Translationally controlled tumor protein exerts a proinflammatory role in acute rejection after liver transplantation
2020-07-07ZhiBinLinPeiJunYangXuanZhangJianLinWangKunLiuKeFengDou
Zhi-Bin Lin , Pei-Jun Yang , Xuan Zhang , Jian-Lin Wang, Kun Liu, Ke-Feng Dou
Department of Hepatobiliary Surgery, Xijing Hospital, The Fourth Military Medical University, Xi’an 710032, China
Keywords:Translationally controlled tumor protein Liver transplantation Acute rejection Lymphocyte
A B S T R A C T Background: Translationally controlled tumor protein (TCTP), which has been verified to have a proinflammatory activity, plays an important role in allergy. However, it remains unclear whether TCTP has an impact on the acute rejection (AR) after liver transplantation.Methods: Three protocols were used to delineate the role of TCTP in AR after liver transplantation. First,in rat orthotopic liver transplantation (OLT), the expression of TCTP was measured by enzyme-linked immunosorbent assay (ELISA), real-time PCR, Western blot and immunofluorescence assays. Second, in mixed lymphocyte reaction (MLR), the role of TCTP in lymphocyte proliferation was measured by carboxyfluorescein succinimidyl ester (CFSE) labeling and the impact of TCTP on inflammatory factor release was detected by cytokine arrays. Third, in human OLT, the level of serum TCTP was detected by ELISA,and the relationship between TCTP and model for early allograft function (MEAF) score was assessed by Spearman’s correlation.Results: In rat OLT, AR resulted in great harm to allografts, manifesting as deterioration of liver function, increasing inflammatory factors and infiltrating lymphocytes. Meanwhile, TCTP was overexpressed in serum and allografts. Higher level of TCTP was associated with higher rejection activity index (RAI).In an MLR protocol, TCTP knockdown inhibited the proliferation of mixed inflammatory cells and significantly suppressed the release of 15 cytokines and chemokines. In human OLT, the serum TCTP was up-regulated within a week after operation. Additionally, the increasing speed of serum TCTP positively correlated with MEAF scores ( r = 0.449; P = 0.0088).Conclusions: Up-regulated TCTP positively affects AR after liver transplantation.
Introduction
Liver transplantation has been widely used as a potent therapy for final-state liver diseases [1 , 2] . Despite that several advances in optimizing the process of liver transplantation have been made,acute rejection (AR) remains to be a major obstacle of favorable outcomes [3] . Immunosuppressants, such as tacrolimus, have been adopted to control the AR injury. However, the effects are rather limited and the complications remain lethal [4 , 5] . Therefore, the underlying mechanism of AR should be further investigated and new therapeutic targets of AR should be developed to improve the prognosis of liver recipients.
Translationally controlled tumor protein (TCTP), which is also referred to as histamine releasing factor (HRF), P23 or fortilin, is highly conserved and abundantly expressed among different kinds of cells and tissues [6] . Studies have proven that TCTP is implicated in various cellular processes, such as cell growth, development, immune response, apoptosis, DNA repair and malignant transformation [7-11] . One of the best characterized functions of TCTP is its proinflammatory activity. Initially, HRF, which is the human homologue of TCTP, was found to be able to stimulate histamine released from basophils [12] . It was subsequently revealed that TCTP primed basophils for the release of IL-4 and IL-13 [13] . Moreover,TCTP plays an independent role in the growth of T and B lymphocytes [14 , 15] . Therefore, it is worthwhile to investigate whether TCTP exerts proinflammatory activity during AR after liver transplantation.
Methods
Animalsandpatients
Brown Norway (BN) and Lewis rats were purchased from Vital River, Inc. (Beijing, China). TCTP+/−mice (C57BL/6 background)were generated using gene targeting technology (TCTP−/−causes embryonic lethality [7] ). Briefly, a targeting vector that contained a series of functional sites was linearized and electroporated into embryonic stem cells derived from the C57BL/6 background, and the targeted embryonic stem cells were injected into the blastocysts of Balb/c mice. Then, the chimeras were mated with C57BL/6 mice, and theTpt1mutation was transmitted to the germ line. Animals were raised in a specific pathogen free (SPF) condition with free access to rodent diet and clean water.
From September 2018 to May 2019, 34 patients undergoing liver transplantation at the Department of Hepatobiliary Surgery, Xijing Hospital of the Fourth Military Medical University were enrolled to the present study. Informed consent was obtained from all of the patients. The serum samples of patients were collected on the day before the orthotopic liver transplantation (OLT), and at day 1, 5 and 7 after OLT. The blood was collected, centrifuged (1 0 0 0 ×g,15 min) and the serum was stored at −20 °C. The levels of alanine aminotransferase (ALT), international normalized ratio (INR)and total bilirubin (TBIL) were recorded. The present study was approved by the Ethics Review Committee of Xijing Hospital (No.KY20172013-1).
Operationandgrouping
Young adult male rats (180-220 g) were subjected to sham operation or OLT. The OLT were conducted based on the modified two-cuff method described by Kamada [16] . The sham operation was laparotomy without liver transplantation. All surgeries were carried out under isoflurane anesthesia. Lewis rats (as donors) and BN rats (as recipients) were chosen to construct the acute rejection(AR) group (L-B), while Lewis rats (as both donors and recipients)were chosen to construct the tolerance group (L-L). The serum and tissue samples of rats were collected on the day before orthotopic liver transplantation (OLT), and at day 1, 3, 5 and 7 after OLT. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Biochemistryandenzyme-linkedimmunosorbentassay(ELISA)
A Chemistry Analyzer (Chemray 240, Rayto, Shenzhen, China)was used to measure the serum levels of alanine aminotransferase(ALT), aspartate aminotransferase (AST), albumin (ALB) and total bilirubin (TBIL) in rats, according to manufacturer’s instructions.The concentration of serum interferon-γ(IFN-γ), interleukin-6 (IL-6), IL-23, tumor necrosis factor-α(TNF-α) and TCTP in rats were measured using ELISA kits purchased from Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China, cat. no. ml002833, ml002828,ml0 03152, ml0 02859 and ml233103, respectively). The concentration of serum TCTP in humans were measured using ELISA kits purchased from Signalway Antibody Co., Ltd. (Maryland, America,cat. no. EK3951).
Immunohistochemistry(IHC)andimmunofluorescenceassay
The livers were fixed overnight in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm-thick sections. The slices were used for the hematoxylin and eosin (H&E) staining and immunofluorescence staining. Immunofluorescence staining for TCTP was performed using antibody purchased from Abcam Co., Ltd.(Shanghai, China, cat. no. ab133568, 1:250) and counterstained with Hoechst 33258 (Sigma-Aldrich, Shanghai, China).
RNAisolationandquantitativereal-timepolymerasechainreaction(PCR)
Total RNA was isolated from livers using Trizol reagent (Invitrogen, Life Technologies, CA, USA), and was reverse-transcribed using a reverse transcription kit (Takara, Kyoto, Japan). Then, the cDNA was analyzed by real-time polymerase chain reaction (PCR) using the SYBR Green PCR master mix (Takara) in a two-step reaction.Each reaction was repeated in triplicate wells.β-actin was used as the reference gene. The expression of the gene was calculated using the 2 - ( △△ Ct) method. The primer sequences for the RT-PCR are presented as following: TCTP (forward primer, GTT GCT CTC CTG GAC TAC CG and reverse primer, TGG TAA GCA GCC AAT TA);βactin (forward primer, TGT CAC CAA CTG GGA CGA TA and reverse primer, GGG GTG TTG AAG GTC TCA AA).
Westernblotanalysis
Protein lysates were prepared in the RIPA Lysis Buffer (Beyotime, Shanghai, China, cat. no. P0013K), which contained the protease and phosphatase inhibitor cocktails (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Total protein extractions were separated by the use of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto the polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA).After blocked for 1 h at room temperature, these membranes were incubated with specific primary antibodies at 4 °C overnight. The antibodies were presented as following:β-actin (1:10 0 0; cat. no.3700; Cell Signaling Technology, Inc., Shanghai, China), and TCTP(1:1 0 0 0 0; cat. no. ab133568; Abcam).
Therejectionactivityindex(RAI)scoring
The calculation of RAI was based on Banff schema, a semiquantitative scoring system for grading AR [17] . Briefly, three components of RAI (portal inflammation, bile duct damage and venous endothelial inflammation) were scored from 0 to 3. Then all the scores were added to combine to an overall score, the RAI.
Mixedlymphocytereaction(MLR)andcarboxyfluorescein succinimidylester(CFSE)lymphocytelabeling
Spleens were collected from animals and minced to prepare single splenocytes suspension. The splenocytes were incubated with erythrocytes lysis buffer (Beyotime) in 37 °C for 8 min and then washed two times with phosphate buffered saline (PBS) and centrifuged (300 ×g, 5 min). Thereafter, lymphocytes were extracted and resuspended in RPMI-1640 containing 10% fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.Stimulator cells and responder cells were used to construct the MLR model -the stimulator cells (lymphocytes form Balb/c mice)were treated with mitomycin C (50 μg/mL) for 20 min to prevent their mitogenic activity; and the responder cells (lymphocytes from either TCTP+/ + or TCTP+/−mice without mitomycin C treatment)were co-cultured with the stimulator cells in a ratio of 1:1. The responder cells were labelled with CFSE (MedChemExpress, New Jersey, USA). The mixed lymphocytes were cultured for three days and the CFSE staining was measured by MoFlo XDP (Beckman Coulter,Maryland, USA) and then analyzed using FlowJo10.5 software (Becton, Dickinson & Company, New Jersey, USA).
Cytokinearray
The supernatant extracted from either MLR consisting TCTPknockdown lymphocytes or the control group were incubated with the Proteome Profiler Mouse Cytokine Array Kit (R&D Systems,Minnesota, USA, cat. no. ARY006) for 24 h, according to manufacturer’s instructions. The relative expression levels of the cytokines were compared using Image Lab Software 6.0.1 (Bio-Rad, California,USA).
Modelforearlyallograftfunction(MEAF)scoring
The following formulas were used to calculate the MEAF score,as reported [18] .
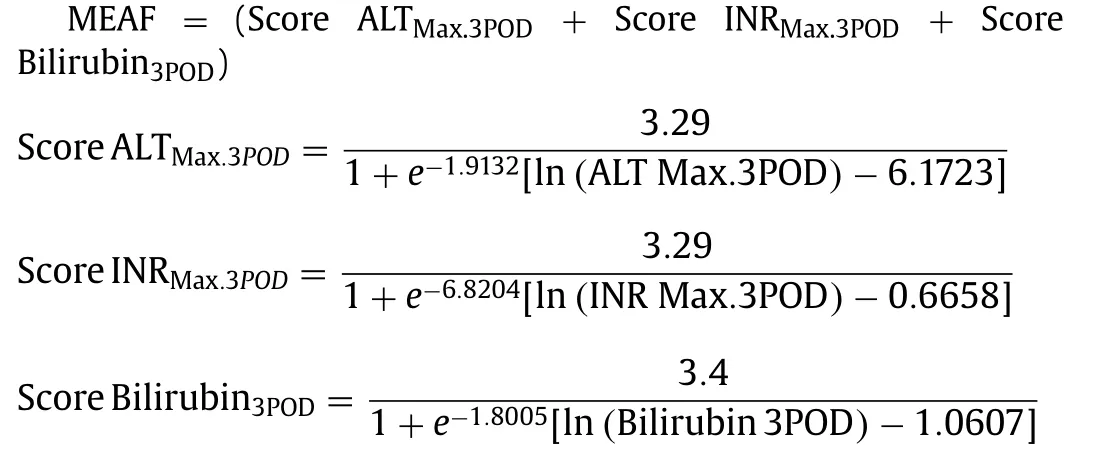
(Max.3POD: maximum values during the first 3 postoperative days; 3POD: values of the postoperative day 3)
Statisticalanalysis
Each experiment has repeated at least triple times. SPSS 23.0 for Windows (IBM Corp., Armonk, NY, USA) and Prism 7.0 (Graph-Pad Software, La Jolla, CA, USA) were used to perform the statistical analysis. All of the data were presented as the mean ±SD. Two-sided Student’st-test and one-way analysis of variance(ANOVA) methods were used to compare two or three different groups of data of normally distributed and homoscedasticity. Twoway ANOVA (multiple comparisons by the usage of Tukey’s post hoc test) was used to compare two groups which involved two or more time-points. ANOVA for repeated measurement was performed to measure serum TCTP levels in patients undergoing liver transplantation. Pearson correlation analysis was used to analyze the correlation between serum TCTP and RAI or MEAF score. AP<0.05 was considered statistically significant.
Results
TheimpactofARonallografts
Among the three groups, only the sham group showed no change in the liver function. Compared with the tolerance group(L-L group), the AR group (L-B group) showed a higher level of AST at postoperative day 7 (POD 7) (483.6 ±112.1 vs 159.3 ±23.5 U/L,P<0.05), a higher level of TBIL at POD 7 (35.5 ± 3.7 vs 19.4 ±3.8 μmol/L,P<0.05) and a lower level of ALB at POD 3 (23.2 ± 1.1 vs 26.5 ± 1.4 g/L,P<0.05, Fig. 1 A). Expectedly, the levels of inflammatory factors altered in the L-L and L-B groups, but not in the sham group. Compared with the LL group, the L-B group showed a higher level of IFN-γat POD 5 (1636.5 ±92.6 vs. 944.5 ±108.2 pg/mL,P<0.01) and POD 7(1698.0 ±222.1 vs. 1115.3 ±115.7 pg/mL,P<0.05), a higher level of IL-6 at POD 1 (137.5 ±9.2 vs. 105.1 ±12.7 pg/mL,P<0.05) and POD 5 (86.3 ±4.2 vs. 41.5 ±10.6 pg/mL,P<0.01), a higher level of IL-23 at POD 3 (72.5 ±6.4 vs. 42.7 ±7.6 pg/mL,P<0.001), a higher level of TNF-αat POD 1 (304.3 ±35.4 vs. 218.5 ±17.6 pg/mL,P<0.05), and a lower level of TNF-αat POD 3 (190.2 ±13.4 vs.278.3 ±21.1 pg/mL,P<0.01) ( Fig. 1 B). Subsequently, H&E staining on the sections of grafts showed that severe inflammatory cell infiltration appeared in the grafts of the L-B group, but not in the L-L group or the sham group ( Fig. 1 C). Taken together, AR caused deteriorative liver function, increasing inflammatory factors and infiltrating lymphocytes in allografts.
Up-regulatedTCTPinARprocess
Compared with the L-L group, higher level of serum TCTP was detected in the L-B group at POD 7 (P<0.05, Fig. 2 A), and higher level of TCTP mRNA was detected in the L-B group on POD 5(P<0.05) and POD 7 (P<0.001, Fig. 2 B). Meanwhile, the level of TCTP protein showed significant increase at POD 7 in the L-B group (P<0.001, Fig. 2 C and D). The results of the immunofluorescence staining confirmed that TCTP was intensively expressed in the cytoplasm of inflammatory cell and hepatocyte nearby in the L-B group ( Fig. 2 E). Taken together, TCTP was overexpressed during AR after liver allotransplantation in a time-dependent manner.
TCTPcorrelatestoRAI
RAI was classified as the mild (RAI 4-5), moderate (RAI 6-7) and severe groups (RAI 8-9). The correlation analysis revealed that serum TCTP level was positively correlated with the RAI score(r= 0.4909,P= 0.0386, Fig. 3 A). Compared with the mild group,the severe group rather than the moderate group showed an increase in TCTP mRNA (P<0.05, Fig. 3 B). While both the moderate group and the severe group showed higher level of TCTP protein(P<0.05,P<0.001, respectively; Fig. 3 C and D). Immunofluorescence staining showed that TCTP was distinctly expressed in the cytoplasm of inflammatory cell and adjacent hepatocyte in the severe group ( Fig. 3 E). Taken together, results revealed that TCTP was overexpressed during AR after liver allotransplantation in a degreedependent manner.
TheimpactofTCTPknockdownonMLR
The knockdown of TCTP gene is shown in Fig. 4 A. Real-time PCR and Western blot showed that TCTP was significantly decreased in lymphocytes from TCTP-knockdown mice (P<0.01, Fig. 4 B and C). Lymphocytes of TCTP+/−mice showed a limited proliferation ( Fig. 4 D). The results of CFSE staining showed that proliferative lymphocytes (CFSE+) decreased in TCTP+/- MLR (P<0.01,Fig. 4 E). Thereafter, results of membrane-based antibody arrays revealed that the level of 15 cytokines and chemokines descended in TCTP+/−MLR ( Fig. 4 F). Taken together, TCTP-knockdown inhibited the proliferation of lymphocytes and suppressed the level of cytokines and chemokines in MLR.
Up-regulatedserumTCTPinhumanliverrecipients
Serum TCTP level was increased after liver transplantation(P<0.001, Fig. 5 A). The positive correlation between the change speed of serum TCTP and the MEAF score was confirmed(r= 0.449,P= 0.0088, Fig. 5 B). In summary, serum TCTP was upregulated after liver transplantation and reflected the early graft function.
Discussion
AR after liver transplantation is mainly characterized by intense inflammation, accompanied with unbalanced secretion of various cytokines [19] . Numerous studies have proven that TCTP, a histamine releasing factor (HRF) [12] , can act as an stimulator of cytokines release, including IL-1, IL-4, IL-6, IL-8 and IL-13, from B lymphocytes or basophils [13 , 14 , 20] . Therefore, TCTP might be involved in the process of AR after liver transplantation.
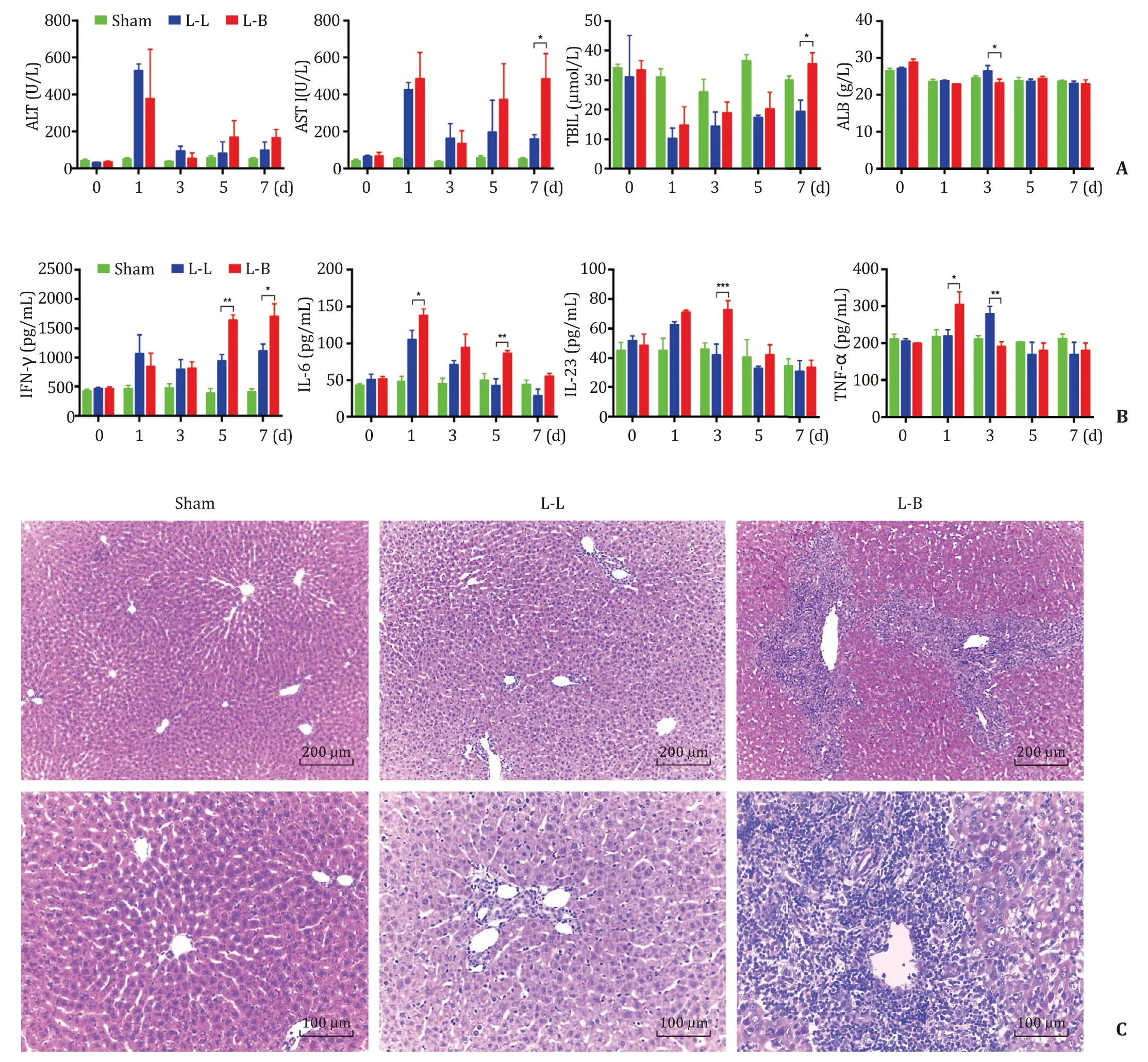
Fig. 1. AR caused destruction in allografts. A: The serum levels of ALT, AST, TBIL and ALB at the different time points; B : The serum levels of IFN- γ, IL-6, IL-23 and TNF- α at different time points. The results were presented as mean ±standard deviation (SD). n = 5. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; C : The representative histological panels of H&E staining in liver sections [Scale bars: 200 μm (upper) and 100 μm (lower)]. ALT: alanine aminotransferase; AST: aspartate aminotransferase; TBIL: total bilirubin; ALB:albumin; IFN- γ: interferon- γ; IL-6: interleukin-6; IL-23: interleukin-23; TNF- α: tumor necrosis factor- α.
AR was characterized by lymphocytes infiltration and inflammatory factor surge [21] . Therefore, to find out the relationship between TCTP and AR after liver transplantation, we investigated the specific role of TCTP in lymphocytes and inflammatory factors.
Lymphocytes exert a dominant part in the immune response.It is well documented that alloreactive effector T lymphocytes can recognize alloantigen through interacting major histocompatibility complex (MHC) on the donor cell in the direct pathway and processing alloantigen self-restrictedly in the indirect pathway,and play a dominant role in the development of AR [22 , 23] .Meanwhile, B lymphocytes is also associated with severe graft rejection [24] . The relationship between TCTP and lymphocytes has been reported: Yang’s study revealed that TCTP and Bcl-xL,a well-known anti-apoptosis protein, were distinctly overexpressed in mature T lymphocytes activated by T-cell antigen receptor (TCR) ligation and CD28 costimulation [25] . Subsequently,Wu’s study found that TCTP deletion significantly reduced the composition of mature CD4+and CD8+Tcells in the lymph nodes and spleens of mutant mice, and TCTP-deficient T lymphocytes were much more vulnerable to cell death than normal cells [15] . And the study of Vonakis revealed that human recombinant histamine-releasing factor (HrHRF) inhibited the secretion of IL-2 and IL-13 from human T cells by preventing gene transcription [26] . Kang’s study demonstrated that rHRF stimulates B cell proliferation and up-regulates the level of IL-1 and IL-6 in B cells [14] .
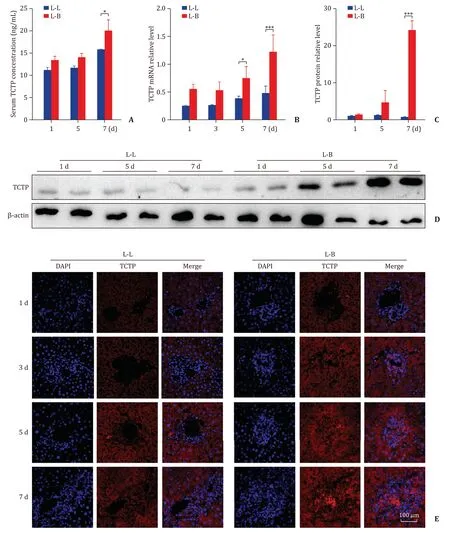
Fig. 2. Up-regulated TCTP in AR process. A-C : The serum level ( A ), the mRNA expression level ( B ) and the protein expression level ( C ) of TCTP in grafts at different time points. The results were presented as mean ±SD. n = 5. ∗P < 0.05; ∗∗∗P < 0.001; D-E : The representative Western blot assays ( D ) and immunofluorescence assays ( E ) for the TCTP protein expression in grafts at different time points. Scale bar: 100 μm. TCTP: translationally controlled tumor protein; AR: acute rejection; SD: standard deviation.

Fig. 3. TCTP correlates to rejection activity index (RAI). The serum TCTP level and RAI of the grafts was significantly correlated in the AR group ( A : P < 0.05). The mRNA( B ) and protein ( C ) expression levels of TCTP in grafts were obtained from the mild, moderate and severe groups (RAI 4-5, 6-7 and 8-9, respectively). The results were presented as mean ±SD. n = 5. ∗P < 0.05; ∗∗∗P < 0.001. The representative Western blot assays ( D ) and immunofluorescence assays ( E ) for the TCTP protein expression in grafts obtained. Scale bar: 100 μm. TCTP: translationally controlled tumor protein; SD: standard deviation.
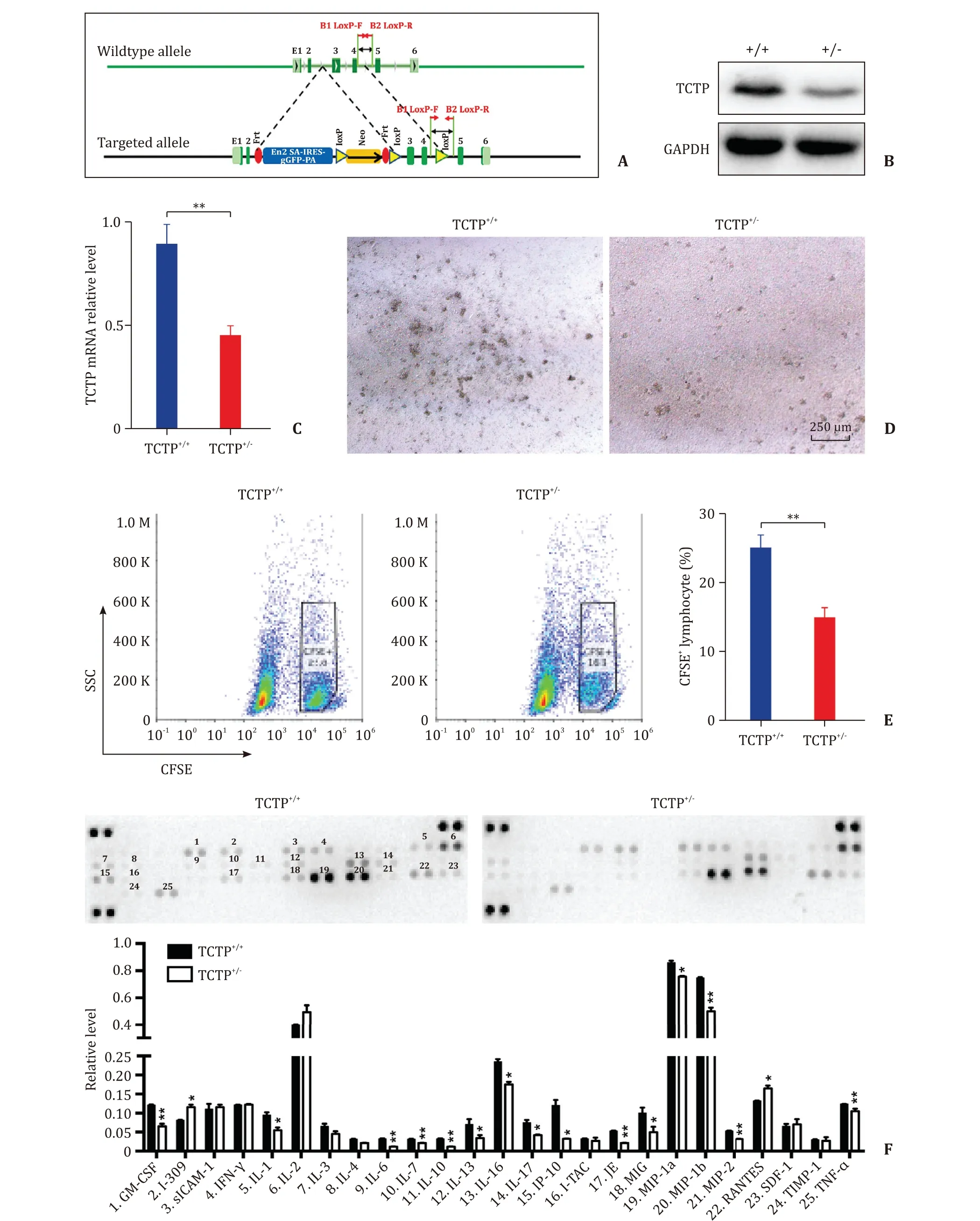
Fig. 4. TCTP-knockdown inhibited the proliferation and reaction of mixed lymphocytes. A : The schematic of the construct to knockdown the TCTP gene ( Tpt1 ); B : Representative Western blot analysis for the level of TCTP in primary lymphocytes; C : RT-PCR analysis for the transcription of TCTP gene in primary lymphocytes; D : The effect of TCTP on proliferation of mixed lymphocytes. Scale bar: 250 μm; E : Representative CFSE staining for the mixed lymphocytes. The numbers stand for the proportion of proliferated recipient cells; F : The concentration of cytokines and chemokines in cell culture supernatant from MLR. The results were presented as mean ±standard deviation(SD). n = 5. ∗P < 0.05; ∗∗P < 0.01 (Two-sided student’s t- test). TCTP: translationally controlled tumor protein; RT-PCR: real-time polymerase chain reaction (RT-PCR); CFSE:carboxyfluorescein succinimidyl ester; MLR: mixed lymphocyte reaction.
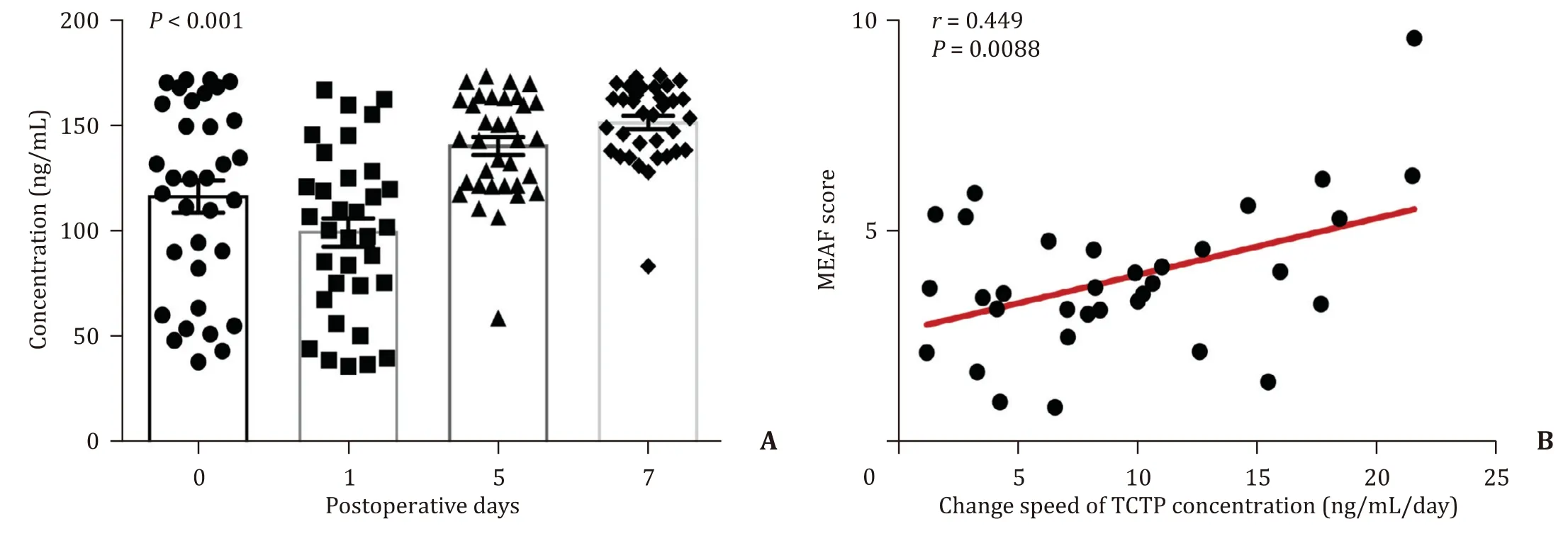
Fig. 5. The up-regulated serum TCTP after human liver transplantation. A : Serum levels of TCTP in liver recipients at different time points ( P < 0.001, compared with day 1);B : The correlation between the average increasing rate of serum TCTP and MEAF score (Pearson correlation analysis). TCTP: translationally controlled tumor protein; MEAF:model for early allograft function.
Our data are consistent with that of Yang’s study [25] , that TCTP was overexpressed in the activated inflammatory cells which infiltrated among hepatocytes during AR process. And the results of immunofluorescence staining indicated that TCTP was also induced in the cytoplasm of hepatocytes near the lymphocyte-infiltrating area, therefore it was speculated that the up-regulated TCTP in the activated inflammatory cells could be released as a cytokine, either to enter into the cytoplasm of adjacent hepatocytes by endocytic pathway, or to stimulate the expression of TCTP in adjacent hepatocytes by acting on specific membrane receptors. Further studies are needed to verify this hypothesis and to investigate the effect of overexpressed TCTP on hepatocytes. And TCTP-knockdown hampers the proliferation of mixed lymphocytes, which are in line with the results of Wu’s study [15] and Kang’s study [14] .
Besides, cytokine arrays showed that TCTP-knockdown had a negative effect on the secretion of most cytokines and chemokines from inflammatory cells in AR. The level of IL-1 and IL-6 was decreased in MLR consisting TCTP-knockdown lymphocytes. This result is similar to that of Kang’s study, which showed that exogenous TCTP stimulates B cell to produce IL-1 and IL-6 [14] .However, the level of IL-2 and IL-13 did not ascend in MLR consisting TCTP+/−lymphocytes, which disaccorded with that of Vonakis’s study: exogenous TCTP inhibited the secretion of IL-2 and IL-13 from human T cells [26] . We considered that despite T cells’dominant role in AR, B cells, dendritic cells and natural killer cells also contribute to the AR process. TCTP-knockdown in these cells might subsequently alter the profile of cytokine secretion of T cell.
Judging from the results of the present study and literatures mentioned above, it can be deduced that after liver transplantation, numerous inflammatory cells with a high level of TCTP would infiltrate into the allograft. Overexpressed TCTP promotes AR in liver allograft by maintaining the proliferation of inflammatory cells and stimulating the release of cytokines and chemokines.However, since MLR is not equal to AR after liver transplantation,more convictive experiments, such as liver transplantation in transgenic mice, are needed to support this conclusion.
The present study also revealed that the highly induced serum TCTP during the progress of AR was correlated to high RAI in allografts, which suggests that serum TCTP has potential to be used to access the injury of allografts after transplantation. Moreover, the change speed of serum TCTP is correlated to high MEAF score in patients receiving liver transplantation. The MEAF, a newly invented model for evaluating early allograft function after liver transplantation, showed a significant relevance with graft outcome and patient prognosis, and has been verified to be a potent model to assess the survival according to the score of recipient [18 , 27-29] .Compared with the categorical early allograft dysfunction (EAD)classification, MEAF has its own advantage. It provides a more accurate graft function assessment than EAD classifications, and helps clinicians to make early decisions on retransplantation benefits [30] . Since the change speed of serum TCTP is positively correlated with MEAF score, serum TCTP can probably serve as a novel biomarker which can reflect on the outcomes of the liver transplant patients. Future research need to be based upon an enlarged sample volume to prove the feasibility of the hypothesis.
In conclusion, the present study is the first to investigate the role of TCTP in liver allotransplantation. The results showed that TCTP is associated with the outcomes of liver allograft in rats in a time-dependent manner and a degree-dependent manner. And TCTP-knockdown has a negative effect on the proliferation and secretory ability of inflammatory cells in an MLR model. Moreover,the change speed of serum TCTP is correlated with MEAF score of liver recipients. The main message of this study is that TCTP exerts a proinflammatory role in liver transplantation.
CRediT authorship contribution statement
Zhi-Bin Lin:Data curation, Formal analysis, Methodology, Investigation, Software, Writing - original draft.Pei-Jun Yang:Data curation, Formal analysis, Methodology, Software.Xuan Zhang:Methodology, Project administration, Resources, Software, Supervision, Funding acquisition.Jian-Lin Wang:Data curation, Formal analysis.Kun Liu:Resources, Software.Ke-Feng Dou:Conceptualization, Funding acquisition, Supervision, Validation, Writing - review & editing.
Funding
This work was supported by grants from the National Key Research and Development Program Funded Projects( 2017YFC1103703 ), the National Basic Research Program of China( 2015CB554100 ), and the National Natural Science Foundation( 81870446 , 81670593 , and 81900571 ).
Ethical approval
This study was approved by the Ethics Review Committee of Xijing Hospital (No. KY20172013-1). Written informed consent was obtained from all participants.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Transjugular intrahepatic portosystemic shunt for a patient with chylothorax in cryptogenic/metabolic cirrhosis
- Differential methylation landscape of pancreatic ductal adenocarcinoma and its precancerous lesions
- Hepatobiliary&Pancreatic Diseases International
- MicroRNAs and long non-coding RNAs in liver surgery: Diagnostic and therapeutic merits
- Alpha-fetoprotein and 18 F-FDG standard uptake value predict tumor recurrence after liver transplantation for hepatocellular carcinoma with portal vein tumor thrombosis: Preliminary experience
- Sequential transcatheter arterial chemoembolization and portal vein embolization before right hemihepatectomy in patients with hepatocellular carcinoma
