Computed tomography vs liver stiffness measurement and magnetic resonance imaging in evaluating esophageal varices in cirrhotic patients: A systematic review and meta-analysis
2020-06-08YueLiLeiLiHongLeiWengRomanLiebeHuiGuoDing
Yue Li, Lei Li, Hong-Lei Weng, Roman Liebe, Hui-Guo Ding
Abstract
Key words: Multidetector computed tomography imaging; Magnetic resonance imaging;Liver stiffness measurement; Liver cirrhosis; Esophageal varices; Meta-analysis
INTRODUCTION
Globally, liver cirrhosis is the most common liver disease and the 11thleading cause of death. Approximately two million people die from liver disease every year and 50%of them die from complications of cirrhosis[1]. Portal hypertension (PH) with esophageal varices (EV) and the following lethal variceal hemorrhage is the most serious and common complication of cirrhosis. The incidence of EV in cirrhotic patients is 7% per year and the five-year cumulative incidence rate reaches 21%[2].Although the treatment of variceal hemorrhage has been improved over the past two decades, the 6-wk mortality is 10%-20%[3]. The confirmation of varices and the most suitable treatment in the early phase is crucial in order to reduce the mortality. To date, endoscopy is regarded as the “gold standard” for diagnosing the presence of varices and predicting bleeding risk. Baveno VI recommends that compensated cirrhotic patients without varices whose etiological factor has been removed should receive endoscopy every 3 years[4]. Endoscopy, however, is invasive and uncomfortable. In addition to endoscopy, hepatic vein pressure gradient (HVPG) is considered as a “gold standard” in estimating PH and for risk stratification of liver cirrhosis. HVPG is superior to liver biopsy in predicting the occurrence of complications in cirrhotic patients, including EV and variceal hemorrhage[5]. It is promising that with the aid of HVPG-guided precise treatment, physicians can diagnose and treat PH similarly to “high blood pressure”[6]. However, HVPG measurement is also invasive and expensive. Therefore, non-invasive and easy-toperform diagnostic techniques to predict complications in cirrhotic patients with PH are required in clinical practice.
So far, several models and parameters based on serum markers[7,8]have been proposed. However, poor reliability has prevented their use in clinical practice.Recently, multiple studies evaluated the accuracy of liver stiffness measurement(LSM), computed tomography (CT), and magnetic resonance imaging (MRI) in the diagnosis of EV and prediction of high-bleeding-risk EV (HREV) in cirrhotic patients.There have, however, been controversies regarding the use of LSM, CT, and MRI as non-invasive diagnostic methods for EV and prediction of HREV in cirrhotic patients.Therefore, the aim of this meta-analysis was to evaluate the value of the imaging methods for the diagnosis of EV and prediction of HREV in clinical practice.
MATERIALS AND METHODS
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[9], and the protocol is registered at PROSPERO (CRD42019126278).
Literature search
A systematic literature research based on PubMed, Embase, Cochrane, CNKI, and Wanfang databases using various combinations of Medical Subject Headings and non-Medical Subject Headings terms was performed independently by two reviewers.The search was limited to original full text articles published in English and Chinese.
The articles reporting the diagnostic value of LSM were searched using key words“LS,” “liver stiffness,” “FibroScan,” “esophageal varices”, and “cirrhosis”, and those reporting the diagnostic value of CT and MRI were searched based on key words“CT,” “computed tomography,” “esophageal varices”, and “cirrhosis” and “MR,”“magnetic resonance,” “esophageal varices”, and “cirrhosis”, respectively.
The last search was performed on April 26, 2019.
Eligibility criteria: The inclusion criteria were: (1) Patients were diagnosed with liver cirrhosis; (2) Endoscopy was performed to confirm the presence and/or grade of EV;(3) Relevant examinations, such as LSM, CT, or MRI, were performed; and (4) The diagnostic accuracy was compared between reference and LSM, CT, or MRI. The exclusion criteria were: (1) Duplicate articles; (2) Reviews; (3) Case reports; (4)Noncirrhotic patients; (5) Patients in whom the presence of varices evaluated was not evaluated by endoscopy; and (6) Lack of accuracy assessment.
Data extraction: The primary data were extracted by two reviewers independently.The study characteristics contained country, study design, age, gender, and etiology of liver cirrhosis. The data included patient number, cut-off value, and the sensitivity and specificity in the diagnosis of EV or HREV. The criteria for HREV based on endoscopy were any of the following[10-12]: (1) Varices diameter ≥ 5 mm and snakelike varices with red color signs; and (2) Large varices (diameter ≥ 10 mm) and nodular and tumor-shaped varices with or without red color signs.
Quality assessment
Two reviewers independently assessed the study quality using the Quality Assessment of Diagnostic Accuracy Studies-2 in RevMan5.3. They calculated the risk of bias as high, low, or unclear with regard to the following aspects: Patient selection,index test, reference standard, and flow and timing. Discrepancies were resolved by discussion between the two reviewers. Each question was judged as “yes”, “no”, or“unclear”.
Statistical analysis
First, true positive (TP) value, false positive (FP) value, false negative (FN) value, and true negative (TN) value were extracted from the original articles. Data analyses were conducted using Stata12.0, MetaDisc1.4, and RevMan5.3.
Second, the heterogeneity of all tested parameters was examined byQ-statistic test andI2index. Heterogeneity was considered significant ifP< 0.05 (Q-statistic test) orI2≥ 50%[13]. When heterogeneity was tested, we further evaluated the threshold effects by calculating the Spearman's correlation coefficient. Threshold effects were considered significant ifP< 0.05. If no threshold effects existed, sources of heterogeneity were analyzed by meta-regression according to study characteristics.Besides, we performed subgroup analysis according to the results of meta-regression.
The analysis was performed using the fixed-effects model or random-effects model if heterogeneity was considered significant. The diagnostic accuracy was evaluated by the area under the summary receiver operating characteristic curve (AUSROC) with 95% confidence interval (CI), summary sensitivity and specificity with 95%CI,summary positive likelihood ratio (PLR) and negative likelihood ratio (NLR) with 95%CI, and summary diagnostic odds ratio (DOR).
Finally, publication bias was evaluated using Deek's funnel plot, withP< 0.05 as having significant publication bias[14].
RESULTS
Literature identification
All analyzed cirrhotic patients were diagnosed by histopathology and/or typical clinical symptoms and laboratory and imaging findings. The etiologies of liver cirrhosis included hepatitis B virus, hepatitis C virus, alcohol, autoimmune hepatitis,nonalcoholic steatohepatitis, and miscellaneous.
LSM: According to the aforementioned search strategy, 898 articles relevant to LSM and cirrhosis were identified. Eighteen best-matched articles were chosen for final meta-analysis[15-32]. The selection process is presented in Figure 1A. Fifteen out of eighteen selected publications[15-17,19-25,27-29,31,32]studied the diagnostic value for EV in 1836 patients. These studies were performed in Asia (n= 6), Europe (n= 7), and Africa(n= 2). In addition, 13[15-18,20-23,26,27,30-32]articles reported the predictive value of HREV in 2388 patients. These studies were performed in Asia (n= 5), Europe (n= 6), and Africa(n= 2), respectively.
CT: According to the search strategy, 17 out of 2192 articles relevant to CT imaging and cirrhosis were chosen for meta-analysis[33-49](Figure 1B). Sixteen articles[33-38,40-49]enrolled 3327 patients (31 groups) and examined the diagnostic value of CT for EV.These studies were performed in Asia (n= 9), North America (n= 3), and Africa (n=4) (Table 1). Besides, 10[34-36,39-43,45,47]articles reported the predictive value of HREV in 2686 patients (23 groups). These studies were performed in Asia (n= 5), North America (n= 3), and Africa (n= 2) (Table 2).
MRI: According to the search strategy, 7 out of 601 articles that evaluated MRI in liver cirrhosis were included in the meta-analysis[50-56](Figure 1C). Four manuscripts reported the diagnostic value of MRI for EV, which included 750 patients (7 groups)[50-52,54]. These studies were performed in Asia (n= 3) and Africa (n= 1).Besides, 4 articles comprising 9 groups and 1053 patients studied the predictive value of HREV[53-56], which were performed in Asia (n= 3) and Europe (n= 1).
The quality of the eligible articles is shown in Figure 2.
Meta-analysis
The results of meta-analysis are shown in Table 3. Significant heterogeneity was observed in all analyses (P< 0.05), except summary sensitivity in diagnosing EV and summary NLR in evaluating both EV and HREV using MRI (P> 0.05). Therefore, the random-effects model was used to combine effect quantity. Threshold effects were not found in all analyses (P> 0.05). CT had the highest AUSROC for the evaluation of EV and HREV (Figure 3A and B).
LSM: Using LSM to diagnose EV, the AUSROC was 0.86 (95%CI: 0.83-0.89,I2=97.43%, Figure 3C), with a summary sensitivity of 0.84 (95%CI: 0.78-0.89,I2=82.63%;Figure 4A) and summary specificity was 0.71 (95%CI: 0.60-0.80,I2= 86.56%; Figure 4B). The summary PLR, NLR, and DOR were 2.91 (95%CI: 2.08-4.06,I2= 82.66%), 0.22(95%CI: 0.16-0.30,I2= 79.49%), and 13.01 (95%CI: 7.83-21.64; Table 3), respectively.
As for the predictive value of LSM for HREV, the AUSROC was 0.85 (95%CI: 0.81-0.88,I2= 97.13%; Figure 3D), with a summary sensitivity of 0.81 (95%CI: 0.75-0.86,I2=70.93%; Figure 4C) and summary specificity of 0.73 (95%CI: 0.66-0.80,I2= 91.65%;Figure 4D). The summary PLR, NLR, and DOR were 3.04 (95%CI: 2.38-3.89,I2=85.63%), 0.26 (95%CI: 0.19-0.34,I2= 68.30%), and 11.93 (95%CI: 7.89-18.03; Table 3),respectively.
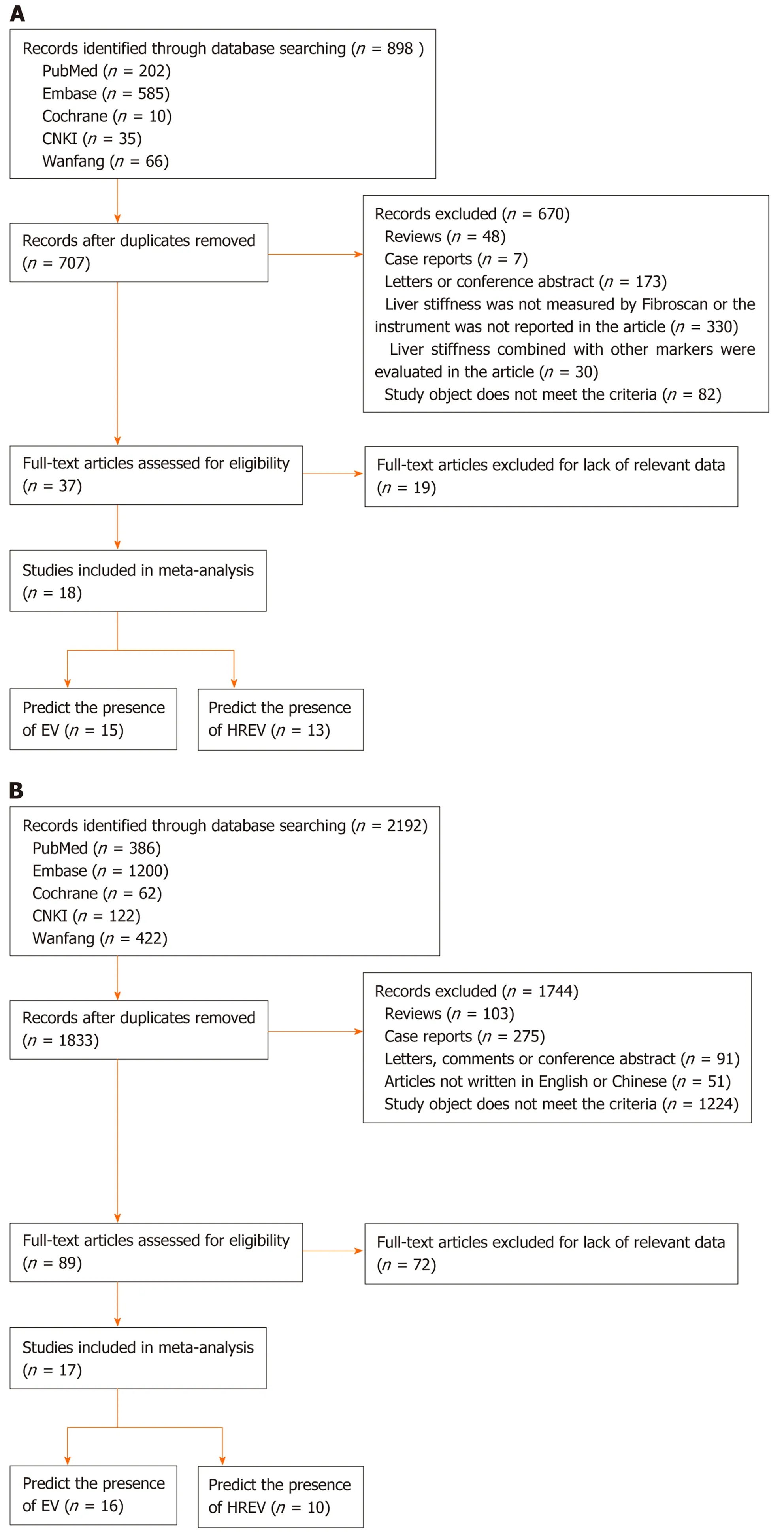
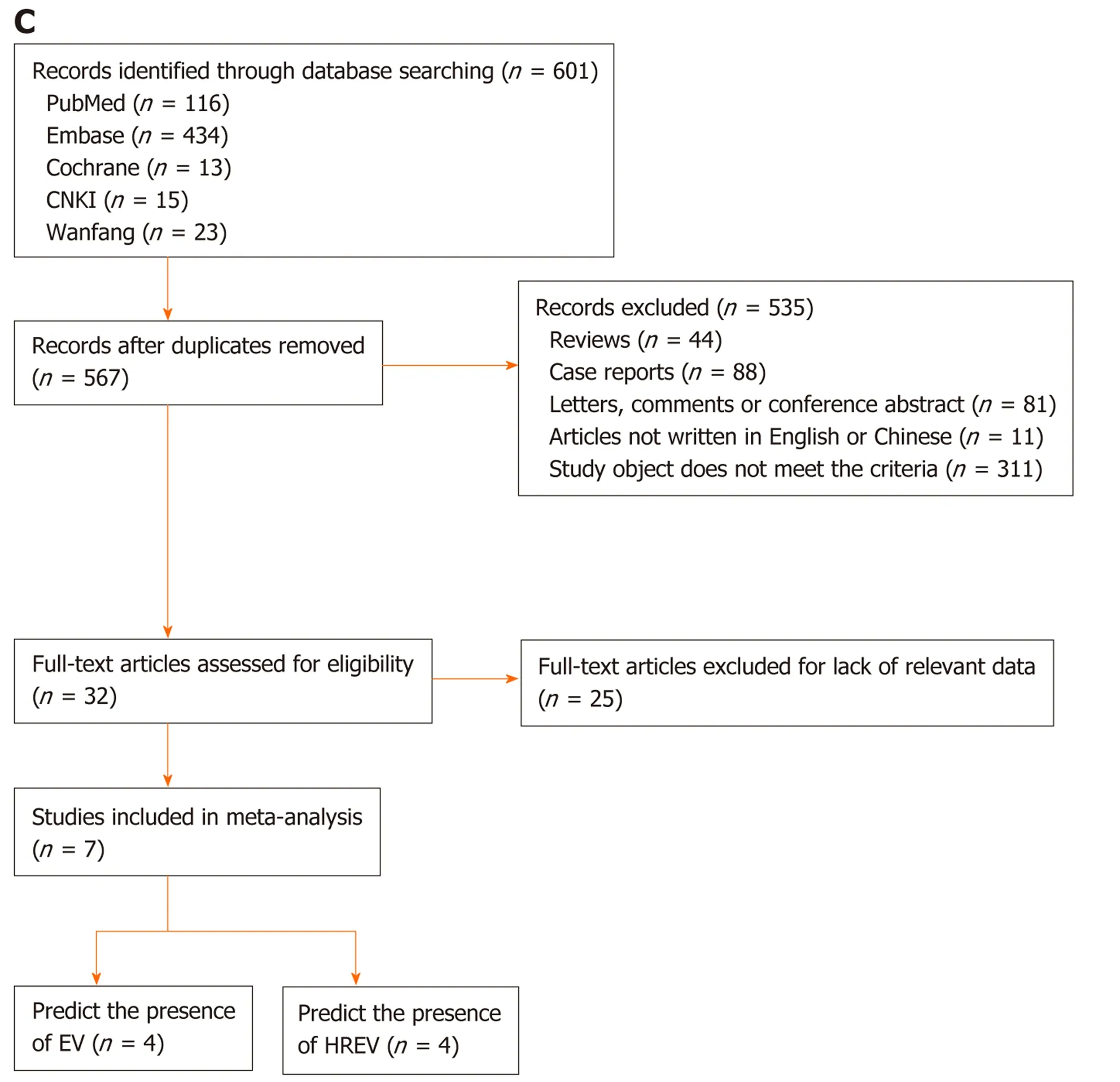
Figure 1 Flow chart of the search and selection of articles. A: Flow chart of the search and selection of articles about liver stiffness measurement; B: Flow chart of the search and selection of articles about computed tomography; C: Flow chart of the search and selection of articles about magnetic resonance imaging.
CT: The AUSROC of CT in the diagnosis of EV was 0.91 (95%CI: 0.88-0.93,I2= 97.17%;Figure 3E), with a summary sensitivity of 0.91 (95%CI: 0.87-0.94,I2= 88.46%) and specificity of 0.75 (95%CI: 0.68-0.82,I2= 80.58%; Figure 5A and B). The summary PLR,NLR, and DOR were 3.67 (95%CI: 2.73-4.94,I2= 83.81%), 0.12 (95%CI: 0.08-0.18,I2=88.94%), and 30.98 (95%CI: 16.02-59.91; Table 3), respectively.
The AUSROC of CT in the prediction of HREV was 0.94 (95%CI: 0.91-0.96,I2=98.30%; Figure 3F), with a summary sensitivity of 0.88 (95%CI: 0.82-0.92,I2= 87.06%)and specificity of 0.87 (95%CI: 0.81-0.92,I2= 93.26%; Figure 5C and D). The summary PLR, NLR, and DOR were 6.90 (95%CI: 4.54-10.49,I2= 91.04%), 0.14 (95%CI: 0.09-0.21,I2= 91.10%), and 49.99 (95%CI: 25.38-98.43; Table 3), respectively.
MRI: The AUSROC of MRI in the diagnosis of EV was 0.86 (95%CI: 0.83-0.89,I2=86.41%; Figure 3G), with a summary sensitivity of 0.81 (95%CI: 0.76-0.86,I2= 33.57%)and specificity of 0.82 (95%CI: 0.70-0.89,I2= 74.53%; Figure 6A and B). The summary PLR, NLR, and DOR were 4.44 (95%CI: 2.74-7.21,I2= 31.66%), 0.23 (95%CI: 0.18-0.28,I2< 0.01%), and 19.58 (95%CI: 11.36-33.66; Table 3), respectively.
As for the prediction of HREV by MRI, the AUSROC was 0.83 (95%CI: 0.79-0.86,I2= 91.64%; Figure 3H), with a summary sensitivity of 0.80 (95%CI: 0.72-0.86,I2=67.03%) and specificity of 0.72 (95%CI: 0.62-0.80,I2= 83.17%; Figure 6C and D). The summary PLR, NLR, and DOR were 2.83 (95%CI: 2.11-3.80,I2= 51.94%), 0.28 (95%CI:0.21-0.38,I2= 43.01%), and 10.00 (95%CI: 6.63-15.09; Table 3), respectively.
Based on this meta-analysis, CT had higher accuracy in evaluating the presence of both EV and HREV with an AUSROC of 0.91 and 0.94, respectively.
Meta-regression
Based on the above results, we further focused on CT for diagnosis of EV and prediction of HREV. We performed meta-regression for CT to examine the source of heterogeneity and found that the accuracy of CT in the diagnosis EV was affected by CT scanner (P< 0.05).
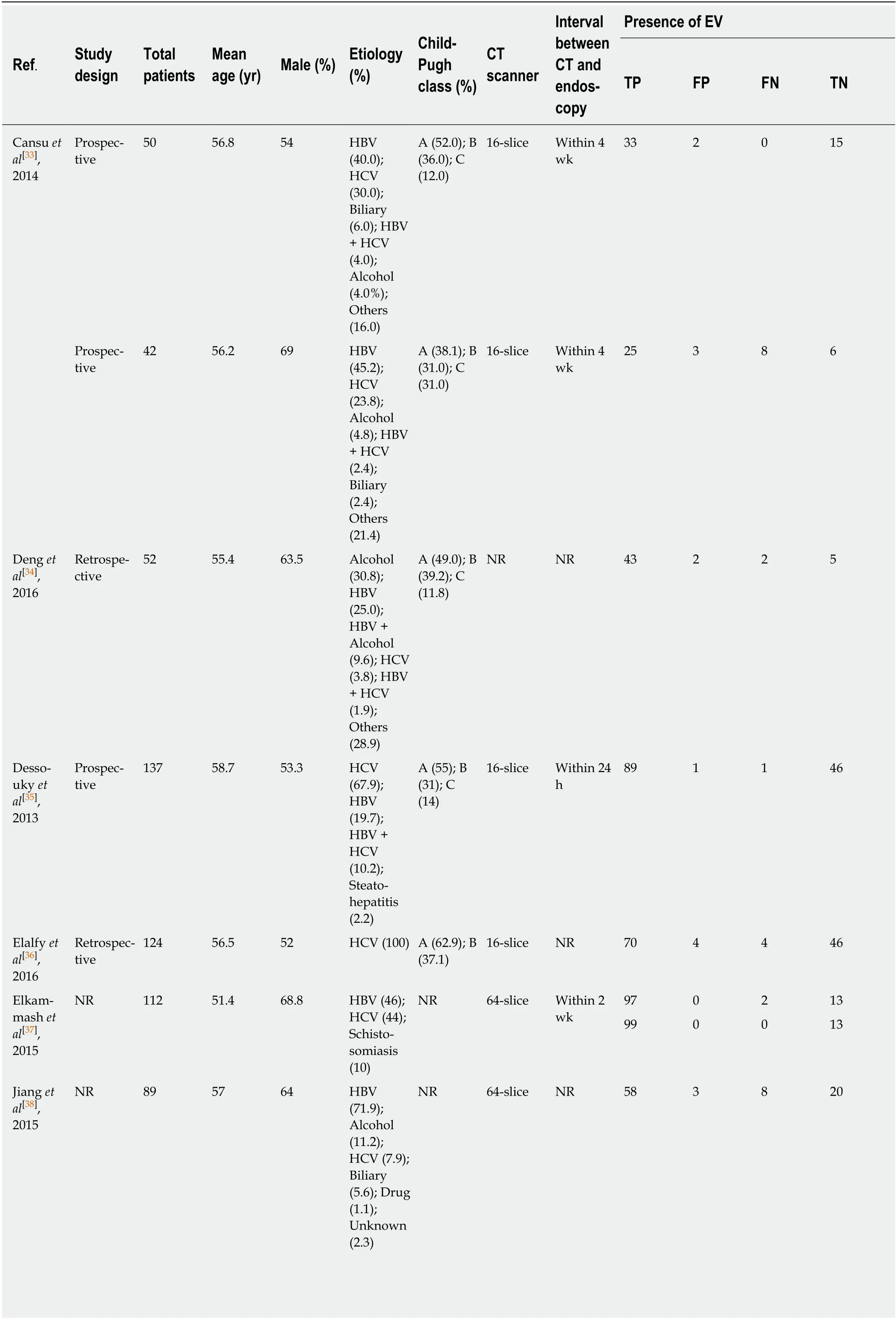
Table 1 Characteristics of articles using computed tomography imaging to diagnose esophageal varices

1Median.2Mean. NR: Not reported; HBV: Hepatitis B virus; HCV: Hepatitis C virus; NASH: Nonalcoholic steatohepatitis; CT: Computed tomography; EV:Esophageal varices; TP: True positive; FP: False positive; FN: False negative; TN: True negative.
CT subgroup analysis
64-slice scannervs16-slice scanner in diagnosis of EV: CT performed with a 64-slice scanner showed better accuracy in EV compared with imaging performed with a 16-slice scanner [AUSROC: 0.98 (95%CI: 0.97-0.99,I2< 0.01%)vs0.94 (95%CI: 0.92-0.96,I2< 0.01%); summary sensitivity: 0.98 (95%CI: 0.91-1.00,I2= 92.01%)vs0.94 (95%CI:0.88-0.97,I2= 73.98%); summary specificity: 0.94 (95%CI: 0.82-0.98,I2= 64.69%)vs0.78(95%CI: 0.65-0.87,I2= 76.48%); and summary DOR: 904.11 (95%CI: 74.85-11000)vs50.75 (95%CI: 16.21-158.911)].I2-values decreased and indicated that there was no significant heterogeneity.
16-slice scanner in prediction of HREV: Based on the diameter of EV, the AUSROC for prediction of HREV using 16-slice CT scanner was 0.96 (95%CI: 0.93-0.97,I2=40.73%). The summary sensitivity, specificity, and DOR were 0.93 (95%CI: 0.89-0.96,I2= 17.26%), 0.94 (95%CI: 0.87-0.97,I2= 78.08%), and 192.47 (95%CI: 71.03-521.49),respectively. There was no statistically significant heterogeneity.
Publication bias
According to Deeks' funnel plot, there was no evidence of significant publication bias(P >0.05).
DISCUSSION
Esophageal variceal hemorrhage is a catastrophic and fatal complication of PH with cirrhosis. The current “gold standard” for the diagnosis of EV and HREV is endoscopy in clinical practice. However, periodic endoscopy is expensive and uncomfortable, and therefore not easily accepted by most patients. The advantages of non-invasive diagnostic tools for evaluating EV and HREV are repeatability and better patient acceptance. We therefore performed a meta-analysis to compare the accuracy of evaluating EV and HREV by three non-invasive diagnostic methods: CT,MRI, and LSM.
In this meta-analysis, we identified 18, 17, and 7 articles evaluating the accuracy of LSM, CT, and MRI for diagnosing EV and predicting HREV, respectively. The analysis showed that CT had the highest accuracy for both EV and HREV. The AUSROC was 0.91 and 0.94, and DOR was 30.98 and 49.99 for evaluating the presence of EV and HREV. Baveno VI consensus recommends that patients with a liver stiffness < 20 kPa on transient elastography and with a platelet count > 150 × 109/L have a very low risk of having varices requiring treatment, and can avoid screening endoscopy. In studies that validate the criteria, up to 100% of patients who met the criteria had an ultimately negative endoscopy, but it showed a relatively low specificity of 61.5%[57]. Rosmanet al[58]investigated the utility of incorporating the CT or MR findings of portosystemic collateral vessels to predict HREV in patients who did not meet Baveno VI criteria. The presence of portosystemic collateral vessels to predict HREV yielded a sensitivity of 0.95 and specificity of 0.36 in these patients.Therefore, the use of additional portosystemic collateral vessels from CT or MRI can further help identify patients with compensatory cirrhosis who do not require endoscopy. The weakness of LSM using transient elastography is decreased applicability in obese patients and patients with ascites. Lippet al[43]evaluated the ability of CT and MRI to detect EV and found that CT is a superior imaging modality to MRI. According to a meta-analysis performed by Denget al[7], Lok score had the highest AUSROC of 0.79, followed by FIB-4, Forns, aspartate aminotransferase-toalanine aminotransferase ratio, and aspartate aminotransferase-to-platelet ratio, for the diagnosis of EV. Aspartate aminotransferase-to-alanine aminotransferase ratio had the highest AUSROC of 0.74, followed by aspartate aminotransferase-to-platelet ratio, Lok, FIB-4, and Forns scores for the prediction of HREV. A significant heterogeneity (I2ranged from 86.41% to 98.30%) was found in their meta-analysis. The CT scanner was significantly associated with heterogeneity in diagnosing EV.Subgroup analysis suggested that the accuracy of CT scanner with more slices was critical for diagnosing EV.
Compared with endoscopy, contrast-enhanced CT or MRI can clearly show the portal vein system and collateral circulation[59,60]. In addition to EV, they can be used for the diagnosis of other complications including hepatocellular carcinoma[61,62]. There is no doubt that endoscopy is irreplaceable. It can diagnose esophageal and gastricvarices as well as other lesions that cause upper gastrointestinal bleeding, such as peptic ulcer. Combined with the ultrasound probe, it was applied to probe the blood vessels around the wall of the esophagus. Zhenget al[63]evaluated endoscopic ultrasound probe examinations for the prediction of recurrence of EV after endoscopic therapies by detecting peri-esophageal collateral veins, perforating veins, and paraesophageal collateral veins. The result showed that peri-esophageal collateral veins can predict 1-year variceal recurrence with a sensitivity of 45% and specificity of 86%when using a diameter of 3.5 mm as cut-off value.
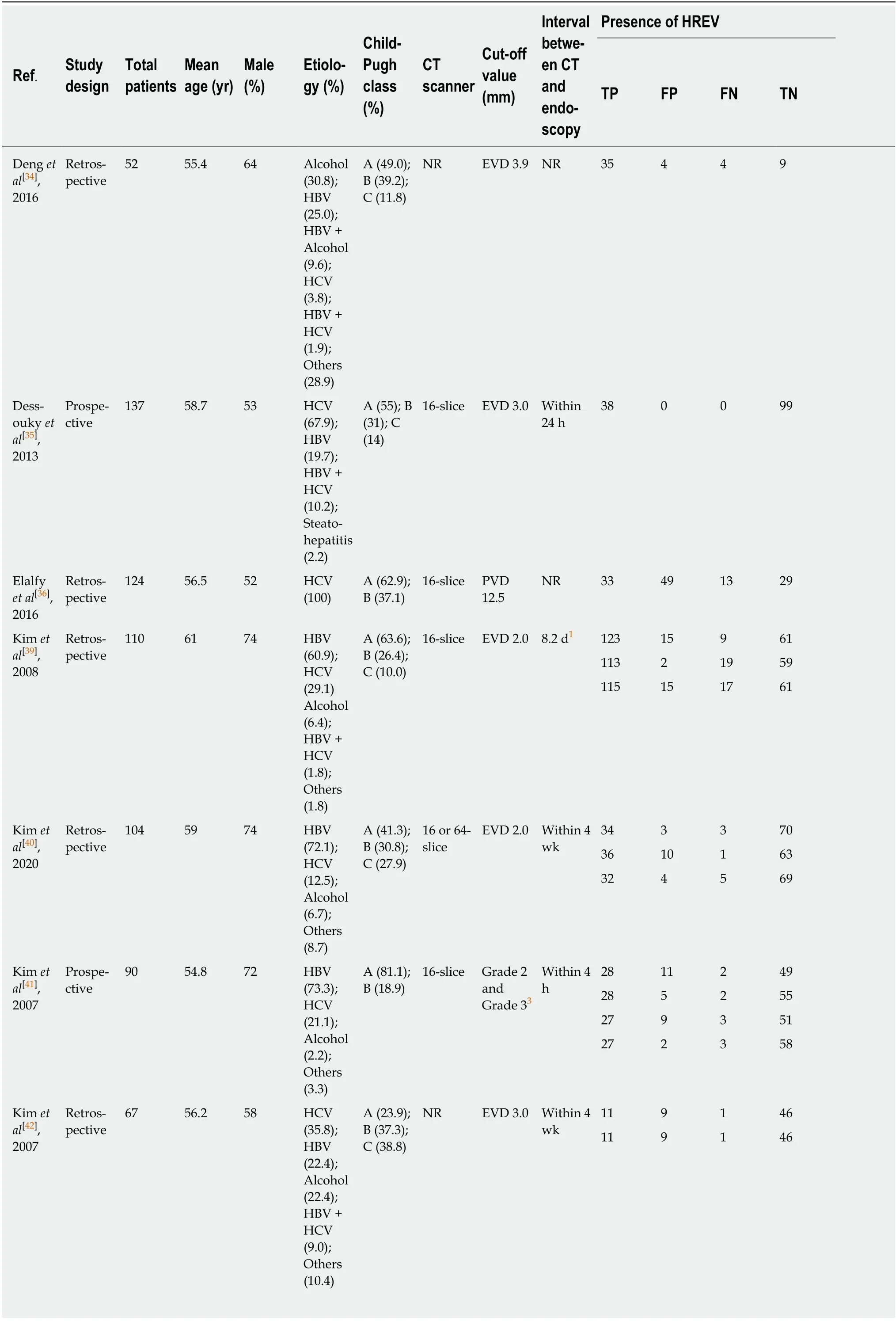
Table 2 Characteristics of articles using computed tomography imaging to predict HREV
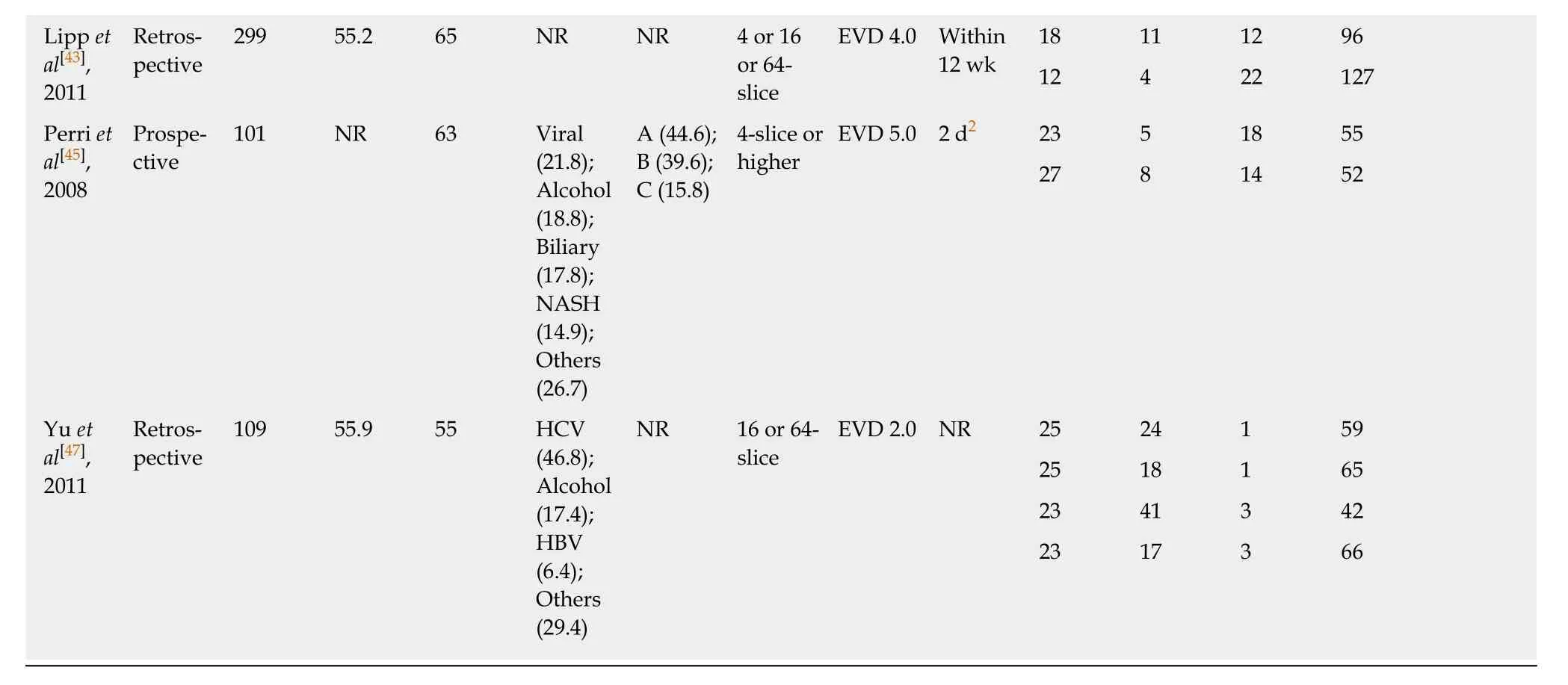
1Mean.2Median.3Grade 2: Varices show beaded appearance; Grade 3: Varices run in oblique course and are tortuous with tumorlike appearance. EVD: Esophageal varices diameter; NR: Not reported; PVD: Portal vein diameter; HBV: Hepatitis B virus; HCV: Hepatitis C virus; NASH: Nonalcoholic steatohepatitis; CT:Computed tomography; HREV: High-bleeding-risk esophageal varices; TP: True positive; FP: False positive; FN: False negative; TN: True negative.
There are several limitations of our analysis that should be taken into consideration. First, we searched the databases for articles only written in English and Chinese, which may miss some articles written in other languages. Second, though the Deek's funnel plot asymmetry test showed no evidence of significant publication bias,there are probably studies of negative outcomes which have not been published.These research results may be missed. Third, the included articles had different definitions or cut-off values of HREV. Thus, no standard diagnostic thresholds for CT,MRI, and LSM were defined. Finally, we regarded endoscopy currently as the “gold standard” for diagnosing EV and HREV, nevertheless, there was no head-to-head controlled study of the above-mentioned non-invasive diagnostic methods in the same series of patients. This indirect comparison brought to a statistical bias, thus might attribute to study heterogeneity. Despite the limitations, new analysis techniques of radiomics are likely to improve diagnostic and predictive accuracy of many diseases. Choiet al[64]developed a deep learning system for accurate staging of liver fibrosis using CT. These promising results should initiate further studies on CT using artificial intelligence and machine learning technology to reduce the need for endoscopy.
In conclusion, based on this meta-analysis, CT has higher accuracy for evaluating both EV and HREV in cirrhotic patients. However, further head-to-head comparisons of these noninvasive diagnostic tools are required to confirm the predictive value in EV and HREV, particularly in view of the future use of artificial intelligence technology.
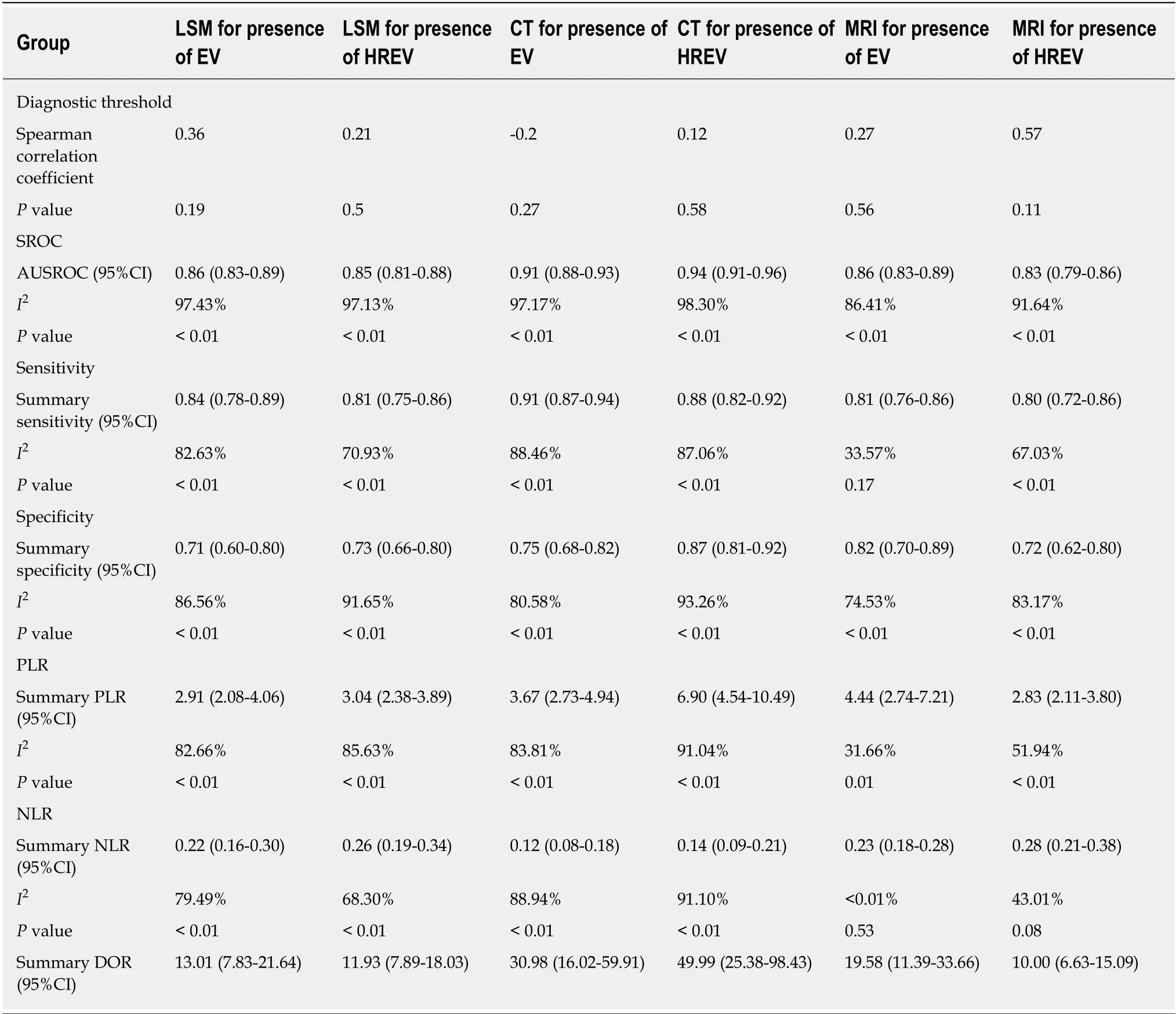
Table 3 Overview of results of meta-analysis

Figure 2 Methodological evaluation according to Quality Assessment of Diagnostic Accuracy Studies-2 of the included articles. A, C, and E: Diagnosis of esophageal varices using liver stiffness measurement, computed tomography, and magnetic resonance imaging, respectively; B, D, and F: Prediction of highbleeding-risk esophageal varices using liver stiffness measurement, computed tomography, and magnetic resonance imaging, respectively. Articles were identified as having a potential bias risk for patient selection and index text.
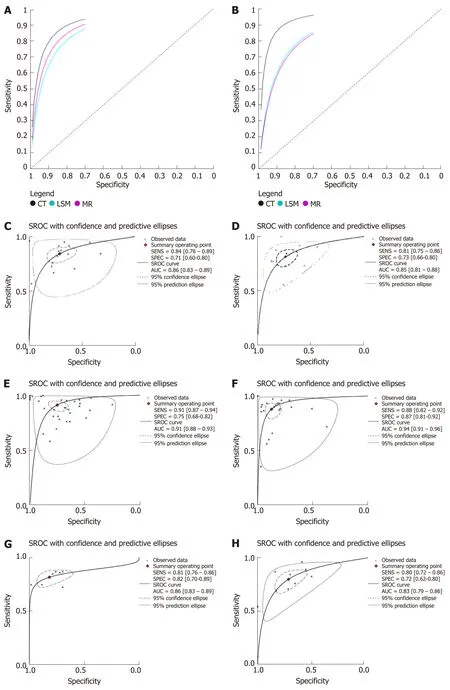
Figure 3 Summary receiver operating characteristic curves. A and B: Summary receiver operating characteristic (SROC) curves of liver stiffness measurement,computed tomography, and magnetic resonance imaging for the diagnosis of esophageal varices (EV) and prediction of high-bleeding-risk EV (HREV); C and D:SROC curves of liver stiffness measurement for the diagnosis of EV and prediction of HREV; E and F: SROC curves of computed tomography for the diagnosis of EV and prediction of HREV; G and H: SROC curves of magnetic resonance imaging for the diagnosis of EV and prediction of HREV.
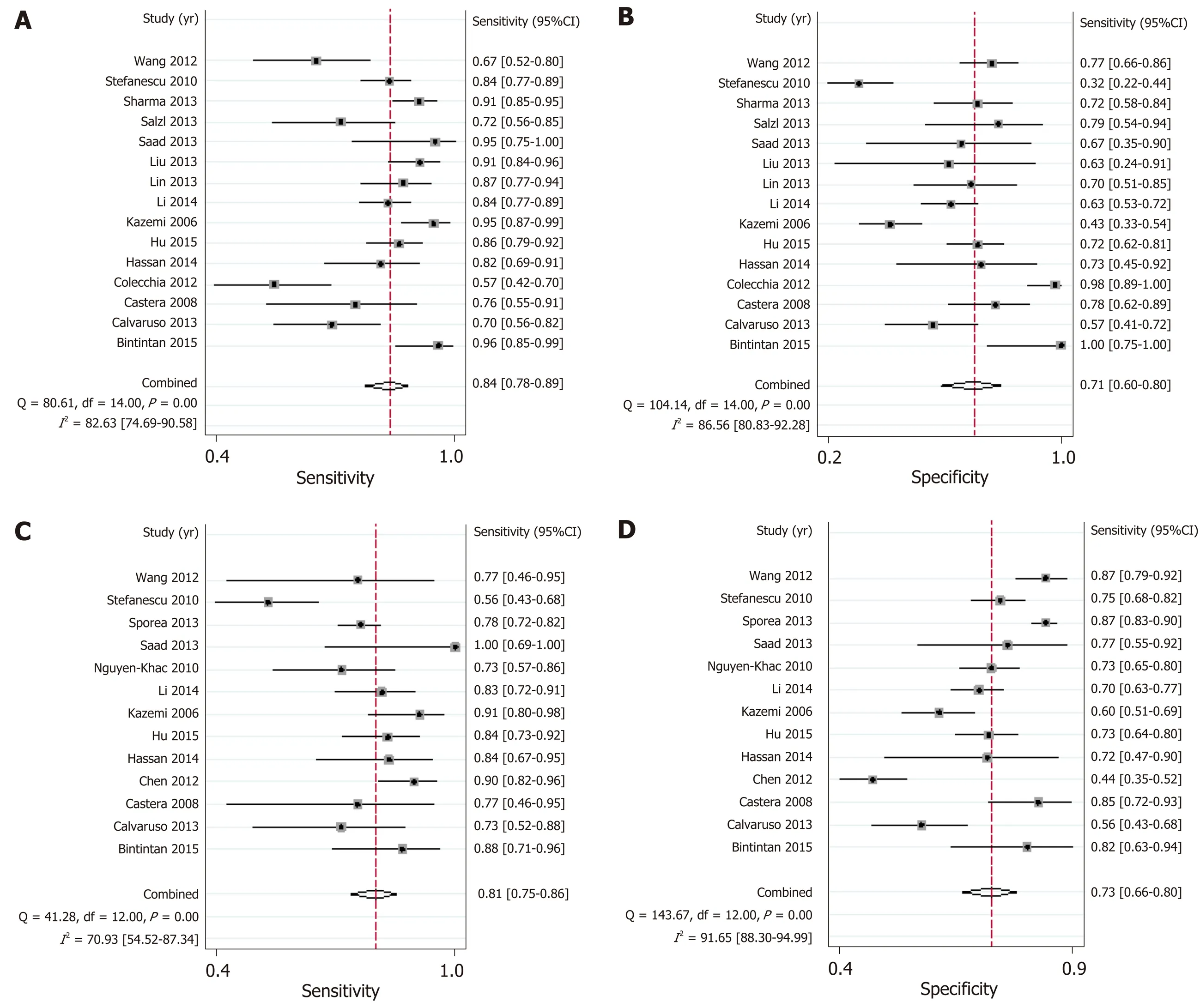
Figure 4 Summary sensitivity and specificity of liver stiffness measurement. A and B: Summary sensitivity and specificity of liver stiffness measurement for the diagnosis of esophageal varices; C and D: Summary sensitivity and specificity of liver stiffness measurement for the prediction of high-bleeding-risk esophageal varices.
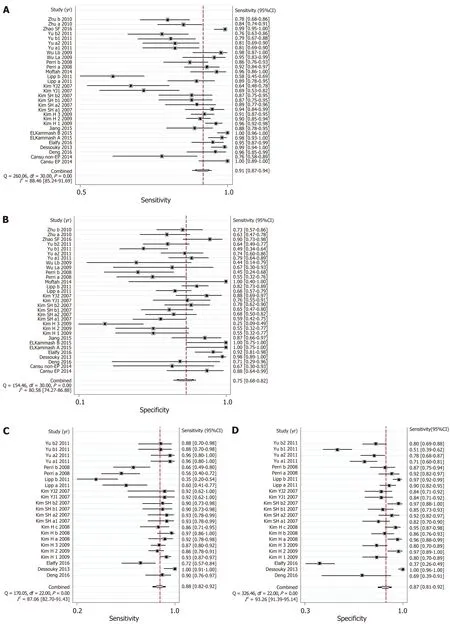
Figure 5 Summary sensitivity and specificity of computed tomography imaging. A and B: Summary sensitivity and specificity of computed tomography for the diagnosis of esophageal varices; C and D: Summary sensitivity and specificity of computed tomography imaging for the prediction of high-bleeding-risk esophageal varices.
ARTICLE HIGHLIGHTS
Research background
The non-invasive and easy-to-perform diagnostic techniques to predict complications in cirrhotic patients are required in clinical practice. Up to now, the clinical use of liver stiffness measurement (LSM), computed tomography (CT), and magnetic resonance imaging (MRI), as non-invasive diagnostic methods to diagnose esophageal varices (EV) and to predict highbleeding-risk EV (HREV) in cirrhotic patients, is controversial.
Research motivation
The LSM, CT, and MRI for the diagnosis of EV and prediction of HREV, promising non-invasive diagnostic methods to predict complications in cirrhotic patients, are required in clinical practice.However, the accuracy, sensitivity, and specificity varied in different studies. The overall accuracy, sensitivity, and specificity of LSM, CT, and MRI in the diagnosis of EV and prediction of HREV in cirrhotic patients have not stated.
Research objectives
This is a very important and interesting systematic review and meta-analysis aimed to determine the overall accuracy and sensitivity of three non-invasive methods to diagnose EV and predict the risk of bleeding in patients with liver cirrhosis.
Research methods
We performed literature searches by using selected keywords in PubMed, Embase, Cochrane,CNKI, and Wanfang databases for full-text articles published in English and Chinese. All statistical analyses were conducted using Stata12.0, MetaDisc1.4, and RevMan5.3. Summary sensitivity and specificity, positive likelihood ratio and negative likelihood ratio, diagnostic odds ratio, and the area under the summary receiver operating characteristic curves that evaluated the accuracy of LSM, CT, and MRI as candidates for diagnosing EV and predicting HREV in cirrhotic patients were analyzed. The random-effects model was used to combine effect quantity.The quality of the articles was assessed using the quality assessment of diagnostic accuracy studies-2 tool. Heterogeneity was examined byQ-statistic test andI2index, and sources of heterogeneity were explored using meta-regression and subgroup analysis. Publication bias was evaluated using Deek's funnel plot.
Research results
Overall, 18, 17, and 7 relevant articles on the accuracy of LSM, CT, and MRI in diagnosing EV and predicting HREV were retrieved. CT had higher accuracy than LSM and MRI in diagnosing EV and predicting HREV with areas under the summary receiver operating characteristic curves of 0.91 (95%CI: 0.88-0.93) and 0.94 (95%CI: 0.91-0.96), respectively. The sensitivities of LSM, CT,and MRI in diagnosing EV and predicting HREV were 0.84 (95%CI: 0.78-0.89), 0.91 (95%CI: 0.87-0.94), and 0.81 (95%CI: 0.76-0.86), and 0.81 (95%CI: 0.75-0.86), 0.88 (95%CI: 0.82-0.92), and 0.80(95%CI: 0.72-0.86), respectively. The specificities were 0.71 (95%CI: 0.60-0.80), 0.75 (95%CI: 0.68-0.82), and 0.82 (95%CI: 0.70-0.89), and 0.73 (95%CI: 0.66-0.80), 0.87 (95%CI: 0.81-0.92), and 0.72(95%CI: 0.62-0.80) , respectively. The positive likelihood ratios were 2.91, 3.67, and 4.44, and 3.04,6.90, and 2.83, respectively. The negative likelihood ratios were 0.22, 0.12, and 0.23, and 0.26,0.14, and 0.28, respectively. The diagnostic odds ratios were 13.01, 30.98, and 19.58, and 11.93,49.99, and 10.00, respectively. A significant heterogeneity was observed in all analyses (P< 0.05).CT scanner was identified to be the source of heterogeneity. There was no significant difference in diagnostic threshold effects (P> 0.05) or publication bias (P >0.05). To determine the risk for bleeding of EV using a non-invasive method might have important clinical applications in daily practice. The study gives an overall view of the problem, and for sure does give clinical details which could be useful in making decisions in everyday practice.
Research conclusions
Based on the meta-analysis of observational studies, CT has higher accuracy in evaluating EV and HREV than LSM and MRI in cirrhotic patients. It is suggested that CT, a non-invasive diagnostic method, is the best choice for the diagnosis of EV and prediction of HREV in cirrhotic patients compared with LSM and MRI.
Research perspectives
The results are very important with significant applications for clinicians in making decisions in daily practice for treatment of cirrhotic patients with portal hypertension. In future, the head-tohead or direct comparisons of these non-invasive methods in the same series of patients are required to confirm the predictive value, especially by using artificial intelligence technique.
ACKNOWLEDGEMENTS
We acknowledge the support in statistical methods review provided by Xiang-Yu Yan, PhD, Capital Medical University School of Public Health, Beijing, China.
杂志排行
World Journal of Gastroenterology的其它文章
- Folic acid attenuates high-fat diet-induced steatohepatitis via deacetylase SlRT1-dependent restoration of PPARα
- Genetic association analysis of CLEC5A and CLEC7A gene singlenucleotide polymorphisms and Crohn's disease
- Hepatitis B virus recurrence after liver transplantation: An old tale or a clear and present danger?
- Natural products that target macrophages in treating non-alcoholic steatohepatitis
- Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor:A case report
- Annexin A2 promotion of hepatocellular carcinoma tumorigenesis via the immune microenvironment
