal, genetic, and environmental factors are involved in its pathogenesis [3 , 4] , but the mechanism is not clear.
ICP is associated with an increased risk of adverse pregnancy outcomes, such as intrauterine fetal death (IUFD), preterm delivery,meconium staining, low APGAR scores, and increased neonatal unit admission [5] . Preeclampsia, gestational diabetes and bleeding disorders are more common in these patients [6 , 7] . A previous study especially concerned about the elevated SBA which cause maternal pruritus and have fetal toxic effects after placental transport [8] .The researches to find specific markers for the early diagnosis of the disease and to decrease the adverse pregnancy outcome are in progress.
SBA-related morphologic changes in the placenta like vasoconstriction in the placental vessels, apoptosis, and loss of apical microvilli cause the disruption in the placental barrier ending in fetal adverse effects [9 , 10] . The failure of transport of bile acids through hepatocyte membranes causes their accumulation in the maternal hepatocytes, and liver dysfunction [6] . Heparan sulfate proteoglycan (HSPG), which has a role in cellular healing process like cytokines and growth factors, forms most of the transmembrane structure [11] . Especially syndecan-1 was shown to be expressed on the syncytiotrophoblasts, and surface epithelium; and to have a role in preventing progression of damage in the liver disease [12 , 13] . On the other hand, glypican-3 expression has not been seen in normal hepatocytes, but was shown to be elevated at the early stages of the hepatocellular diseases [14] .
SBA enters to maternal circulation after placental passage, and is excreted via the gastrointestinal system. IUFD risk ratio is 1.2%in the ICP patients without regard to the use of ursodeoxycholic acid (UDCA) and may happen even if the fetal biophysical score and Doppler parameters are normal [15] . This may suggest that the deposited bile acids in maternal hepatocytes, and the disordered bile acid transportation in placental and maternal hepatocytes may be the reason of un-improved gestational outcome despite the use of UDCA, which decreases SBA.
This study aimed to evaluate the serum levels of syndecan-1 and glypican-3 in ICP, and their value on the prediction of the adverse perinatal outcome as well as the response to UDCA treatment.
Methods
Studypatients
Approximately 4300 births are delivered in our hospital annually. There were 56 ICP patients in our clinic from January 2017 to January 2019. The prevalence of ICP was 0.65% each year. The diagnostic criteria of ICP were: a) pruritus, starting on late second, or the third trimester without other etiologies; b) elevated SBA ( ≥10 mmol/L) and/or two times or more elevation in serum liver transaminases; and c) improvement in laboratory and clinical parameters after delivery [1 , 16] . ICP patients were excluded if any of the following criteria were present: multiple pregnancies, allergic or dermatological diseases, placenta previa, type I or II diabetes mellitus, chronic hypertension or preeclampsia, thromboembolic disease, hyper- or hypothyroidism, fetal chromosomal aneuploidy and/or malformations, chronic liver or kidney disease, cholelithiasis, hyperemesis gravidarum, or viral infections affecting liver function tests. The criteria for healthy controls were: pregnancy of 20-35 years of age; normal serum levels of liver enzymes [AST (9-32 U/L), ALT (7-35 U/L)], bile acids (SBA<10 mmol/L); absence of any acquired or congenital liver or bile system disease (hepatitis B,hepatitis C, cirrhosis, liver transplantation, cystic hepatitis, hemangioma etc.); no use of medication that may elevate liver enzymes;no use of immunosuppressive treatment; absence of chronic disease or ulcerative colitis; absence of previous surgeries like cholecystectomy; and absence of cholangitis, cholecystitis, pancreatitis or bile stones. The patients of the control group who had a preterm delivery or preterm rupture of membranes were also excluded. We included 88 pregnant patients, 44 patients with ICP, and 44 healthy controls.
Assessmentofclinicalandlaboratoryparameters
All of the patients included in the study were evaluated at the beginning of the study, and their anthropometric measures, demographics, and medical properties were recorded. The patients admitted with pruritus were evaluated for ICP, with their blood[complete blood count (CBC), liver enzymes, and hepatitis markers]and urine tests. The patients having a two-fold increase in their liver enzymes were sampled for their fasting and postprandial SBA,and examined with ultrasonography for their hepatobiliary system.UDCA (3 ×250 mg/d) was applied to the patients who had been diagnosed as ICP [17] . These patients were reevaluated with the clinical and laboratory parameters after seven days from the onset of the treatment. The patients still having the same or worse pruritus, having the same or increased liver enzymes, and having no more decrease than 50% in fasting-postprandial SBA were evaluated for the fetal well-being (fetal well-being was assessed by cardiotocography, Doppler ultrasonography, and biophysical profile until delivery in order to determine if any unfavorable evidence was present). Betamethasone for two doses (24 h apart) was applied for fetal lung maturation for the patients who did not complete 37th gestational week [18] . The patients having an improvement in their clinical and/or laboratory results were scheduled for delivery at the 37th week. The exact pathology and mechanism of IUFD in ICP is not known. Non-stress test and/or biophysical profile are used to prevent IUFD in the antenatal period, but these tests do not have predictive value [16 , 19 , 20] . American College of Gastroenterology (ACG) recommends delivery at the 37th gestational week in ICP [21] . The indications for early or late preterm birth in ICP are not clear. South Australia Maternal and Neonatal Community of Practice (SAMNCP) recommend delivery in ICP when the SBA level is above 100 mmol/L and there are strong clinical signs [1] . The Society for Maternal-Fetal Medicine (SMFM) has not defined the exact criteria but suggested iatrogenic induction of delivery in the light of previous obstetric outcomes and antenatal follow-up tests [22] . We induced the delivery of the patients whose symptoms were getting worse, whose liver enzymes had elevated, and/or whose fetal well-being parameters (non-stress test,biophysical profile) were getting worse.
The venous blood was collected in similar gestational weeks in both groups (33.14 ±1.52 weeks). Routine laboratory automated techniques were used to determine serum ALT and AST. SBA was determined by spectrophotometric enzymatic method, and CBC parameters were measured by automated blood counter Cell-Dyn 3700 automated hemocytometer (Abbott, IL, USA). All serum samples were collected after centrifugation at 50 0 0 rpm for 15 min and stored at -80 °C until the day of analysis. All participants were followed until delivery. Serum syndecan-1 and glypican-3 levels were assessed at the time of the initial diagnosis of ICP. Syndecan-1 levels were analyzed using enzyme-linked immunosorbent assay(ELISA) kit (Wuhan USCN Business Co., Ltd, China), and the results were presented as ng/mL.
In addition, any maternal or fetal complications occurred during routine obstetric follow-up were recorded. Adverse perinatal outcome criteria were as follows: (a) 1 and 5-min APGAR score<7 (this was stringent for the acceptance of adverse perinatal outcome); (b) premature delivery; (c) fetal birth weight<2500 g; and(d) amniotic fluid with meconium [16 , 23] .
Statisticalanalysis
IBM SPSS Statistics for Windows, version 22.0 (SPSS Inc.,Chicago, IL, United States) was used for statistical analysis. Data were shown as mean ±standard deviation or median (range) or a number of cases and (percentage), where applicable. Variables were tested for normality by the Kolmogorov -Smirnov test. Student’st-test compared the mean differences between groups. We used the Mann-WhitneyUtest for non-parametric groups. Pearson’s Chi-square test analyzed nominal data. Multivariate logistic regression analysis was used to determine if there is a relationship between adverse perinatal outcome, unresponsiveness and serum biochemical parameters, syndecan-1, glypican-3 and clinic features.Spearman’s correlation test was used to analyze the correlation between serum syndecan-1, glypican-3 and AST, ALT, fasting and postprandial SBA in ICP. APvalue<0.05 was considered statistically significant.
Results
Clinicalcharacteristics
A total of 88 participants (44 ICP patients and 44 healthy controls) were enrolled in the study. The baseline characteristics, anthropometric, and laboratory parameters of ICP and controls are presented in Table 1 . The groups were comparable for age, BMI,weight gained during pregnancy, gravity, systolic blood pressure,diastolic blood pressure, type of delivery, and sex of babies. The ICP group significantly delivered at earlier weeks (35.31 ±2.52 weeks) than the control group (38.31 ±2.46 weeks;P<0.001).The blood was collected at 33 (33.14 ±1.52) weeks in both groups.Fetal weight was heavier in the control group (3208.18 ±329.06 vs. 2461.20 ±802.71;P<0.001). Preterm delivery rate was higher in the ICP group than the control group (61.4% vs. 2.3%;P<0.001)( Table 1 ). Patients in the ICP group had significantly higher AST,ALT, syndecan-1, and glypican-3 levels than the control group(P<0.001,P<0.001,P= 0.003,P= 0.004, respectively) ( Table 1 ).
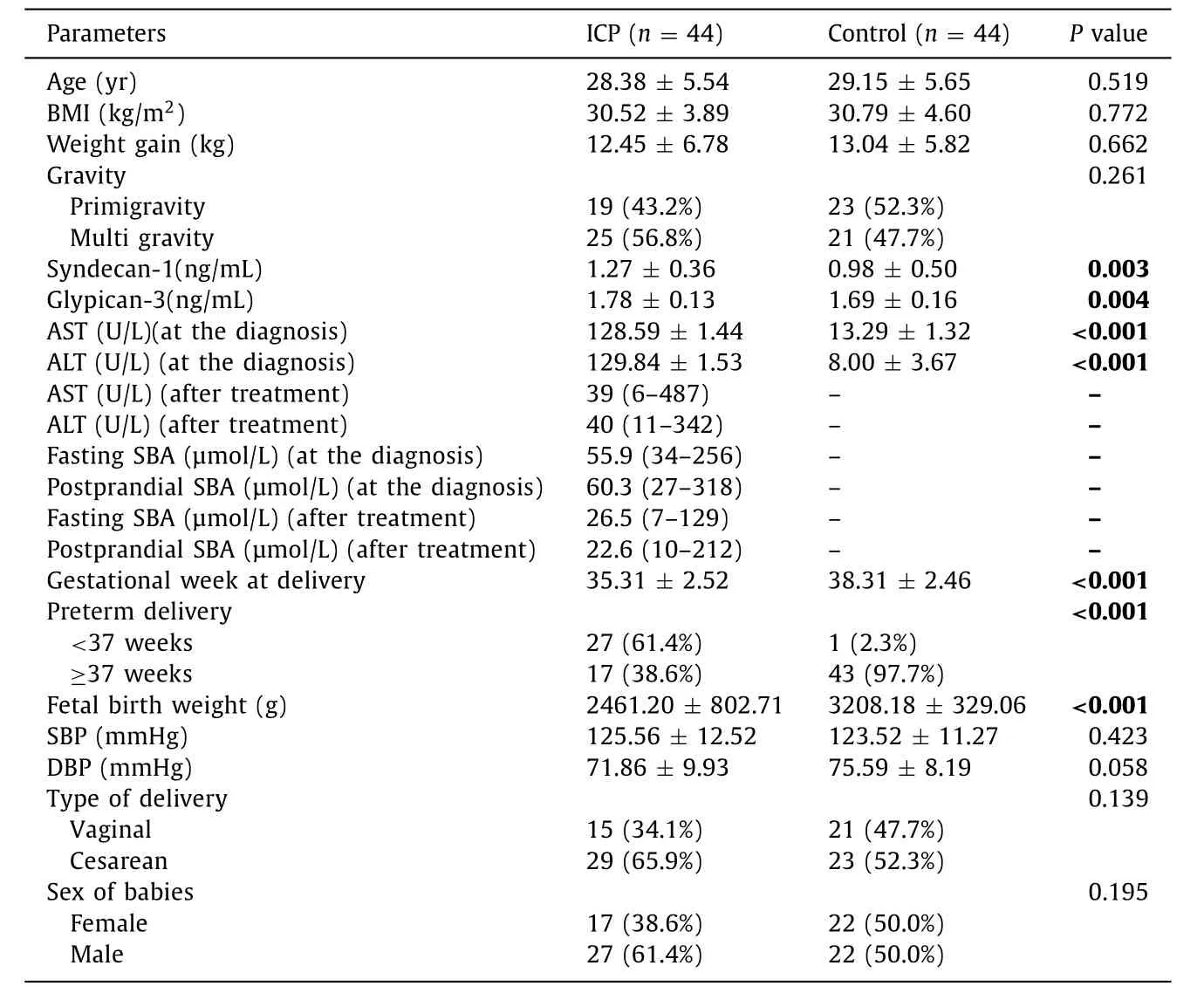
Table 1 Baseline characteristics, anthropometric and laboratory parameters of the groups.
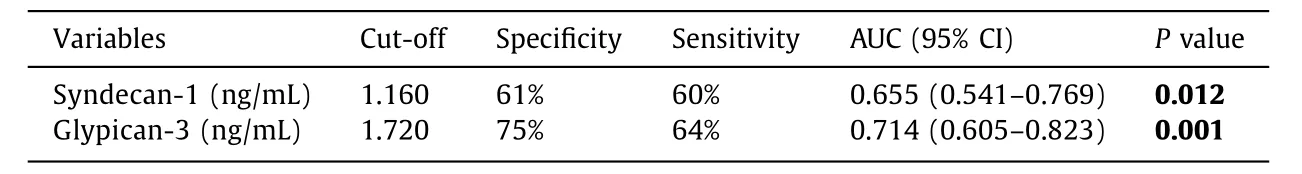
Table 2 Best cut-off value, sensitivity, specificity, and AUC of syndecan-1 and glypican-3 in ICP.
Serum syndecan-1 and glypican-3 levels were re-evaluated with ROC analysis. Cut-off levels were determined, and AUC was calculated ( Table 2 , Fig. 1 ).
Evaluationofriskfactorsbyregressionanalysis
Further analysis was performed by applying logistic regression analysis to assess the association between serum level of syndecan-1 and glypican-3 and ICP. Additionally, we evaluated the risk factors of adverse perinatal outcome in the ICP group. Elevated serum syndecan-1, glypican-3, ALT and postprandial SBA were significantly associated with ICP in pregnant women (OR = 4.715,95% CI: 1.554-14.310,P= 0 0 06; OR = 8.465, 95% CI: 3.372-21.248,P= 0.007; OR = 1.382, 95% CI: 1.131-1.690,P= 0.002; OR = 3.392,95% CI: 1.003-12.869,P= 0.026; respectively) ( Table 3 ). Only elevated serum glypican-3 and postprandial SBA levels were significantly associated with adverse perinatal outcome in ICP patients(OR = 4.275, 95% CI: 2.726-5.635,P= 0.039; OR = 3.026, 95% CI:1.069-13.569,P= 0.037; respectively) ( Table 3 ).
Univariate regression analysis was applied to determine the risk factors related to unresponsiveness to the treatment, and the significant parameters were analyzed with multivariate regression analyses. Elevated levels of syndecan-1 and glypican-3 were the risk factors suggesting late recovery, both for clinical, and laboratory parameters, in ICP patients (OR = 7.464, 95% CI: 2.130-26.153,P= 0.017 vs. OR = 6.194, 95% CI: 2.951-13.002,P= 0.025, respectively) ( Table 4 ). The age of the mother, the weight gained during pregnancy, gestational week of the patient, or the levels of AST,ALT, fasting- and postprandial SBA at the diagnosis of ICP, were not significant in predicting the response to the treatment ( Table 4 ).
Further analysis was also performed to determine whether there was a correlation between serum syndecan-1, glypican-3 levels, and AST, ALT, fasting- and postprandial SBA in ICP. No association was observed ( Table 5 ).
Discussion
We found that syndecan-1, glypican-3, ALT and AST levels were significantly higher in the ICP group when compared to the healthy controls. The increase in the ratio of terminal villi and the capillary surface area in the placenta are noteworthy in ICP [16] . Previous studies have shown that syndecan-1 is highly expressed in placental microvilli, and have a role in cell adhesion, proliferation,intracellular signaling, angiogenesis, lipid metabolism, and wound healing [24-26] . Stanford et al. [27] had shown that syndecan-1 is expressed in hepatocytes and microvilli of intestinal basal mem-brane, and takes place in the clearance metabolism of triglyceriderich lipoproteins. Syndecan-1 is also overexpressed in the hepatocytes in infection which suggested that syndecan-1 is correlated to hepatocyte proliferation, apoptosis, and increase in ALT levels [28] .Nam et al. [13] reported that syndecan-1 mitigates liver damage in the case of acetaminophen toxicity.
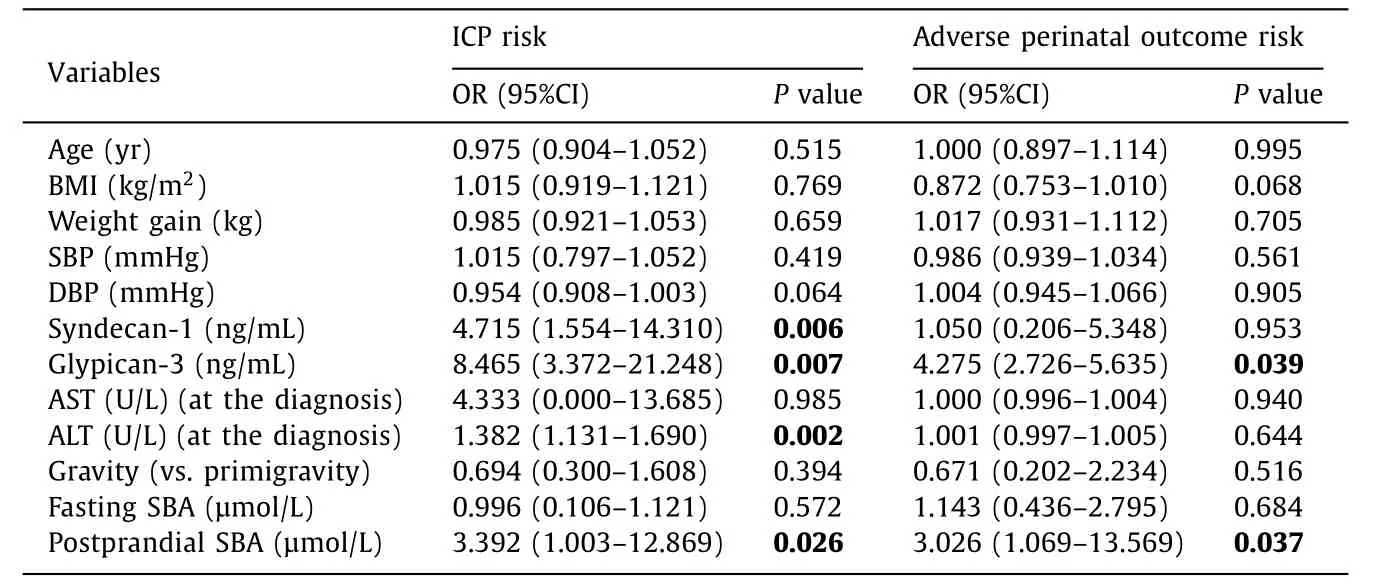
Table 3 Regression analysis for prediction of intrahepatic cholestasis of pregnancy and adverse perinatal outcome risk in the patient group.
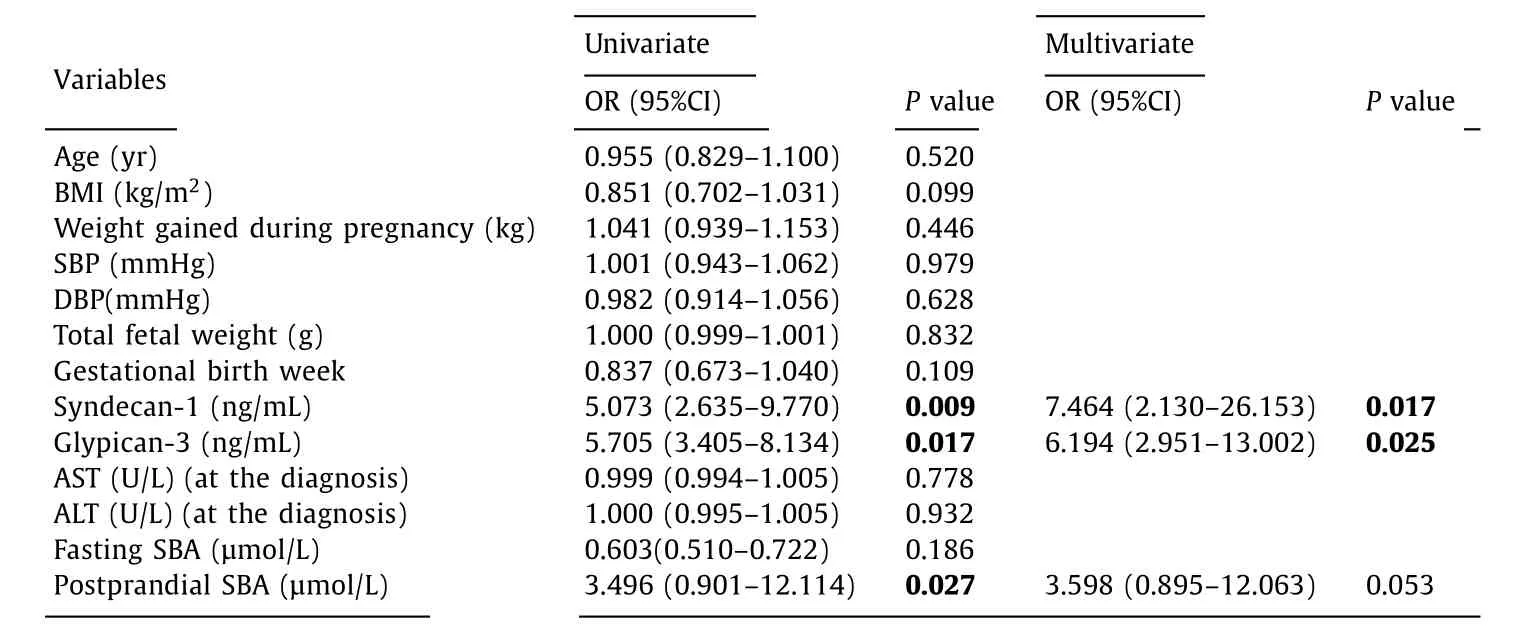
Table 4 Logistic regression analyses of the risk factors related to unresponsiveness to the treatment in the patient group.
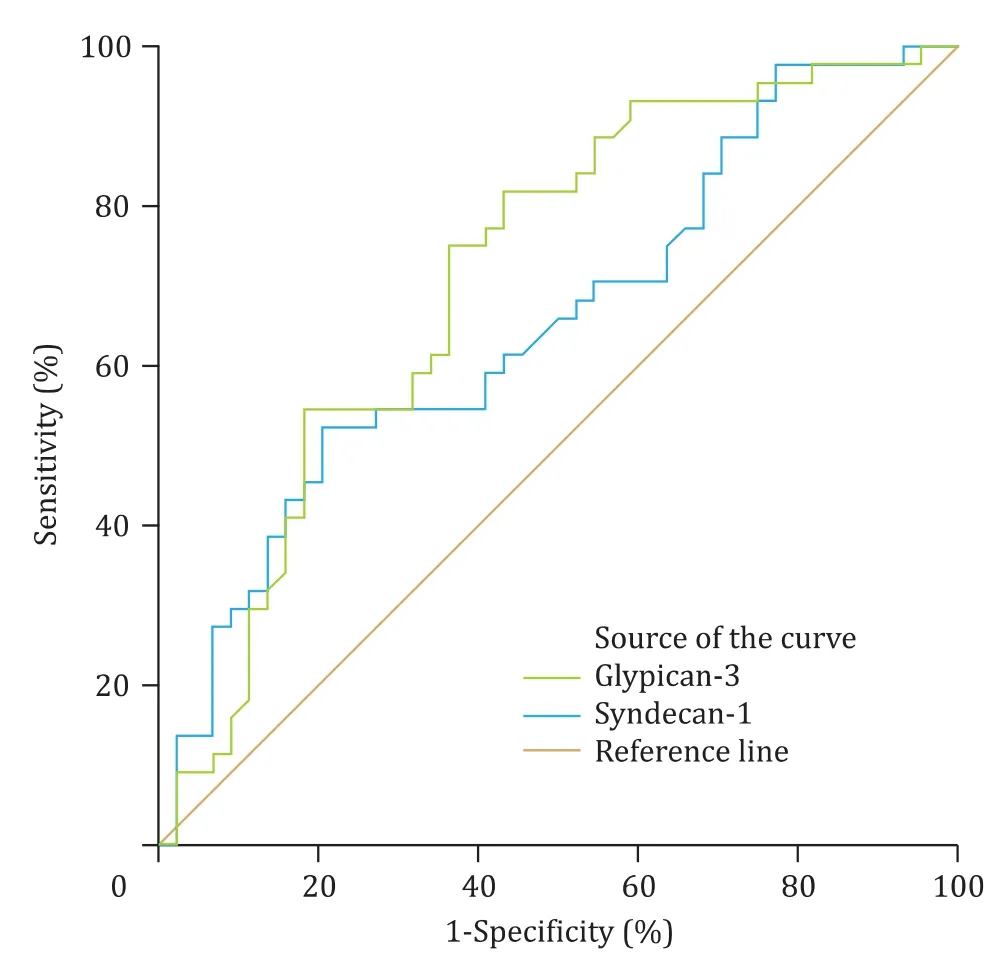
Fig. 1. Syndecan-1 and glypican-3 ROC curves in pregnancy cholestasis.
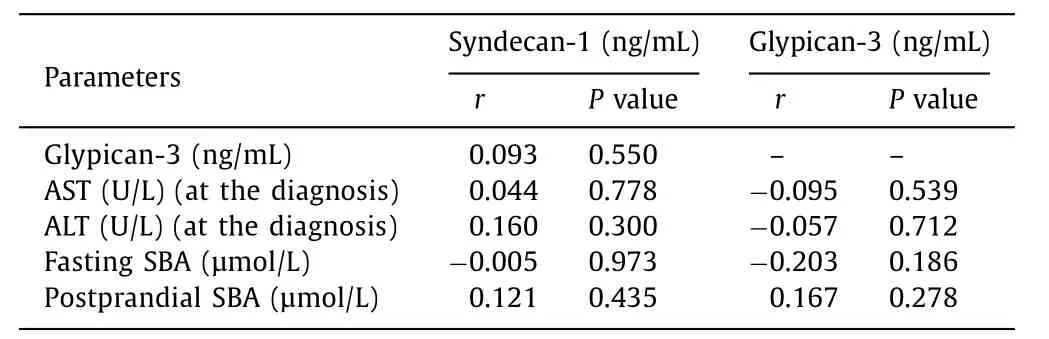
Table 5 Correlation analysis between syndecan-1, glypican-3, AST, ALT, fasting- and postprandial serum bile acid in ICP.
Glypican-3 is an oncofetal protein found in fetal liver, not in the adult liver. The expression of glypican-3 is increased in hepatocellular carcinoma, and it might be used as the target molecule in cancer treatment [29] . Glypican-3 is also an indicator of liver damage [30] . As far as we know, this is the first study on syndecan-1 and glypican-3 in ICP. The studies mentioned above may support the role of glypican-3 in liver damage characterized by the increase in SBA and ALT in ICP. The synchronous elevation of syndecan-1 might regulate the activity of both maternal liver and placenta.
UDCA decreases SBA and corrects the clinical presentation of the patients in ICP [1] . Even if the clinical presentation and the laboratory findings are improved by UDCA treatment, it is not effective in changing the rates of unexplained IUFD, preterm delivery, and adverse perinatal outcome [31 , 32] . The decreased SBA and liver enzymes do not predict the adverse perinatal outcome,and no other determinant (biochemical or ultrasonographical) have been found to suggest adverse outcome and help in the decision of delivery [1] . There are reports of persistent ICP cases ending in postpartum maternal cirrhosis, and hepatic fibrosis despite UDCA treatment [33] . Our study found that high level of syndecan-1 and glypican-3 was related to inadequate response to 7-day UDCA treatment.
All of the metabolic, structural, and functional changes in the placenta take place in the villi which form the maternal-fetal barrier. HSPG is highly observed in the structure of the villi [34] .Syndecan-1 is expressed in the villi of syncytiotrophoblasts of human placenta, and its levels are stable during the gestational weeks [34] . Lorenzi et al. [35] demonstrated the expression of syndecan-1 at the basolateral cell surface of the amniotic epithelium, and the strong relation of it on the structure and function of amnion and chorionic plate. Szabo et al. [36] showed the increased density of syndecan-1 in the placentas of patients with preeclampsia. Glypican-3 is expressed in the cardiomyocytes of neonatal rats [37] . The SBA was accused of accumulating in the fetal cardiomyocytes and fetal lungs, causing IUFD in ICP patients [38 , 39] .On the other hand, studies are proposing the lack of connection between increased levels of SBA and adverse perinatal outcome [3] .In the present study, we observed increased levels of glypican-3 and postprandial SBA were associated with adverse perinatal outcome in the patients of ICP.
Although syndecan-1 has not been studied in ICP, according to the other studies, we speculated that ICP may affect the clearance of syndecan-1. Inadequate clinical and laboratory response to UDCA treatment might be due to hepatocyte dysfunction, which is related to the increase in syndecan-1 levels. We suggested that elevated syndecan-1 and glypican-3 levels, together with the increased levels of liver transaminases and postprandial SBA, can be used to evaluate the therapeutic response in ICP. Besides, the demonstration of relation of glypican-3, together with increased SBA, with adverse perinatal outcome in ICP, leads to its use as a marker of raging disease in both maternal liver and fetus.
In conclusion, syndecan-1 and glypican-3 might be powerful determinants in predicting adverse perinatal outcome in patients of ICP, and they can be used to evaluate the response to the treatment.
CRediT authorship contribution statement
Ba ¸s ak Gümü¸s Güler:Conceptualization, Investigation, Writing -original draft.Sibel Özler:Conceptualization, Investigation, Writing- original draft, Writing - review & editing.
Funding
None.
Ethical approval
This study was approved by the Ethics Committee of the Liv Hospital Affiliated to University of ˙Istinye, Turkey ( 2018-003/015 ).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Transjugular intrahepatic portosystemic shunt for a patient with chylothorax in cryptogenic/metabolic cirrhosis
- Differential methylation landscape of pancreatic ductal adenocarcinoma and its precancerous lesions
- Hepatobiliary&Pancreatic Diseases International
- MicroRNAs and long non-coding RNAs in liver surgery: Diagnostic and therapeutic merits
- Alpha-fetoprotein and 18 F-FDG standard uptake value predict tumor recurrence after liver transplantation for hepatocellular carcinoma with portal vein tumor thrombosis: Preliminary experience
- Translationally controlled tumor protein exerts a proinflammatory role in acute rejection after liver transplantation
