Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor:A case report
2020-06-08YukiAdachiHiromitsuHayashiToshihikoYusaToruTakematsuKazukiMatsumuraTakaakiHigashiKensukeYamamuraTakanobuYamaoKatsunoriImaiYoichiYamashitaHideoBaba
Yuki Adachi, Hiromitsu Hayashi, Toshihiko Yusa, Toru Takematsu, Kazuki Matsumura, Takaaki Higashi,Kensuke Yamamura, Takanobu Yamao, Katsunori Imai, Yo-ichi Yamashita, Hideo Baba
Abstract
Key words: Ectopic hepatocellular carcinoma; Differential diagnosis; Retroperitoneal tumor; Case report
INTRODUCTION
An ectopic hepatocellular carcinoma (EHCC) is defined as an HCC arising from hepatic parenchyma located in an extrahepatic organ or tissue[1]. It can occur in various sites near the liver; for example, gallbladder, hepatic ligaments, omentum,retroperitoneum, and thorax[2].
The incidence of ectopic liver has been reported to be between 0.24% and 0.47% at laparoscopy or autopsy[3-5]. Thus, EHCC is a very rare disease and it is difficult to diagnosis preoperatively, which leads to a clinical issue. The clinical features of EHCC are still not fully elucidated. Here, we report a case of EHCC mimicking a retroperitoneal tumor, and a review the literature concerning EHCC.
CASE PRESENTATION
Chief complaints
The case was an 81-year-old woman positive for a hepatitis C virus (HCV) antibody(HCV-RNA was not detectable), and she had no remarkable chief complaints.
History of present illness
She was followed up regularly by a nearby outpatient clinic as she was an asymptomatic hepatitis C virus carrier, and the abdominal ultrasonography at that clinic revealed the tumor. She was referred to our department for further examination and treatment.
History of past illness
She was positive for HCV antibodies, but HCV-RNA was not detectable. She had previously undergone laparoscopic cho-lecystectomy for cholelithiasis and thyroidectomy at another hospital (details unknown).
Physical examinations
The abdomen was sof t and flat. She has a past history of laparoscopic cholecystectomy. Any other digestive symptoms such as abdominal pain or weight loss were not observed (Eastern Cooperative Oncology Group: 0).
Imaging examinations and laboratory examinations
The abdominal ultrasonography revealed the retroperitoneal tumor (6 cm in size)located at the dorsal side of the pancreas head. On the laboratory tests, serum alphafetoprotein (AFP) and AFP-L3% were 30.1 ng/mL and 83.1%, respectively. The protein induced by vitamin K absence or antagonist-II (PIVKA-II) level was 17 mAU/mL, carcinoembryonic antigen level was 3.5 ng/mL, and CA19-9 level was 9.6 U/mL. The contrast enhanced-computed tomography scan showed that the retroperitoneal tumor had enhancement in the arterial phase and was washed out in the venous phase (Figure 1A). Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging displayed enhancement in the arterial phase and a defect in the hepatobiliary phase (Figure 1B). The positron emission tomography-computed tomography revealed strong accumulation into the tumor (Standardized Uptake Value max: 13.8) (Figure 1C).
TREATMENT
Cytology by endoscopic ultrasound-guided fine needle aspiration showed a poorly differentiated carcinoma with unknown origin (Figure 2 and Table 1). We performed tumor extirpation and sampled the surrounding lymph nodes. Intraoperative findings showed that the tumor was disconnected to the liver and the head of pancreas (Figure 3). The resected gross specimen was 7.5 cm × 6.5 cm × 3.5 cm in size and encapsulated in membrane, and the cut surface was reddish-yellow with intratumoral hemorrhage(Figure 4). On microscopic examination, the tumor was composed of polygonal cells and had hyperchromatic nuclei with prominent nucleoli and granular eosinophilic cytoplasm. Very little pancreatic tissue was seen on the surface, so it was assumed that the tumor had not invaded the pancreas. The morphological histologic diagnosis was poorly differentiated carcinoma (Figure 5). On immunohistochemical staining of the tumor, Hep Per-1 and Glypican 3 were negative, but Arginase-1 (Arg-1) was focally positive. Moreover, AE1/AE3 was partly positive, CAM 5.2 was positive,synaptophysin was negative, S100 was negative, p53 was negative, and β-catenin was positive (Figure 6 and Table 2). On post-operative laboratory tests, the levels of AFP and AFP-L3% had decreased to 1.7 ng/mL and 27.3%, respectively.
FINAL DIAGNOSIS
Finally, we diagnosed the tumor as EHCC.
OUTCOME AND FOLLOW-UP
Currently, eight months have passed, but she is still alive without recurrence.
DISCUSSION
We searched ''ectopic hepatocellular carcinoma'' in PubMed, and 24 case reports were available in full text (Supplementary Table 1)[1,2,4-25]. Preoperative examination revealed no tumor in the mother liver in any patients[1,2,4-25]. There were two cirrhotic cases (one case was viral hepatitis and one case was unknown)[11,25]. Of the 24 cases, 18 underwent surgery[1,2,4,6,8,9,11,15-25]and two received adjuvant therapy[1,19]. There were six cases of recurrence (four cases in the mother liver and two cases in the abdominal cavity)[1,9,16,22,23,25]. Preoperative serum AFPs were often relatively elevated. In addition,AFP L3% was measured in only three cases, but a significant increase was observed in all three cases[11,17,22]. Seven cases had a hepatitis B virus infection[1,6,7,9,13,16,23]and only one case had a hepatitis C virus infection[5]. Immunohistochemical staining showed 17 cases of Hep Per-1 staining, one of which was negative[2,4-8,10-12,14-18,21-23]. There were 14 cases of AFP staining, three of which were negative[1,6,8,10,11,13,15-19,21,22,25]. EHCC is associated with a relatively long-term survival after resection, so surgical treatment should be considered initially if the tumor can be resected. Recurrence often occurs in the mother liver, and it is necessary to follow-up regularly and perform imaging testing after surgery, similar to the follow-up after surgery for HCC.
EHCC is one of the rare carcinomas defined as an HCC arising from ectopic liver tissue, and it is usually discovered incidentally at autopsy or during laparoscopy[3].Ectopic liver tissue was recognized within the gallbladder, spleen, pancreas, adrenal gland, portal vein hepatic ligament, diaphragm, thorax, retroperitoneum, and omentum. The reported incidence of an ectopic or accessory liver is approximately 0.56% and the gallbladder is the most common location[6,7].
Liver development starts in the middle of the third week of embryonic life. The hepatic diverticulum (liver bud) is formed from the foregut and becomes a hepatocellular cord. Subsequently, a bile duct, a gallbladder, and a gallbladder duct develop from a connection part between the hepatic diverticulum and the foregut.The pancreas is composed of two types of buds: A ventral pancreatic bud that develops from the bile duct and a dorsal pancreatic bud that arises from the foregut.The liver parenchyma differentiates from the hepatocellular cord. The expression of the ability of the foregut to differentiate into liver tissue is blocked by the ectoderm and mesoderm of the heart. However, the function of these inhibitory factors is blocked in the area where the liver would sprout in the future by fibroblast growth factor 2 secreted from the mesoderm of the heart and adjacent angiogenic endothelial cells. Ectopic liver tissue on the gallbladder wall and around the pancreas comprises liver parenchyma due to the influence of fibroblast growth factor 2 when the bile duct and dorsal pancreatic bud are formed from the foregut and when the ventral pancreatic bud is formed from the bile duct.
In previous reports, four out of 24 cases were found in the pancreas, but only one of them was present in the head of the pancreas, and this case was found on the ventral side of the pancreas head[6,10,18,21]. The current report represents the first case that was found on the dorsal side of the pancreatic head. Because it was located on the dorsal side of the pancreas and close to the caudate lobe, it was difficult to diagnose ectopic HCC before surgery. The initial differential diagnosis was malignant lymphoma or pancreatic head mass. It was believed to be an HCC that developed in the caudate lobe. In our literature review, the median overall survival of 24 cases is 18 mo[1,2,4-25].There are 6 cases with recurrence after tumor resection, whose median overall survival and recurrence-free survival were 18.5 (range 6-48) and 7.5 (range 2-30) mo,respectively[1,9,16,22,23,25]. Among 18 patients who underwent surgery, one cases received postoperative adjuvant therapy[19]. In the case, the adjuvant chemotherapy using cisplatin + etoposide + bleomycin was performed for the EHCC over the left subphrenic space, and the case is without recurrence during 8 mo after surgery[19]. In six cases who did not undergo surgery, three cases received chemotherapy (sorafenib,cisplatin+etoposide+bleomycin, or unknown regimen), two cases underwent just a biopsy and one case inserted a biliary stent as a palliative care[5,7,10,12-14]. The survival outcomes in the two cases with multiple EHCCs in the spleen treated by cisplatin +etoposide + bleomycin and with multiple EHCCs in the thoracic and abdominal cavities treated by sorafenib, are 34 and 13 mo, respectively[7,14]. Although tumor resection had been performed in the majority of EHCC (18 of 24 cases, 75%), the clinical benefit of tumor resection for EHCC is still unclear from our literature review.Further accumulation of EHCC cases need to elucidate the epidemiologic aspects of EHCC.
Some reports have noted that EHCC is observed in about 7%-30% of cases of ectopic liver[2,9]. Carcinogenesis is a multistep process that appears to be accelerated within these tissues. It is theorized that due to the lack of a normal vascular and ductal system, the foci of ectopic liver tissue may be metabolically handicapped,leading to longer exposure to various carcinogenic factors[6,7,10]. The underlying microenvironment would cause persistent cellular stress, which may result in cell death and compensatory cell proliferation. An increased cell turnover may lead to genetic mutations and subsequent development of carcinoma[11]. The reason is that both morphological features are similar, and if the carcinoma cell is poorly differentiated or undifferentiated, it will be extremely difficult to distinguish HCC from adenocarcinoma cells morphologically. The effectiveness of Arg-1 against poorly differentiated HCC. In particular, Arg-1 is the most sensitive and specific marker(greater than 90%) of hepatocellular differentiation and should be the first-line marker of HCCvsother tumors[12,13].
CONCLUSION
The preoperative diagnosis of EHCC is often very difficult. Specific tumor markers can be useful to diagnose EHCC preoperatively if there is any possibility of another tumor from radiological findings. Early surgical treatment for EHCC would provide favorable long-term outcomes.
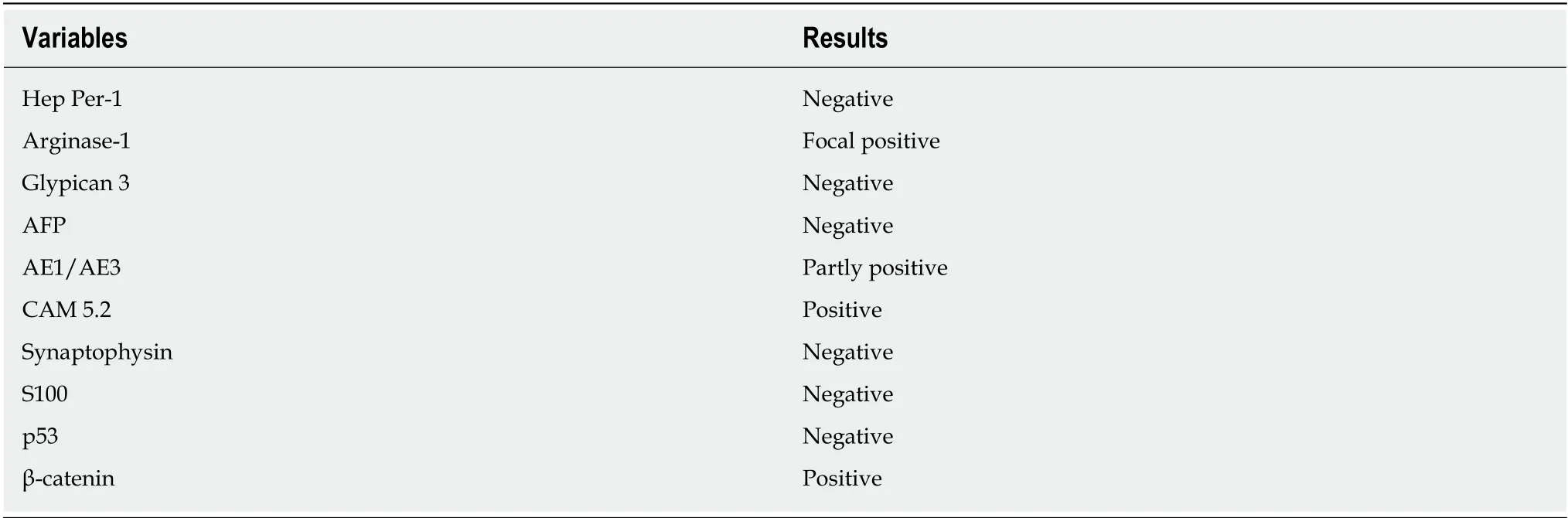
Table 2 Details of immunohistochemical staining of the resected tumor specimen

Figure 2 The microscopic examination of the tumor by endoscopic ultrasound-guided fine needle aspiration. Hematoxylin and eosin stain, magnification ×200 (left) and × 400 (right). Microscopic examination of the tumor confirmed poorly differentiated carcinoma.

Figure 3 The tumor is located at between the hepatic portal region and the dorsal of the pancreatic head and has no connection to the surrounding organ.

Figure 4 Macroscopic features of the excised hepatocellular carcinoma demonstrating a solid multinodular tumor with a fibrous capsule and intratumoral hemorrhage.
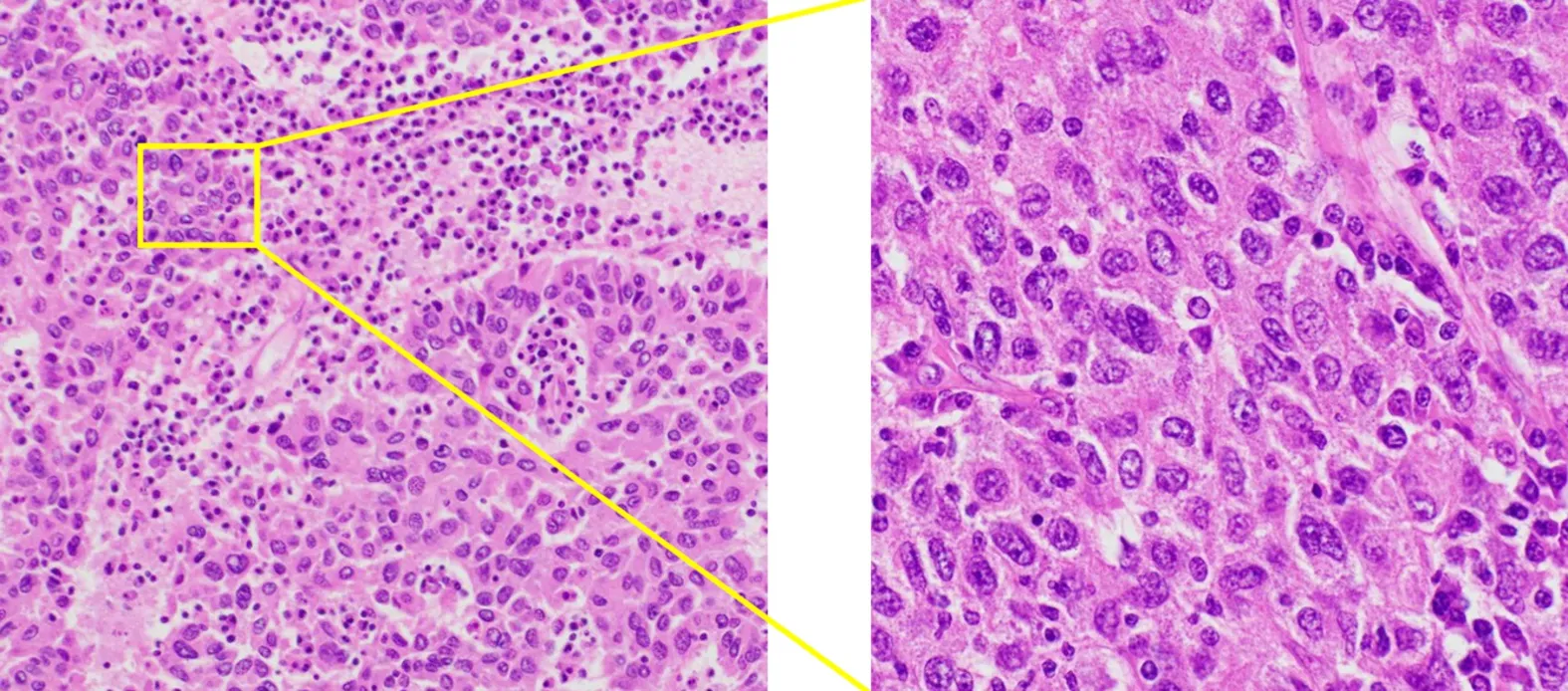
Figure 5 The microscopic examination of the tumor. Microscopic examination of the tumor confirmed poorly differentiated carcinoma morphologically similar to the tumor biopsy by endoscopic ultrasound-guided fine needle aspiration Hematoxylin and eosin stain, magnification × 100 (left) and × 400 (right).
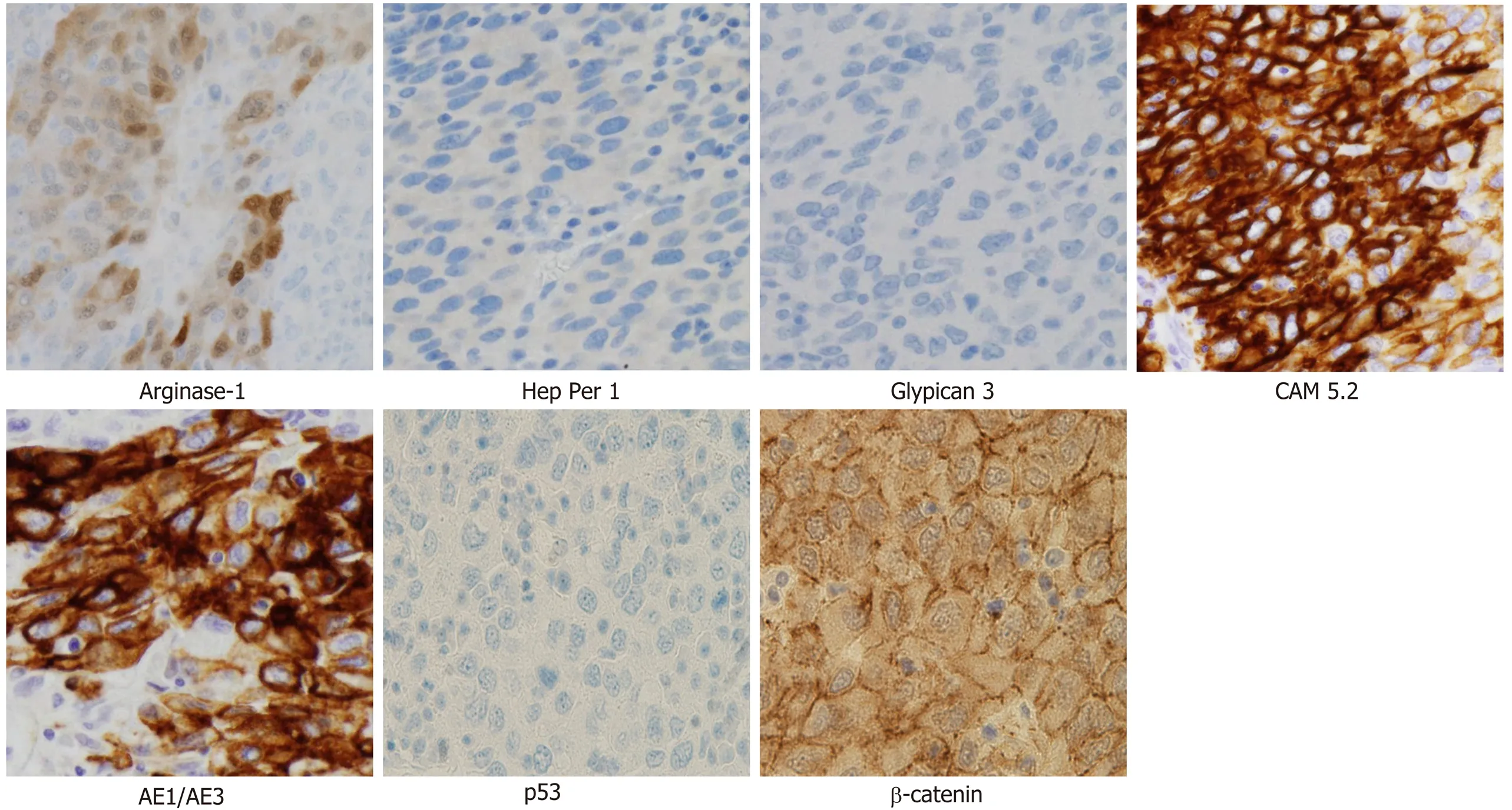
Figure 6 lmmunohistochemical findings. Tumor cells are negative for Hep Per-1, Glypican 3 and p53, but focal positive for Arginase-1. Moreover, CAM 5.2,AE1/AE3, and β-catenin are positive (Magnification × 400).
ACKNOWLEDGEMENTS
I would like to thank Hiromitsu Hayashi for suggesting the topic investigated in this paper. I am grateful to Yo-Ichi Yamashita for assistance with the useful discussions and Hideo Baba for carefully proofreading the manuscript.
杂志排行
World Journal of Gastroenterology的其它文章
- Folic acid attenuates high-fat diet-induced steatohepatitis via deacetylase SlRT1-dependent restoration of PPARα
- Genetic association analysis of CLEC5A and CLEC7A gene singlenucleotide polymorphisms and Crohn's disease
- Hepatitis B virus recurrence after liver transplantation: An old tale or a clear and present danger?
- Natural products that target macrophages in treating non-alcoholic steatohepatitis
- Annexin A2 promotion of hepatocellular carcinoma tumorigenesis via the immune microenvironment
- Computed tomography vs liver stiffness measurement and magnetic resonance imaging in evaluating esophageal varices in cirrhotic patients: A systematic review and meta-analysis
