Natural products that target macrophages in treating non-alcoholic steatohepatitis
2020-06-08ChunLinLiWenJunZhouGuangJiLiZhang
Chun-Lin Li, Wen-Jun Zhou, Guang Ji, Li Zhang
Abstract Nonalcoholic steatohepatitis (NASH) is the progressive subtype of non-alcoholic fatty liver disease and potentiates risks for both hepatic and metabolic diseases.Although the pathophysiology of NASH is not completely understood, recent studies have revealed that macrophage activation is a major contributing factor for the disease progression. Macrophages integrate the immune response and metabolic process and have become promising targets for NASH therapy.Natural products are potential candidates for NASH treatment and have multifactorial underlying mechanisms. Macrophage involvement in the development of steatosis and inflammation in NASH has been widely investigated. In this review, we assess the evidence for natural products or their active ingredients in the modulation of macrophage activation, recruitment, and polarization, as well as the metabolic status of macrophages. Our work may highlight the possible natural products that target macrophages as potential treatment options for NASH.
Key words: Nonalcoholic steatohepatitis; Macrophages; Natural products; Inflammation;Metabolism
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a common liver disease worldwide[1].Approximately one-quarter of the population suffer from NAFLD[1], and approximately 30% of patients with NAFLD progress to the inflammatory subtypenonalcoholic steatohepatitis (NASH)[2]. NASH is characterized by steatosis,inflammation, and fibrosis, and serves as a potential risk factor for hepatocellular carcinoma[3]. Lifestyle interventions, such as dieting and exercise, are the general recommendation for NAFLD[4]. Weight control is of great importance, and a weight loss of 7% to 10% can histologically attenuate NASH in patients[5]. Even without weight loss, patients with NAFLD benefit from exercise by improving insulin sensitivity and reducing hepatic lipid content[3,6,7]. However, not all patients are willing or suitable for such interventions, thus making pharmacological agents urgently needed. Although the pathophysiology and treatment of NASH have been extensively investigated, authorized pharmacological agents that are specific for NASH are not yet available.
Macrophages are versatile innate immune cells. As scavengers, they engulf wornout cells and debris. As secretory cells, they produce a wide array of powerful chemical substances, such as enzymes and complement proteins. In addition,macrophages can present antigens and, along with dendritic cells, initiate adaptive immune response. Tissue macrophages are mainly derived from embryonic progenitors and blood monocytes[8]. Since macrophages obtain phagocytosis and immunoregulating properties, they are involved in tissue development and homeostasis with high plasticity[9]. According to their functions, macrophages are generally divided into two subpopulations, namely, classically activated (M1-type)macrophages and alternatively activated (M2-type) macrophages. The microenvironment determines the phenotype, and the dynamic self-metabolism state inversely regulates its response to the microenvironment. For instance, high levels of lipopolysaccharide (LPS) and interferon γ (IFN-γ) promote M1-type macrophage polarization, whereas interleukin (IL)-4, IL-10, and IL-13 promote M2-type macrophage polarization[9-11]. The predominant phenotype may change at different periods of disease. M1-type macrophages become dominant during inflammation and injury, whereas M2-type macrophages are abundant in the tissue repair and recovery periods.
Macrophages are the main source of inflammatory mediators in the liver, and the activation of macrophages also induces insulin resistance and metabolic dysfunctions.In addition, high titers of immunoglobulin G exist in 40% of adult NAFLD/NASH patients, 60% of pediatric NASH patients, and diet-induced NASH animals,suggesting that adaptive immune responses also take an active part in NASH development[12-14]. The versatile macrophages integrate metabolic and inflammatory responses, as well as adaptive immunity, thus serving as critical targets for the treatment of NASH[15]. Natural products are potential candidates in NASH therapy owing to their safety and multitarget properties, and a series of natural products are reported to modulate macrophages, which may contribute to their effects in preventing or treating NASH.
ROLE OF LIVER MACROPHAGES IN NASH
NASH is characterized by infiltration of inflammatory cells in the liver, and liver macrophages play a central part in the process[16]. Liver macrophages consist of resident Kupffer cells (KCs) and recruited macrophages derived from circulating monocytes. KCs and recruited macrophages have different features in the progression of NASH[17]. KCs are the first line of defense in the liver, and endogenous and exogenous pathogens induce KC activation. The activated KCs clear pathogens depending on their phagocytic activity. Simultaneously, KCs secrete proinflammatory cytokines and chemokines, promote the inflammatory response, and recruit peripheral blood monocytes to the liver. With the progression of disease,monocyte-derived macrophages become the dominant macrophages in the liver[17].Generally, macrophage activation serves as a protector by engulfing pathogens and secreting cytokines or mediators in the early stage of host immunity[18]. However,continuous stimulation induces cell death, liver injury, and related diseases[19].
NATURAL PRODUCTS THAT TARGET MACROPHAGES IN NASH TREATMENT
Natural products regulating macrophage activation
In NASH, pathogen-associated molecular patterns or damage associated molecular models such as gut-derived endotoxins, adipose tissue-derived adipokines, and debris from injured or dead hepatocytes induce KC activation, activated KCs secrete chemokines to recruit monocytes to the liver, and the expanding liver macrophage pool may further promote liver injury[20,21]. KC depletion has been reported to protect mice from hepatic steatosis and insulin resistance upon high fat diet (HFD) feeding,suggesting that KCs play an important role in NAFLD development[22]. Several natural products are reported to inhibit KC activation. Sparstolonin B is an ingredient ofSparganium stoloniferum, and administration of Sparstolonin B to HFD-fed mice decreases the expression of cluster of differentiation 68 (CD68) and chemokine 2(CCL2) in KCs and reverses NASH features accordingly[23]. Curcuminoids, extracted from the plantCurcuma domestica Val., are found to inhibit KC activation in LPStreated BALB/C mice[24]. In carbon tetrachloride-induced acute liver injury rats,S.miltiorrhizaextract administration obviously suppresses p38 and nuclear factor-kappa B (NF-κB) signaling in KCs[25]. A six-week supplementation of methanolic extract ofGraptopetalum paraguayensewas reported to reduce nitric oxide, tumor necrosis factor(TNF)-α, and IL-6 generation and improve liver inflammation and fibrosis in dimethylnitrosamine- or carbon tetrachloride-induced NASH rats[26].
Excess lipids in the liver may cause lipotoxicity, and lipid intermediate metabolites such as palmitate, ceramides, and free cholesterol are crucial contributors to macrophage activation, oxidative stress, and apoptosis[27-29]. Natural products with the function of reducing lipotoxicity are candidates for NASH treatment. The tuber ofAlisma orientalis (Sam.) Juzep. is a commonly used herbal medicinal material, and administration of its extract prevents endoplasmic reticulum stress and lipogenic gene expression in palmitate-stimulated HepG2 cells as well as in diet-induced NAFLD mice[30,31]. Serine palmitoyltransferase is a key enzyme in ceramide metabolism, and the fungal compound myriocin inactivates serine palmitoyltransferase by forming a C18 aldehyde and prevents sphingolipid biosynthesis in hepatocytes[32].
In addition to endogenous liver stress, liver macrophage activation can also be mediated by extrahepatic stimuli, such as gut-derived endotoxins, translocated bacteria and microbiota metabolites, and adipose-derived cytokines. Blocking or alleviating these stimuli is expected to suppress macrophage activation and improve the NASH phenotype[27,33-36]. Certain natural products may affect the structure of the gut microbiome, and the related metabolites are reported in NASH treatment. Gallic acid is a naturally abundant plant phenolic compound in vegetables and fruits; it partially reshapes gut dysbiosis, reduces the choline metabolites dimethylamine and trimethylamine, and prevents NAFLD development in HFD-fed mice[37]. The natural plant alkaloid berberine can be found in plants, such asCoptis chinensis Franch. andPhellodendron chinense Schneid. Short-term berberine exposure in mice reshapes gut microbiota by reducingClostridiumclusters XIVa and IV, and shows beneficial effects on NASH mice[38]. Indb/dbmice, administration ofdendrobiumextract increases theBacteroidetestoFirmicutesratio and the relative abundance ofPrevotellaandAkkermansia, and reduces the relative abundance ofS24-7,Rikenella, andEscherichia coli., thus alleviating hepatic steatosis in mice[39]. Certain natural products are found to improve NAFLD/NASH through modulation of adipokines. Dihydromyricetin is the main ingredient of the edible medicinal plantAmpelopsis grossedentata. In a doubleblind clinical trial, dihydromyricetin treatment reduces resistin levels and improves insulin intolerance in patients with NAFLD[40]. Korean Red Ginseng is found to increase adiponectin and reduce pro-inflammatory TNF-α levels in patients with NAFLD[41]. Total alkaloids ofRubus alceaefolius Poirhave beneficial effects on NAFLD by reducing serum leptin and resistin and increasing adiponectin levels in HFDinduced rats[42]. Additionally, the edible plantsOpuntia ficus indica(nopal),umbelliferone, and piperine have been reported to improve insulin resistance and oxidative stress by upregulating serum adiponectin and downregulating leptin levels in obese animals[43-45].
Natural products regulating liver macrophage recruitment
In NASH, classical LY6Chigh(mice) and CD14+(human) monocytes are recruited to the inflamed area in the liver through chemokines[46]. CCL2 is present at a very low level in the physiological state but is significantly increased in NASH. The interaction of CCL2 with its receptor C-C motif receptor 2 (CCR2) is required for monocyte migration to the liver, and knockout of CCL2 or CCR2 significantly reduces macrophage accumulation and mitigates NASH severity in mice[21]. Therefore,inhibiting macrophage recruitment to the liver is considered an effective strategy for NASH treatment. Chemokine antagonists have been found in natural products,suggesting that natural products play a positive role in this process[47]. Flavonoids derived from modified apple reduce the transcription ofCCR2, chemokine ligand 10,andCCR10in mice[48]. Dietary broccoli can reverse dextran sulfate sodium-evoked CCR2 upregulation in mice[49]. Berberine reduces CCL2 levels and inhibits macrophage recruitment in HFD-fed rats[50]. In high refined carbohydrate-containing diet-fed BALB/c mice, supplementation with crude extract ofRudgea viburnoides(Cham.)benth. (Rubiaceae) leaves lowers hepatic CCL2, reduces macrophage recruitment, and improves the inflammatory response in NASH animals[51]. In HFDinduced NASH mice andApoE-/- mice, administration of Long ya Aralia chinensis Lderived total saponins ofAralia elata(Miq) Seem for 12 wk decreases CCL2, blocks the inosital-requiring enzyme-1α (IRE1α)-mediated c-Jun N-terminal kinase pathway and significantly improves hepatic steatosis[52].
Natural products regulating macrophage polarization
Polarization of macrophages is determined by the local environment[53]. The inflammatory microenvironment with LPS and IFN-γ induces macrophage polarization to the pro-inflammatory M1-type, characterized by increased proinflammatory cytokines, chemokines, and reactive nitrogen and oxygen intermediates[54]. IL-4, IL-10, and IL-13 induce polarization towards the antiinflammatory M2-type (e.g., M2a, M2b, and M2c) characterized by increased scavenger receptors and enhanced phagocytosis activity[11,55]. In addition, PPAR-γ regulates M2-type polarization, and low levels of IFN-γ or high levels of CSF-1 induce recruited monocytes to differentiate into M2-type macrophages[56,57]. Certain stimuli may switch macrophages from M1-type to M2-type, orvice versa[53,58-60]. Failure to appropriately control this switch may cause progression of the disease[61]. In NASH,rapid and abundant pro-inflammatory macrophages are required and of benefit in the early stage; however, the constant existence of pro-inflammatory macrophages results in aggravated inflammation and fibrogenesis[62,63]. A series of natural products have been proven to regulate macrophage polarization and thus alleviate NASH and related complications. Celastrol is found to attenuate lipid accumulation and improve insulin sensitivity in NAFLD mice and regulate macrophage polarization through mitogen-activated protein kinase-NF-κB pathways in mice[64]. Smiglaside A is a phenylpropanoid glycoside isolated fromSmilax riparia, and it has been found to upregulate M2-type and downregulate M1-type macrophage biomarkers in LPSstimulated RAW264.7 cells and mouse peritoneal macrophages[65]. Asperlin isolated from marineAspergillus versicolorLZD4403 fungus significantly reduces the expression of pro-inflammatory mediators such as inducible nitric oxide synthase, IL-1β, and TNF-α, and increases expression of IL-4 and IL-10 in LPS-stimulated RAW264.7 cells[66]. The pentacyclic triterpene lupeol regulates macrophage polarization by reducing pro-inflammatory and increasing anti-inflammatory cytokines in intestinal epithelial cells[67]. Baicalin upregulates IL-10, arginase 1, and IFN regulatory factor 4 (IRF4), downregulates TNF-α, IFN regulatory factor 5, IL-6,and IL-23, and enhances the phagocytosis and efferocytosis of macrophages, thus promoting macrophage polarization to the M2-type in mice with inflammatory bowel disease[68,69]. TheSalvia miltiorrhizaingredient tanshinone IIA andTabebuia avellanedae Lorentz ex Grisebextract were found to promote M2-type macrophage polarization in colitis mice[70,71]. Emodin can be found in Chinese herbs such asRheum palmatumandPolygonum multiflorum; it bidirectionally modulates the polarization of primary mouse macrophages, inhibits pro-inflammatory genes when challenged with LPS/IFN-γ, but increases pro-inflammatory genes under IL-4 stimulation in macrophages[10].Inactivation of the Notch signaling pathway contributes to M2-type polarization[72].Natural products such asTrichosanthes kirilowiilectin and oridonin are reported to deactivate Notch signaling, induce M2-type macrophage polarization, and inhibit the inflammatory response in rodents[73,74].
NATURAL PRODUCTS THAT MODULATE METABOLIC STATUS OF MACROPHAGES
The liver is an important metabolic organ and provides a favorable environment for macrophages[19,75,76]. As immune cells have high plastic functions, macrophages autonomously change their self-metabolism state to adapt to the microenvironment[77-79]. Alterations in the metabolic state influence the energy supply as well as the function and phenotype of macrophages[80]. Metabolic pathways in macrophages include amino acid metabolism, glycolysis, mitochondrial oxidative phosphorylation (OXPHOS), pentose phosphate pathway, fatty acid synthesis, and fatty acid oxidation[81]. Activated macrophages are characterized by abnormal amino acid metabolism, upregulated glycolytic metabolism, and damaged OXPHOS[80,82-84].M1-type macrophages display activated pentose phosphate pathway and a broken tricarboxylic acid (TCA) cycle[85]. M2-type macrophages show enhanced OXPHOS and normal TCA cycle function[86-88]. The damaged TCA cycle promotes the accumulation of succinate and citrate, followed by the generation of IL-1β, and thus contributes to the M1-type macrophage response[89].
Macrophages acquire energy to support their functions; M2-type macrophages obtain energy mainly from OXPHOS, whereas M1-type macrophages obtain energy through glycolysis. Glycolysis is inefficient at ATP generation, so the process is enhanced, and substrate production is accelerated to guarantee the functions of M1 macrophages in the inflammatory state[90]. Accumulated substrates act as stimulants that strengthen the macrophage response and activate other signaling pathways.Pyruvate is one of the end products of glycolysis, and an increase in pyruvate dehydrogenase kinase-2 (PDK2) and pyruvate dehydrogenase phosphorylation decreases pyruvate/acetyl-CoA conversion, reactive oxygen species secretion, and IL-1β production[91-93]. Several natural products are reported to affect the metabolic status of macrophages in NASH treatment.Ampelopsis brevipedunculata(Vitaceae) berries are a medicinal plant for treating liver disease, and its ethanol extract decreases pyruvate,superoxide dismutase, and dimethyl sulfoxide levels in ferrous iron-stimulated liver injury rats[94].Aim Scutellariae RadixandCoptidis Rhizomaare found to upregulate pyruvate kinase activity in the liver, and thus improve the dysfunctional lipid metabolism in diabetic rats[95]. Hyacinth bean (Dolichos lablab L) ameliorates pyruvatederived amino acid metabolism and prevents obesity in HFD-fed mice[96]. PDK1 is associated with the M1-type response and aerobic glycolysis, and inhibition of PDK1 promotes M2-type polarization[97]. It has been reported that methanol extracts of
Mycetia cauliflora Reinw. (Rubiaceae) andDipterocarpus tuberculatus Roxb.(Dipterocarpaceae) target PDK1 and suppress the NF-κB signaling pathway in LPSstimulated RAW264.7 cells[98,99]. Pyruvate kinase M2 inhibits LPS-induced M1-type polarization while evoking M2-type polarization by inhibiting IL-1β and increasing IL-10 generation. Natural products that regulate pyruvate kinase M2 may also benefit NASH therapy, and further studies are needed to explore such agents[100].
SUMMARY AND PERSPECTIVES
Macrophages play a pivotal role in NASH development. Macrophages in the liver integrally regulate immune and metabolic responses and have become attractive targets for NASH treatment. Natural products are important candidates for NASH and are involved in regulating macrophage activation, recruitment, and polarization.Inversely, metabolic status affects the function of macrophages, and enzymes that modulate metabolic processes can also be regulated by natural products (Figure 1,Table 1).
There are plenty of reports about natural products for treating liver-related diseases, and on the basis of the available experimental results, curcumin, berberine,flavonoids, sparstolonin B, baicalin, and emodin are among the most promising agents in NASH treatment. Actually, several natural products are already under clinical investigation. Curcumin is currently in phase II/III clinical trials, expecting to improve liver steatosis, fibrosis, and liver inflammatory mediators in NAFLD patients[101,102]. Administration of berberine plus lifestyle intervention has been proven to reduce body weight, hepatic fat content, and serum lipid profiles, improve insulin sensitivity, and increase brown adipose tissue mass in NAFLD patients[103,104].
Although the effects of natural products on NASH are confirmed, available studies lack consensus standards and specifications, leading to the evaluation system being in an immature state and the potential mechanisms remaining unclear. The variance of patient choice and adherence, dosing methods, as well as test cycle may cause inconclusive results, and large-scale, multicenter random control trials are needed. In addition, many natural products show low bioavailability, thus strategies in promoting drug utilization or improving dosage form (nanoparticle and biological vector) need to develop. Considering the complex pathology of NASH, natural products are quite feasible to solve the problems. However, more work should be done to connect and integrate the two complex systems.
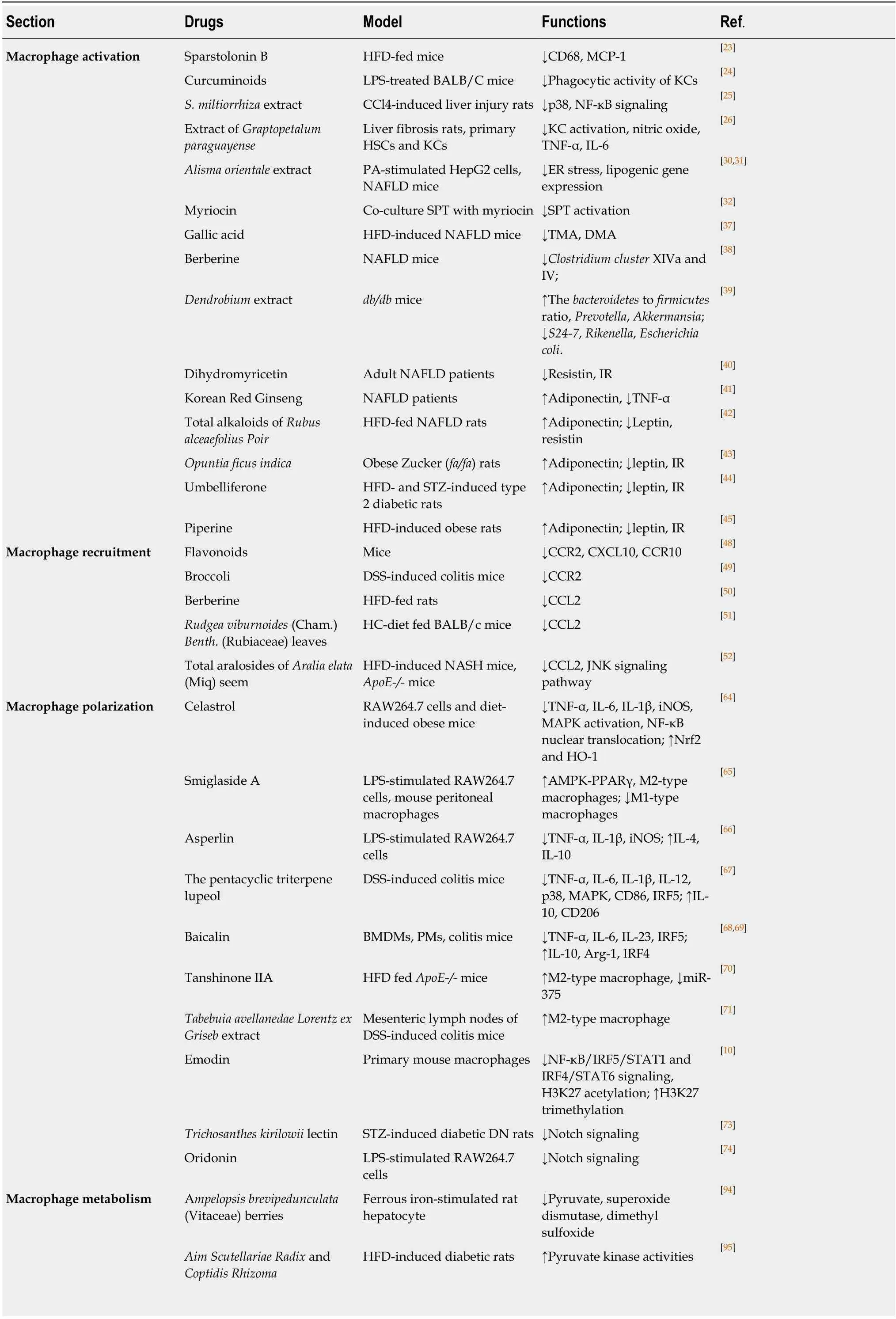
Table 1 Natural products that target macrophage for nonalcoholic steatohepatitis therapy

HFD: High fat diet; LPS: lipopolysaccharide; KCs: Kupffer cells; CCl4: Carbon tetrachloride; HSCs: Hepatic stellate cells; NAFLD: Non-alcoholic fatty liver disease; SPT: Serine palmitoyltransferase; DMA: Dimethylamine; TMA: Trimethylamine; CCR2: C-C motif receptor; CXCL10: Chemokine ligand 10; DSS:Dextran sulfate sodium; HC: High refined carbohydrate; JNK: c-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinase; iNOS: Inducible nitric oxide synthase; IRF: Interferon regulatory factor; PDK: Pyruvate dehydrogenase kinase; IL: Interleukin; TNF: Tumor necrosis factor; CCR2: C-C motif receptor 2; MCP: Monocyte chemotactic protein; NF-κB: Nuclear factor-kappa B.
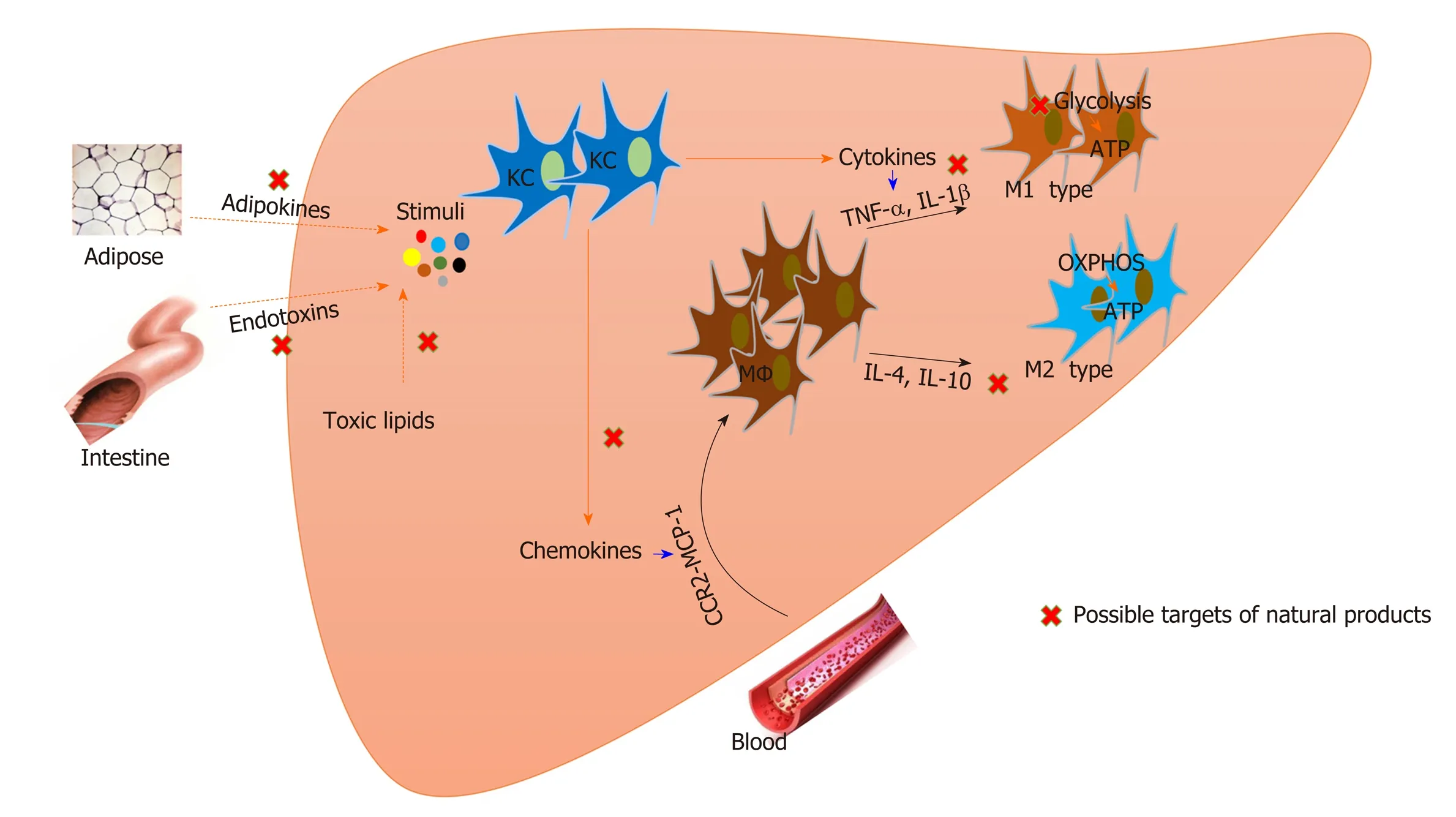
Figure 1 Natural products that target macrophages for nonalcoholic steatohepatitis treatment. Both resident Kupffer cells and recruited macrophages are involved in the pathogenesis of nonalcoholic steatohepatitis. Modulation of macrophage activation, polarization, and recruitment by natural products contributes to nonalcoholic steatohepatitis improvement. Metabolic status affects the function of macrophages, and natural products also regulate macrophage metabolism. KC:Kupffer cell; MΦ: Macrophage; OXPHOS: Mitochondrial oxidative phosphorylation; IL: Interleukin; TNF: Tumor necrosis factor; CCR2: C-C motif receptor 2; MCP:Monocyte chemotactic protein.
杂志排行
World Journal of Gastroenterology的其它文章
- Medical management of metabolic and cardiovascular complications after liver transplantation
- New role for ceramide in hypoxia and insulin resistance
- Role of gut microbiota on intestinal barrier function in acute pancreatitis
- Prognostic significance of hepatic encephalopathy in patients with cirrhosis treated with current standards of care
- Optimal proximal resection margin distance for gastrectomy in advanced gastric cancer
- Computed tomography vs liver stiffness measurement and magnetic resonance imaging in evaluating esophageal varices in cirrhotic patients: A systematic review and meta-analysis
