Study on the value of parecoxib sodium preemptive analgesia for laparoscopic surgery based on postoperative pain and stress mediator secretion
2020-07-04QingBoHanYongMinLiYangLiuPingXuanGuo
Qing-Bo Han, Yong-Min Li, Yang Liu, Ping-Xuan Guo
Anesthesiology Department, Kailuan General Hospital of Tangshan Hebei Province, Tangshan, Hebei Province, 063000
Keywords:Laparoscopic surgery Parecoxib sodium Preemptive analgesia Pain Stress mediator
ABSTRACT Objective: To investigate the effect of parecoxib sodium preemptive analgesia on postoperative pain and stress response in patients with laparoscopic surgery. Methods: 118 patients with asymptomatic gallbladder polyps who underwent elective laparoscopic surgery in our hospital between January 2018 and January 2019 were divided into the control group (n=59) and the preemptive analgesia group (n=59) by random number table. Control group received routine total intravenous anesthesia, and preemptive analgesia group received intravenous injection of parecoxib sodium 0.7mg/kg during anesthesia induction. The differences in serum levels of pain mediators [prostaglandin E2 (PGE2), substance P (SP) and neuropeptide Y (NPY)], inflammatory factors [interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-12 (IL-12)] as well as stress mediators [cortisol (Cor), norepinephrine (NE) and epinephrine (E)] at before surgery (T0), 30min after extubation (T1), 6h after surgery (T2) and 24h after surgery (T3) were compared between the two groups of patients. Results: At T0, there was no significant difference in VAS score as well as inflammatory factor or stress mediator levels between the two groups (P>0.05). At T1, T2 and T3, VAS scores of the preemptive analgesia group were lower than those of the control group; serum IL-1β, IL-6, IL-12 and TNF-α levels were lower than those of the control group; serum Cor, NE and E levels were lower than those of the control group (P<0.05). Conclusion: Parecoxib sodium preemptive analgesia has a positive effect on reducing postoperative pain and systemic stress in patients with laparoscopic cholecystectomy.
1. Introduction
Laparoscopic cholecystectomy has been successfully applied in the treatment of patients with chronic cholecystitis and gallbladder polyps. It is with small incision and relatively convenient operation, but it still causes severe postoperative pain and even severe circulation fluctuation to some patients, which not only affects postoperative recovery, but may also lead to serious complications. The use of analgesics in the process of anesthesia plays an important role in maintaining the stability of intraoperative circulation and helping the smooth operation, as well as in alleviating postoperative pain. Parecoxib sodium is a selective COX-2 inhibitor, which is used for the short-term treatment of postoperative pain [1-2]. It has been successfully applied in the analgesic intervention of senile fracture [3], anorectal diseases [4] and other surgical operations. The influence of its application timing on analgesic effect is also the focus of current clinical research. In this study, we discussed the effect of Parecoxib sodium application timing on the postoperative pain and body stress status of patients with laparoscopic cholecystectomy so as to determine the best timing of Parecoxib sodium analgesia and to provide reference for the selection and application timing of analgesics for follow-up surgical patients.
2.Information and methods
2.1 General information
118 patients with asymptomatic gallbladder polyps undergoing elective laparoscopic surgery in our hospital between January 2018 and January 2019 were collected as the research subjects, they signed informed consent and relevant documents, and there were 60 cases of men and 58 cases of women who were 42-69 years old, with body mass index of 22-26kg/m2, and with the American society of anesthesiologists (ASA) grade Ⅰ or Ⅱ. Inclusion criteria: (1) with no previous history of laparoscopic surgery; (2) meeting the indications for elective laparoscopic surgery; (3) > 18 years old and < 80 years old; (4) with good nutritional status and able to tolerate the trauma of laparoscopic surgery. Exclusion criteria: (1) complicated with other diseases that might affect systemic functions, such as malignant tumors, coronary heart disease, etc.; (2) with hyperalgesia or hypalgesia; (3) pregnant or breast-feeding women. The included patients were divided into control group and preemptive analgesia group, each with 59 cases. There was no significant difference in the basic data of gender, age, body mass index or course of disease between the two groups (P>05), as shown in Table1.
2.2 Anesthesia method
After entering the operating room, patients in both groups were routinely indwelled with external cubital vein needle and monitored for vital signs (including electrocardiogram, non-invasive arterial pressure, and digital oxygen saturation, etc.). Intravenous anesthesia induction was as follows: midazolam (produced by Jiangsu Nhwa Pharmaceutical Co., Ltd., patch No. 20180319) 0.04mg/kg, propofol (produced by Hebei Yipin Pharmaceutical Co., Ltd., patch No. 20180420) 1.5mg/kg, remifentanil (produced by Jiangsu Nhwa Pharmaceutical Co., Ltd., patch No. 20180714) 2μg/kg, and vecuronium bromide (produced by Hubei Keyi Pharmaceutical Co., Ltd., patch No. 20180612) 0.1mg/kg. After muscle relaxation was satisfactory, the trachea was intubated, ventilator was connected and set to mechanical ventilation mode. Specific parameters were as follows: tidal volume 7-9ml/kg, respiratory rate 10-12 times /min, inspiratory/expiratory 1:2, and end-expiratory pco2 35-40mmhg. Intraoperative continuous intravenous infusion of propofol 4-8mg/(kg·h) and intermittent 30min intravenous injection of vecuronium bromide 5mg were provided. Narcotic drugs were stopped when sewing the skin. The tracheal tube was removed after the patients were awake, and regained swallowing reflex, spontaneous breathing and tidal volume.
Patients in the preemptive analgesia group were intravenously injected with 0.7mg/kg parexib sodium (produced by Pfizer Pharmaceutical Co., Ltd., batch no. 20180303) (diluted to 5ml with sterile normal saline) during anesthesia induction, while patients in the control group were intravenously injected with 0.7mg/kg parexib sodium during skin suture. Patients in both groups chose not to have self-controlled analgesia after surgery, and those who couldn’t tolerate postoperative pain were given intramuscular injection of pethidine (produced by Shenyang First Pharmaceutical Co., Ltd., Northeast Pharmaceutical Group, batch number 20180921) 75mg.
2.3 Pain and stress mediators
Visual analogue scale (VAS) was used to evaluate the subjective pain degree of patients in the two groups before surgery (T0), 30min after extubation (T1), 6h after surgery (T2) and 24h after surgery (T3). The total score was 0-10 points, and the higher the score, the more severe the pain. 2.0ml of peripheral venous blood specimen was collected respectively in the above time points, and ELISA method was used to determine the prostaglandin E2 (PGE2), substance P (SP), neuropeptide Y (NPY), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-12 (IL-12), tumor necrosis factor α(TNF-α), cortisol (Cor), norepinephrine (NE) and epinephrine (E) levels.
2.4 Statistical methods
The study data were collected and recorded into SPSS20.0 for calculation. The statistical value P<0.05 indicated statistically significant difference. Chi-square test was used to compare counting data between the two groups. The independent sample t test was used for the comparison of measurement data between the two groups, and the paired t test was used for the comparison within the same group.
3.Results
3.1 Comparison of serum pain mediators PGE2, SP and NPY between the two groups
The differences in serum PGE2, SP and NPY levels were not statistically significant between the two groups at T0 (P>0.05). Compared with those at T0, serum levels of above pain mediators of both groups were higher; compared with those of the control group,serum levels of above pain mediators of the preemptive analgesia group were lower at T1, T2 and T3, and the differences were statistically significant (P<0.05), shown in Table 2.
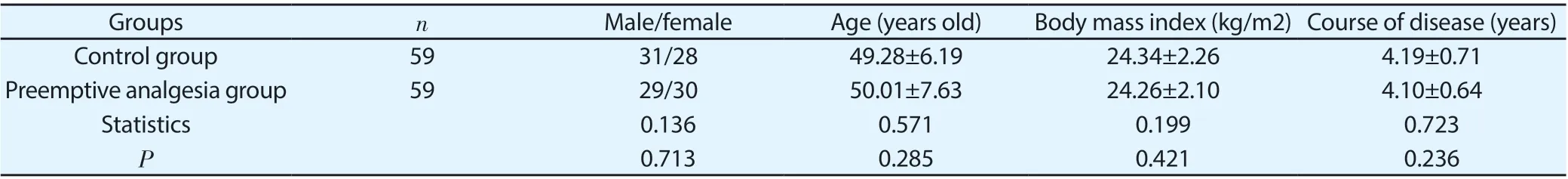
Table 1 Comparison of general information
Table 2 Comparison of perioperative pain mediator levels between two groups (±s)

Table 2 Comparison of perioperative pain mediator levels between two groups (±s)
Groups n Time points PGE2(pg/mL) SP(μg/mL) NPY(ng/mL)Control group 59 T0 38.49±7.14 21.35±4.58 131.92±22.19 T1 48.93±8.25 28.82±5.24 146.28±23.18 T2 60.14±9.47 36.27±6.25 189.39±32.28 T3 54.51±7.77 30.19±6.58 155.67±22.39 Preemptive analgesia group 59 T0 39.11±8.24 21.09±5.12 130.28±21.44 T1 44.51±7.78 24.27±6.12 137.11±25.59 T2 52.11±9.72 29.39±6.58 159.39±29.29 T3 47.89±9.14 27.74±5.14 142.79±31.29 t/P between groups at T0 0.437/0.663 0.291/0.772 0.408/0.684 t/P between groups at T1 2.994/0.003 4.338/0.000 2.521/0.013 t/P between groups at T2 4.545/0.000 5.823/0.000 5.236/0.000 t/P between groups at T3 4.239/0.000 2.254/0.026 2.571/0.011
Table 3 Comparison of perioperative inflammatory factor levels between two groups (±s)

Table 3 Comparison of perioperative inflammatory factor levels between two groups (±s)
Groups n Time points IL-1β IL-6 IL-12 TNF-α Control group 59 T0 4.18±0.57 3.22±0.51 11.05±1.87 8.62±0.97 T1 7.09±0.85 5.18±0.63 18.52±2.88 11.31±1.68 T2 9.61±1.34 6.94±0.83 24.39±4.50 16.48±2.19 T3 7.46±0.83 6.20±0.74 19.01±2.43 13.50±1.85 Preemptive analgesia group 59 T0 4.22±0.54 3.19±0.48 11.10±1.79 8.70±0.92 T1 6.12±0.75 4.36±0.57 15.07±1.88 10.04±1.73 T2 7.50±0.88 5.32±0.61 20.46±3.17 12.85±1.79 T3 6.03±0.72 4.99±0.63 15.85±1.62 10.97±1.66 t/P between groups at T0 0.391/0.348 0.329/0.371 16.808/0.000 0.460/0.323 t/P between groups at T1 6.573/0.000 7.414/0.000 7.705/0.000 4.045/0.000 t/P between groups at T2 10.110/0.000 12.080/0.000 5.484/0.000 9.858/0.000 t/P between groups at T3 9.997/0.000 9.563/0.000 8.311/0.000 7.818/0.000
Table 4 Comparison of perioperative stress mediator levels between two groups (±s)
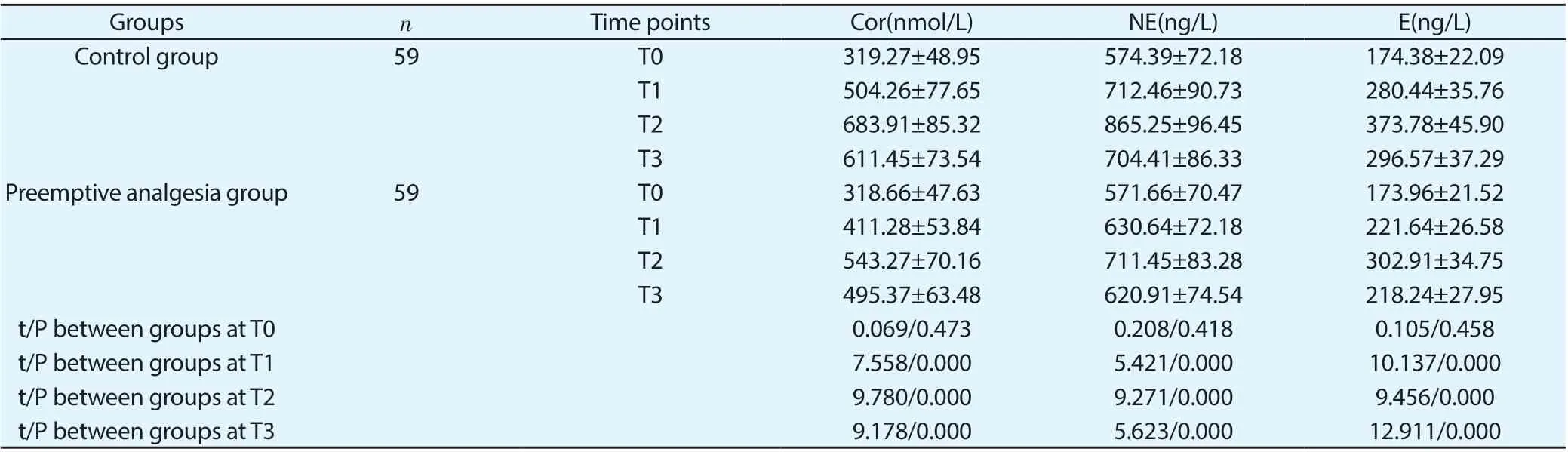
Table 4 Comparison of perioperative stress mediator levels between two groups (±s)
Groups n Time points Cor(nmol/L) NE(ng/L) E(ng/L)Control group 59 T0 319.27±48.95 574.39±72.18 174.38±22.09 T1 504.26±77.65 712.46±90.73 280.44±35.76 T2 683.91±85.32 865.25±96.45 373.78±45.90 T3 611.45±73.54 704.41±86.33 296.57±37.29 Preemptive analgesia group 59 T0 318.66±47.63 571.66±70.47 173.96±21.52 T1 411.28±53.84 630.64±72.18 221.64±26.58 T2 543.27±70.16 711.45±83.28 302.91±34.75 T3 495.37±63.48 620.91±74.54 218.24±27.95 t/P between groups at T0 0.069/0.473 0.208/0.418 0.105/0.458 t/P between groups at T1 7.558/0.000 5.421/0.000 10.137/0.000 t/P between groups at T2 9.780/0.000 9.271/0.000 9.456/0.000 t/P between groups at T3 9.178/0.000 5.623/0.000 12.911/0.000
3.2 Comparison of serum inflammatory factors between the two groups
The differences in serum IL-1β, IL-6, IL-12 and TNF-αlevels were not statistically significant between the two groups at T0 (P>0.05). Compared with those at T0, serum levels of above inflammatory factors of both groups were higher; compared with those of the control group, serum levels of above inflammatory factors of the preemptive analgesia group were lower at T1, T2 and T3, and the differences were statistically significant (P<0.05), shown in Table 3.
3.3 Comparison of serum stress mediators between the two groups
The differences in serum Cor, NE and E levels were not statistically significant between the two groups at T0 (P>0.05). Compared with those at T0, serum levels of above stress mediators of both groups were higher; compared with those of the control group, serum levels of above stress mediators of the preemptive analgesia group were lower at T1, T2 and T3, and the differences were statistically significant (P<0.05), shown in Table 4.
4.Discussion
Noxious stimuli such as surgery can promote the generation of inflammatory response and release of a large number of algogenic substances, and cause peripheral and central nociceptor sensitization,
lower pain threshold and enhanced response to noxious stimuli, which is also an important reason for the acute pain in patients after surgery. Many basic studies have confirmed that parexib sodium has a good analgesic effect on neuropathic pain [5] and bone cancer pain [6]. After entering the blood, the drug is metabolized into the active component of Valdecoxib, which inhibits prostaglandin synthesis through specific inhibition of cycloxygenase-2 (COX-2), and finally achieves analgesic effect [7-8]. Parecoxib sodium has been successfully used for postoperative analgesia of surgery, but the timing of drug use remains controversial. In recent years, the concept of preemptive analgesia has been put forward, which is to give analgesia before the occurrence of noxious stimulation to prevent peripheral and central sensitization, so as to reduce the postoperative pain of patients and the demand for analgesics.
In this paper, we investigated the effect of the application timing of parexib sodium on postoperative pain in patients undergoing laparoscopic cholecystectomy. Patients in the control group received intravenous injection of parexib sodium during the skin suture while patients in the preemptive analgesia group received intravenous injection of parexib sodium during the induction of anesthesia. In the process of pain induced by noxious stimuli, the synthesis and secretion of various pain mediators directly mediate the generation of pain. PGE2, SP and NPY are known pain mediators that can reduce pain threshold and promote pain signal transduction. The increase in serum PGE2, SP and NPY after surgery is directly related to the generation of incision pain. In this study, the serum contents of the above pain mediators were analyzed after using parecoxib sodium for preemptive analgesia, so as to evaluate the analgesic value of preemptive analgesia. The contents of PGE2, SP and NPY in both groups increased at different time points after surgery, indicating that the surgical trauma caused the secretion of pain mediators. The contents of the above pain mediators in the preemptive analgesia group were lower than those in the control group at different time points after surgery, indicating that the use of parexib sodium during the anesthesia induction period can effectively inhibit the introduction of noxious stimuli and reduce the release of pain mediators, thus playing a more effective analgesic role.
Surgical trauma can directly stimulate mononuclear macrophages and induce them to secrete a large number of inflammatory factors, and inflammatory response can promote the occurrence of central sensitization and synergistically reduce the pain threshold of patients [9-11]. In this paper, the levels of inflammatory factors such as IL-1β, IL-6, IL-12 and TNF-α in both groups at different time points after surgery were all increased to different degrees compared with those before surgery, which was consistent with the results of previous studies at home and abroad. Studies [12-13] indicate that NF-κB is activated during trauma to promote the expression of inflammatory factors and induce the production of COX-2, and COX-2 in turn mediates the synthesis and secretion of more inflammatory factors, which forms a vicious cycle. In this paper, the levels of above inflammatory factors of preemptive analgesia group at different time points were all lower than those of control group, explaining that using parexib sodium during anesthesia induction period can effectively reduce the body's inflammatory response, and given that COX-2 stimulates inflammation and inflammatory response promotes pain, it is speculated that parexib sodium may play the anti-inflammatory role to achieve partial analgesic action. Both surgery and anesthesia will stimulate the hypothalamus - pituitary - adrenal cortex axis of the body, result in the mass secretion of stress mediators such as Cor, NE and E into the blood, the excitement of sympathetic nerve as well as the occurrence of systemic stress response, and further lead to increased glycogen decomposition and gluconeogenesis, and elevated blood glucose, which are not conducive to postoperative recovery [14-16]. A number of studies [17-19] have confirmed the positive role of parexib sodium in inhibiting the body's stress response, which is speculated to be related to its alleviation of the stress response caused by pain. In this paper, the serum levels of Cor, NE and E in both groups of patients at different time points after surgery were higher than those before surgery, while those in the preemptive analgesia group were relatively lower. It was speculated that the preemptive application of parexib sodium could more effectively inhibit the stress response of the body, which was consistent with its stronger analgesic effect. To sum up, the following conclusions can be drawn: preemptive analgesia with parexib sodium can effectively reduce the postoperative pain of patients with laparoscopic cholecystectomy, which may be related to its role of reducing the inflammatory stress response of the body, and is expected to provide references for the selection of the application time of parexib sodium in the future. There are also some defects in this study, such as limited postoperative observation time, which need to be further improved and clarified by follow-up studies.
杂志排行
Journal of Hainan Medical College的其它文章
- Clinical effect of preventive nursing on the rate of deep vein thrombosis in patients with lung cancer: A meta-analysis
- A network pharmacology approach to explore action mechanisms of Bi xie and Tu fuling for treating gouty arthritis
- Changes of serum β2-MG, Cys C and urine mAlb levels in patients with ureteral calculi before and after extracorporeal shock wave lithotripsy and their clinical significance
- Study on the effect of Baiban Ointment on the whole gene expression profile of vulvar sclerosing lichen
- To investigate the effects of butylphthalide on reducing neuronal apoptosis in rats with cerebral infarction by inhibiting the JNK / P38 MAPK signaling pathway
- Study of KAP status and influencing factors of Protective Behaviour on COVID-19 among Hainan Mobile Phone Users
