Alpha-fetoprotein and 18 F-FDG standard uptake value predict tumor recurrence after liver transplantation for hepatocellular carcinoma with portal vein tumor thrombosis: Preliminary experience
2020-07-07ZheYngFngZhouLuoShuoWngJnLerutLiZhungQiYongLiXioXuShuSenZheng
ZheYng Fng-Zhou Luo Shuo Wng Jn Lerut Li Zhung Qi-Yong Li Xio Xu Shu-Sen Zheng ∗
a Department of Hepatobiliary and Pancreatic Surgery, Department of Liver Transplantation, Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine, Hangzhou, China
b Division of Hepatobiliary Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
c National Clinical Research Center of Infectious Diseases, Hangzhou, China
d Starzl Unit of Abdominal Transplantation, University Hospitals Saint Luc, Université catholique Louvain, Brussels, Belgium
Keywords:Hepatocellular carcinoma Liver transplantation Portal vein tumor thrombosis Alpha-fetoprotein Standard uptake value
A B S T R A C T Background: Portal vein tumor thrombosis (PVTT) is regarded as a contraindication for liver transplantation (LT) in hepatocellular carcinoma (HCC). However, some of these patients may have a favorable prognosis after LT. In this study, we evaluated the biological behavior of HCC with PVTT using tumor biomarker (alpha-fetoprotein, AFP) and 18 F-FDG positron emission tomography (tumor standard uptake value) to identify a subset of patients who may be suitable for LT.Methods: Seventy-five HCC-PVTT liver recipients transplanted during February 2016 and June 2018 were analyzed. Different pre-transplant prognostic factors were identified by univariate and multivariate analyses. PVTT status was identified following Vp classification (Vp1-Vp4).Results: Three-year recurrence-free survival and overall survival rates were 40% and 65.4% in Vp2-Vp3 PVTT patients, 21.4% and 30.6% in Vp4 PVTT patients ( P < 0.05). Total tumor diameter > 8 cm, pretransplant AFP level > 10 0 0 ng/mL and intrahepatic tumor maximal standard uptake value (SUVmaxtumor > 5) were independent risk factors for HCC recurrence and overall survival after LT in Vp2-3 PVTT patients. Low risk patients were defined as total tumor diameter ≤8 cm; or if total tumor diameter more than 8 cm, with both pre-transplant AFP level less than 10 0 0 ng/mL and intrahepatic tumor SUVmax less than 5, simultaneously. Twenty-two Vp2-3 PVTT HCC patients (46.8%) were identified as low risk patients, and their 3-year recurrence-free and overall survival rates were 67.6% and 95.2%, respectively.Conclusions: Patients with segmental or lobar PVTT and biologically favorable tumors defined by AFP and 18 F-FDG SUVmax might be suitable for LT.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common types of cancer worldwide [1] . Invasion of portal and hepatic veins is one of the most unfavorable prognostic factors [2] . Liver resection is always a preferential treatment modality [3] . For patients with portal or hepatic vein invasion, liver resection could still acquire good prognosis as shown by a Japanese national study and Chinese multi-centers research [4 , 5] . Patients with severe cirrhosis however might not tolerate resection and liver transplantation (LT) may be the only treatment for these patients. The intention-to-treat (ITT) transplant survival benefit allows physicians and surgeons to better select HCC patients waiting for LT [6] .Current criteria for LT in HCC patients, including Milan criteria,The University of California at San Francisco (UCSF) criteria, Up-toseven criteria, Hangzhou criteria, and Time-Radiological-response-Alpha-fetoprotein-Inflammation (TRAIN) score, consider macrovascular invasion as an absolute contraindication [7-11] . However, it has been shown that some of these patients have favorable outcome [12 , 13] . In a multi-center study in Korea, HCC-portal vein tumor thrombosis (PVTT) patients with macrovascular invasion but having a preoperative alpha-fetoprotein (AFP) + protein induced by vitamin K absence/antagonist-II (PIVKA-II) ≤300 had a 5-year recurrence-free survival (RFS) of nearly 50% after LT [14] . Therefore, it was postulated that selected tumors can be treated with LDLT with an acceptable outcome regardless how advanced morphology is.
In the current study, the value of biomarkers PIVKA-II, AFP,platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio(NLR) and tumor standard uptake value (SUV) defined by positron emission tomography (PET) were evaluated to predict the prognosis after LT in HCC-PVTT patients. These parameters were evaluated next in relation to the extent of PVTT in order to identify those patients who would eventually benefit most from LT.
Methods
Seventy-five PVTT-HCC patients who underwent liver transplantation in our centers from February 2016 to June 2018 were enrolled in this study. Patients who had a confirmed diagnosis of HCC with extrahepatic metastasis or with invasion of hepatic vein and inferior vena cava were excluded from this study. HCC diagnosis was confirmed after transplantation by pathology. The criteria of PVTT were based on pre-transplant radiological characteristics (including enhanced CT scan, ultrasound, and MRI) and pathology of the specimen. PVTT was classified as follows [3] : (1)Vp1: tumor thrombus distal to the second branch of the portal vein; (2) Vp2: tumor thrombus in the second branch of the portal vein; (3) Vp3: tumor thrombus in the first branch of portal vein; (4) Vp4: main portal trunk tumor thrombus. All patients received modified piggyback liver transplantation. “No touch”hepatectomy technique was adopted to minimize tumor cell seeding. This research was approved by the institutional ethics committee. All patients signed informed consents. Complete clinical and laboratory data were available before operation and during follow-up.
The follow-up strategy and diagnostic criteria of tumor recurrence are as follows [15] : recipients were followed up closely from the date of operation to death or the last follow-up (December 31, 2019) . The monitoring approaches of recurrence include tumor biomarkers (AFP and PIVKA-II) and imaging using ultrasonography and thoraco-abdominal CT scan performed every 3 months during the first 2 years after LT and every 6 months after 2 years.Recurrence was identified by elevation of tumor biomarkers (AFP or PIVKA-II) and positive imaging findings. The median follow up was 19.1 months (1.9-42.4 months), and 27 (36%) patients have a follow-up of more than two years.
Minimal immunosuppression is desirable in liver transplantation to reduce side effects and to promote the process of tolerance induction [16] . In the present study, post-transplant immunosuppressant was tacrolimus based (6-10 ng/mL within 3 months,3-5 ng/mL between 6 and 12 months, and less than 3 ng/mL after 12 months) and glucocorticoid was given 10 0 0 mg intraoperatively with steroid-free regimen post-transplantation. Basiliximab was administrated 20 mg at the first and fourth day postoperation. Mycophenolate mofetil or mycophenolic acid was administered in case of renal dysfunction.
Treatment options were discussed by a multidisciplinary team of surgeons, hepatologists, radiologists and oncologists. Treatment modalities for recurrent HCC included surgical, non-surgical locoregional and systemic therapy. Resection was suggested to every patient whenever feasible. Transarterial chemoembolization (TACE)and/or radiofrequency ablation (RFA) were proposed to patients with unresectable intrahepatic recurrence. External beam radiotherapy was used in case of bone metastasis. Systemic chemotherapy and/or molecular targeted therapy (sorafenib, regorafenib,lenvatinib) was administered for multifocal intra- and extrahepatic recurrence.
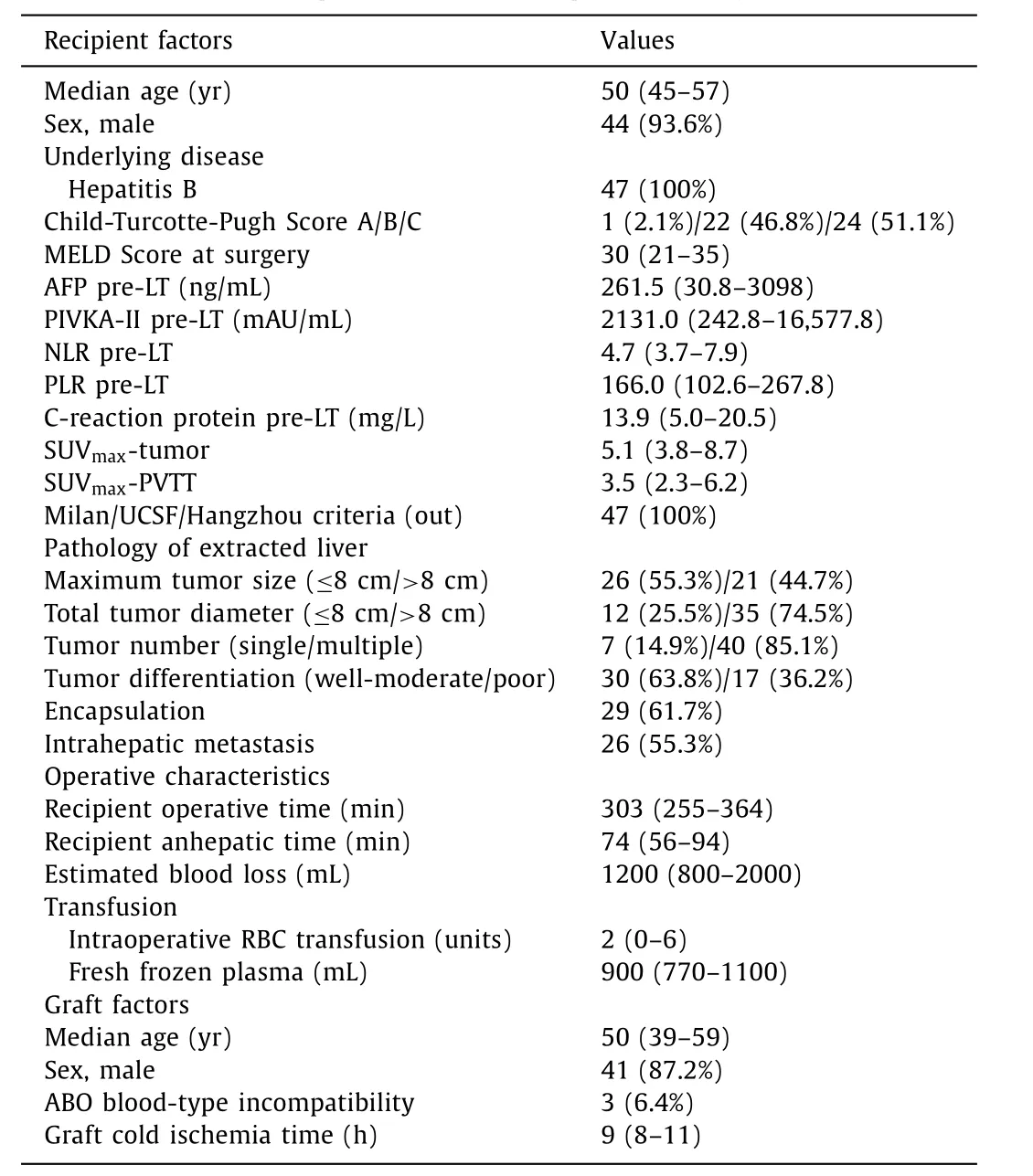
Table 1 Characteristics of 47 HCC patients with PVTT (Vp2-3) following LT.
The primary endpoint was overall survival (OS), which was defined from the operation day to the last follow-up or death. RFS was defined as a period from the date of surgery to the date of tumor recurrence first diagnosed. Continuous variables were described as medians and interquartile ranges (IQR), and categorical variables were described as frequencies and percentages. OS and RFS were analyzed by the Kaplan-Meier method and log-rank test.Independent prognostic indicators were assessed using Cox’s proportional hazard model. AP<0.05 was considered statistically significant.
Results
PVTT was as follows: 47 (62.7%) patients with Vp2-3 PVTT, and 28 (37.3%) patients with Vp4 PVTT. Among the 47 HCC patients with Vp2-3 PVTT, demographic, biochemical, operative and explant pathology are shown in Table 1 . The median age of patients at the time of transplantation was 50 years (IQR 45-57). Forty-four(93.6%) patients were male. Hepatitis B virus (HBV) infection was the underlying liver disease. The median Model for End-Stage Liver Disease (MELD) score was 30 (IQR 21-35); The Child-Turcotte-Pugh score was as follows, 1 (2.1%) patients with grade A, 22 (46.8%)with grade B and 24 (51.1%) with grade C. The median18F-FDG uptake (maximum) of the intrahepatic tumor and PVTT were 5.1(IQR 3.8-8.7) and 3.5 (IQR 2.3-6.2), respectively. The pre-transplant systematic inflammatory index such as NLR, PLR and CRP were 4.7 (IQR 3.7-7.9), 166.0 (IQR 102.6-267.8) and 13.9 (IQR 5.0-20.5)mg/L, respectively. Concerning the pathology of extracted liver, 17(36.2%) patients had poorly differentiated HCC, 40 (85.1%) had multiple tumors, 29 (61.7%) had tumor encapsulation and 35 (74.5%)had accumulated tumor size larger than 8 cm. The median operative and anhepatic time were 303 (IQR 255-364) min and 74 (IQR 56-94) min, respectively. Estimated blood loss was 1200 mL and intraoperative red blood cell (RBC) transfusion was 2 units. The median graft cold ischemia time was 9 (IQR 8-11) hours. Three(6.4%) patients received ABO incompatible LT.
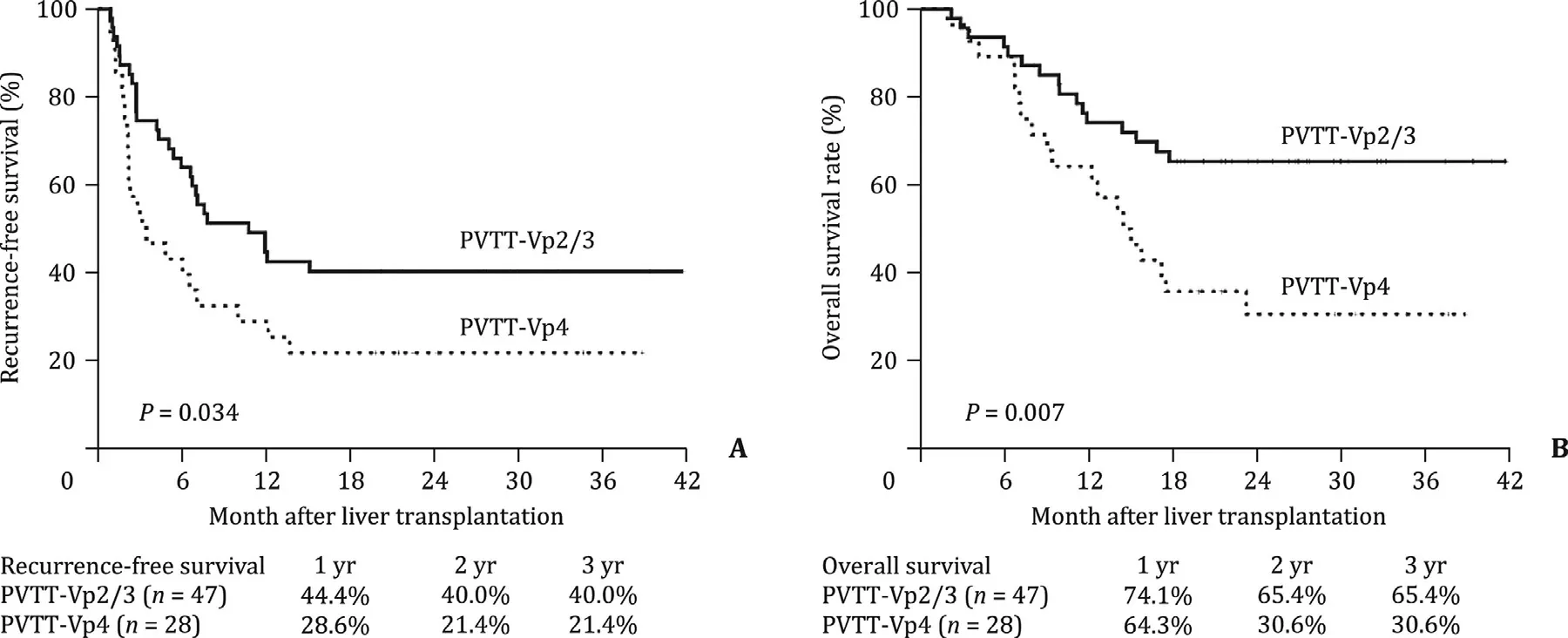
Fig. 1. Kaplan-Meier recurrence-free survival and overall survival after liver transplantation for hepatocellular carcinoma stratified by PVTT status (Vp2/3 or Vp4).
The 1-, 2-, and 3-year RFS rates were 44.4%, 40.0% and 40.0% for patients with Vp2-3 PVTT, and 28.6%, 21.4% and 21.4% for patients with Vp4 PVTT ( Fig. 1 A;P= 0.034). Similarly, the 1-, 2-, and 3-year OS rates were 74.1%, 65.4% and 65.4% for patients with Vp2-3 PVTT,and 64.3%, 30.6% and 30.6% for patients with Vp4 PVTT ( Fig. 1 B;P= 0.007).
Vp2-3 PVTT HCC patients in the univariate analysis, pretransplant AFP ≥10 0 0 ng/mL, PIVKA-II ≥40 mAU/mL, intrahepatic tumor SUV max ≥ 5, maximum tumor size>8 cm and total tumor diameter>8 cm were risk factors for HCC recurrence.Pre-transplant AFP ≥ 10 0 0 ng/mL, NLR ≥ 7, C-reactive protein≥15 mg/L and intrahepatic tumor SUV max ≥5 were also related to the overall survival ( Table 2 ). In the multivariate analysis, total tumor diameter>8 cm, pre-transplant AFP ≥10 0 0 ng/mL and intrahepatic tumor SUV max ≥5 were independent risk factors for post-LT HCC recurrence and overall survival ( Table 3 ).
The prognoses of 47 patients with Vp2-3 PVTT were analyzed.Base on the three major independent predictors, we defined five groups for these patients with segmental or lobar PVTT: Group 1(n= 12), total tumor diameter ≤8 cm; Group 2 (n= 10), total tumor diameter>8 cm, pre-transplant AFP<10 0 0 ng/mL and intrahepatic tumor SUV max<5; Group 3 (n= 10), total tumor diameter>8 cm, pre-transplant AFP>10 0 0 ng/mL and intrahepatic tumor SUV max<5; Group 4 (n= 7), total tumor diameter>8 cm, pre-transplant AFP level<10 0 0 ng/mL and intrahepatic tumor SUV max>5; and Group 5 (n= 8), total tumor diameter>8 cm, pre-transplant AFP level>10 0 0 ng/mL and intrahepatic tumor SUV max>5. Among the five groups, group 1 and group 2 achieved the best prognosis. There was no significant difference in cumulative survival between group 1 and group 2 (P= 0.470),and between group 3 and group 4 (P= 0.302). The prognosis of patients in groups 1 and 2 was significantly better than that in groups 3, 4, and 5 (P<0.001) ( Fig. 2 ).
Different risk groups of patients with Vp2-3 PVTT were then established. Group A (n= 22) represented to low risk patients fulfilling one of the two following items: (a) Total tumor diameter≤8 cm; (b) total tumor diameter>8 cm, with pre-transplant AFP level<10 0 0 ng/mL and intrahepatic tumor SUV max<5. Group B (n= 17) represented to moderate risk patients with total tumor diameter>8 cm, pre-transplant AFP level<10 0 0 ng/mL or intrahepatic tumor SUV max<5; Group C (n= 8) represented to high risk patients with total tumor diameter>8 cm, and both pre-transplant AFP level>10 0 0 ng/mL and intrahepatic tumor SUV max>5. For patients in low risk group, the 1-, 2-, and 3-year OS and RFS rates were 95.2%, 95.2%, 95.2%, and 72.4%, 67.6%,67.6%, respectively, whereas these rates in moderate risk group were 76.5%, 58.8%, 58.8%, and 29.4%, 23.5%, 23.5%, respectively,and in the high-risk patients were even lower (allP<0.001,Fig. 3 ).
Discussion
Hepatocellular carcinoma tends to invade the portal venous system, resulting in PVTT. The incidence of macroscopic PVTT in HCC patients at the time of first diagnosis ranges from 10% to 40%,and the median overall survival is 2.7-4 months in patients untreated for HCC and PVTT [3 , 17] . According to the Barcelona Clinic Liver Cancer (BCLC) staging system, PVTT is regarded as an advanced stage of HCC and molecularly targeted agents (sorafenib and lenvatinib as the first-line drug) are recommended, which result in a median overall survival of 8.4-14.4 months [18 , 19] . However, more aggressive treatments (including surgical resection, radiotherapy, and TACE) are administrated for selected patients with PVTT-HCC in the Asia-Pacific regions, which lead to a better prognosis compared to palliative treatment [3-5] . In China, HCC is often originated from HBV-related cirrhosis and LT is considered a possible curative treatment modality. However, all the present LT selection criteria have defined PVTT as a contraindication because of poor prognosis [7-11] . Several single-center and multicenter studies in Korea found that, if the PVTT was not in the main portal vein and the AFP and PIVKA-II scores were low, living donor liver transplantation (LDLT) could be performed with an improved prognosis [13 , 14 , 20] . In addition, LDLT after combined TACE and 3-dimensional conformal radiotherapy for selected HCC patients with macrovascular invasion showed acceptable oncologic outcomes, with 3-year OS rate of 60.5% [21] . In the present study,we analyzed 47 liver recipients who had PVTT HCC before LT. The Vp4 PVTT group had a bad outcome and the shortest PFS, while the 3-year OS in Vp2-3 PVTT group reached 60%. This indicates that patients with segmental or lobar PVTT might be suitable for LT.
In recent years,18F-FDG and11C-acetate PET/computed tomography have been widely used in the diagnosis of HCC and evalua-tion of tumor metastasis [22] .18F-FDG, a glucose analogue, is the most commonly used tracer in oncology. Well-differentiated tumor appears to have a similar FDG uptake pattern as normal liver tissue, which might show a low level of SUV, whereas increased FDG uptake is observed in poorly differentiated tumors [23] . Many studies reported about the value of 18 F-FDG uptake in LT. Yang et al. proposed positive PET-status, meaning18F-FDG uptake is higher than normal liver tissue, correlated with poor biological behavior and microvascular invasion [24] . A recent multi-center LDLT study in Korea proposed composite criteria for predicting post-transplantation recurrence using clinical and FDG PET/CT factors. For HCC patients with tumor size<6.0 cm, tumor number<8, AFP<465 ng/mL, and maximum tumor-to-background ratio(TBR max )<2.8, the RFS did not differ significantly from those fulfilling Milan, UCSF or Up-to-seven criteria [25] . Hong et al. reported PET-positivity and serum AFP>200 ng/mL as independent prognostic factors of HCC recurrence [26] . In the present study, intrahepatic tumor SUV max>5 was found to be a significant indicator of poor prognosis. Combined with total tumor diameter and pre-transplant AFP level, three subgroups with different recurrent risks were stratified in Vp2 and Vp3 PV patients. We demonstrated that patients with total tumor diameter≤8 cm, or AFP<10 0 0 ng/mL and SUV max<5 might be suitable for LT.
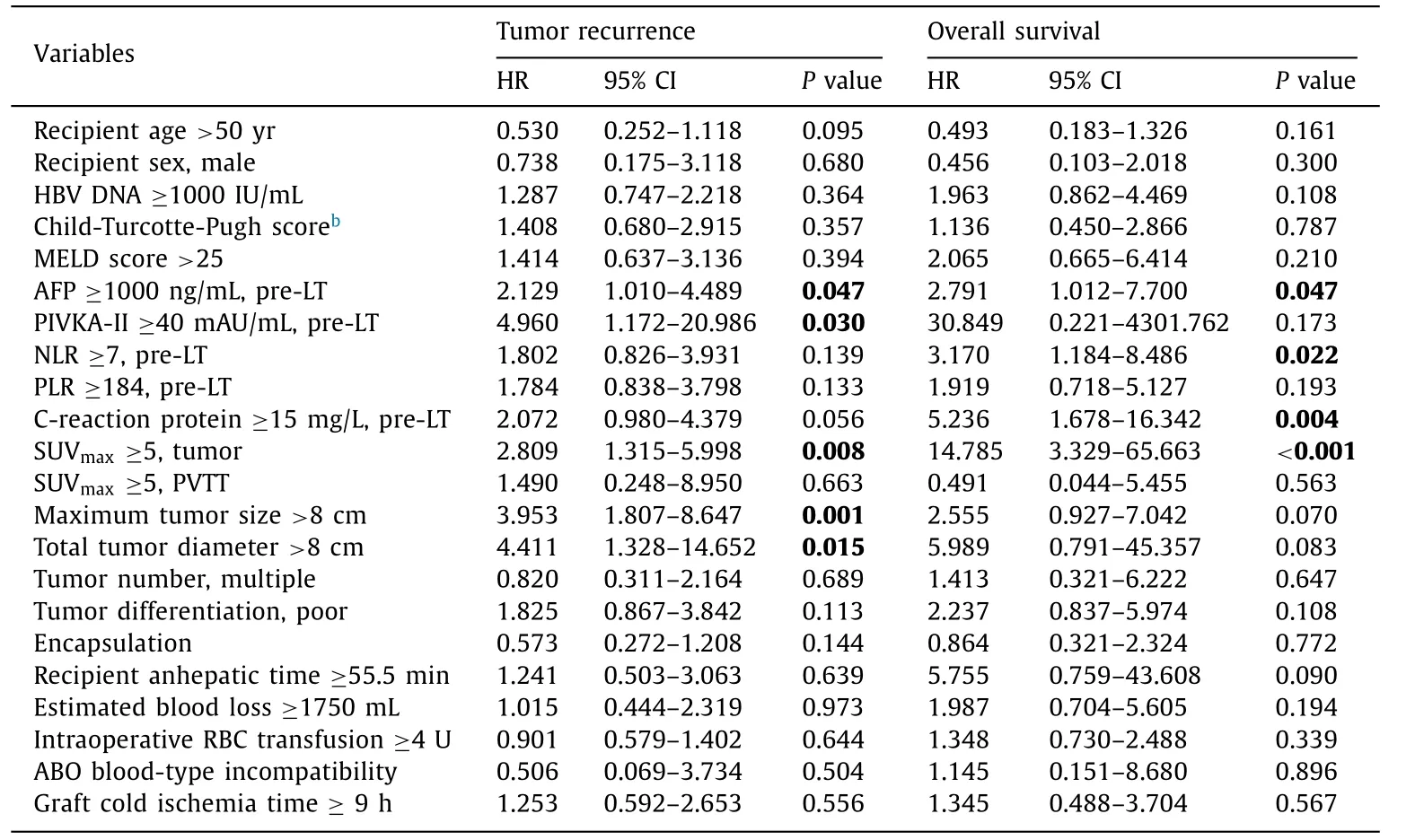
Table 2 Risk factors on posttransplant HCC recurrence and overall survival in patients with PVTT (Vp2-3) a .

Table 3 Multivariate analysis evaluating risk factors on posttransplant HCC recurrence and overall survival in the subset of patients with PVTT-Vp2/3 a .
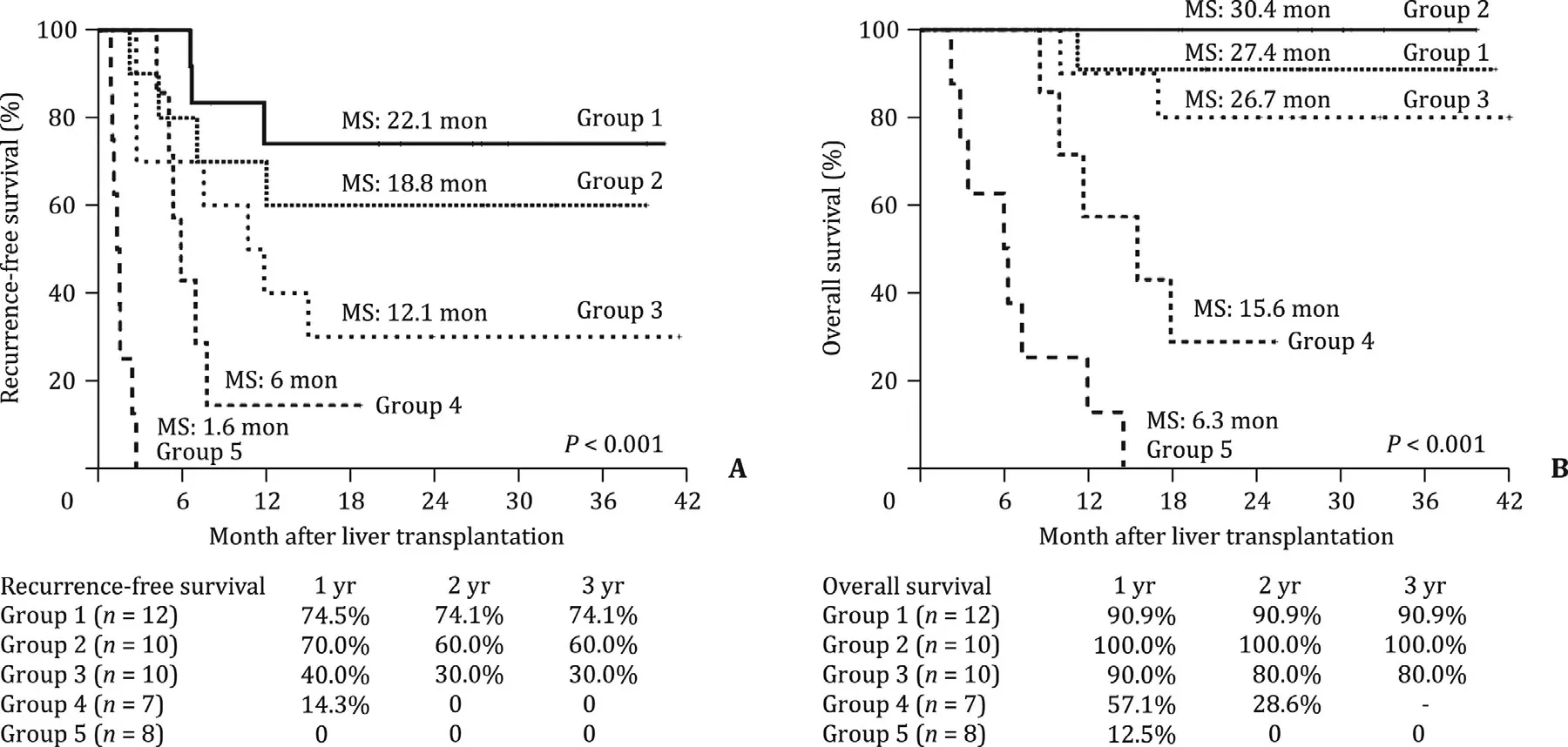
Fig. 2. Comparison of the ( A ) recurrence-free survival and ( B ) overall survival rates in the subgroup of patients with PVTT Vp2 and Vp3 by combination of total tumor diameter, pre-transplant AFP level and tumor 18 F-FDG standard uptake value. MS: median survival.
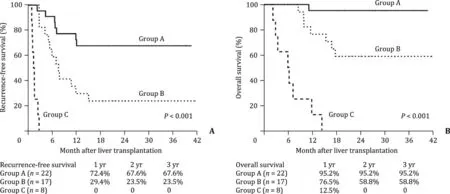
Fig. 3. Comparison of the ( A ) recurrence-free survival and ( B ) overall survival rates in the subgroup of patients with PVTT Vp2 and Vp3 by combination of accumulative tumor size, pre-transplant AFP level and intrahepatic tumor 18 F-FDG standard uptake value.
This study is important because in patient selection, we combined tumor size and the PVTT status with levels of 18 F-FDG standard uptake value and serum AFP. These combinations exhibited the highest predictive value for RFS and OS. Secondly we further analyzed by stratifying PVTT into segmental, lobar and main status, and combined with tumor markers.
The limitation of this is the retrospective nature which might have bias in patient selection. Another limitation is small patient number and short follow-up. Prospective, long-term and largescale studies are required to validate our conclusion.
In conclusion, main trunk PVTT remains a contraindication for LT. Segmental or lobar PVTT is acceptable for LT in highly selected patients. AFP level and18F-FDG standard uptake value should be considered when selecting patients.
Acknowledgments
We expressed our gratitude to all surgeons and nurses for their help in collecting patient information. The authors also would like to thank Bulat Abdrakhimov for correcting this paper.
CRediT authorship contribution statement
Zhe Yang:Conceptualization, Data curation, Funding acquisition, Resources.Fang-Zhou Luo:Data curation, Software, Writing- original draft, Writing - review & editing.Shuo Wang:Conceptualization, Data curation, Investigation, Resources.Jan Lerut:Validation, Writing - review & editing.Li Zhuang:Data curation, Formal analysis, Resources.Qi-Yong Li:Formal analysis, Resources.Xiao Xu:Data curation, Validation.Shu-Sen Zheng:Project administration, Resources, Funding acquisition, Supervision, Validation.
Funding
This study was supported by grants from the National S&T Major Project ( 2017ZX10203205 ), the Medical Science and Technology Project of Zhejiang Province ( 2014KYA082 ),the Fundamental Research Funds for the Central Universities( 2018FZA7002 ), and the Shulan Talent Foundation.
Ethical approval
This study was approved by the institutional ethics committee.All patients’ consents have been acquired.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Transjugular intrahepatic portosystemic shunt for a patient with chylothorax in cryptogenic/metabolic cirrhosis
- Differential methylation landscape of pancreatic ductal adenocarcinoma and its precancerous lesions
- Hepatobiliary&Pancreatic Diseases International
- MicroRNAs and long non-coding RNAs in liver surgery: Diagnostic and therapeutic merits
- Translationally controlled tumor protein exerts a proinflammatory role in acute rejection after liver transplantation
- Sequential transcatheter arterial chemoembolization and portal vein embolization before right hemihepatectomy in patients with hepatocellular carcinoma
