Hepatitis B virus recurrence after liver transplantation: An old tale or a clear and present danger?
2020-06-08IlariaLenciMartinaMilanaGiuseppeGrassiTommasoManziaCarloGaziaGiuseppeTisoneRobertaAngelicoLeonardoBaiocchi
Ilaria Lenci, Martina Milana, Giuseppe Grassi, Tommaso M Manzia, Carlo Gazia, Giuseppe Tisone,Roberta Angelico, Leonardo Baiocchi
Abstract Hepatitis B virus (HBV) recurrence after liver transplantation (LT) has been described more than 50 years ago. Similarly, to other clinical conditions, in which impairment of host immune defense favors viral replication, early reports described in details recurrence and reactivation of HBV in liver transplant recipients. The evidence of a possible, severe, clinical evolution of HBV reappearance in a significant percentage of these patients, allowed to consider,for some years, HBV positivity a contraindication for LT. Moving from the old to the new millennium this picture has changed dramatically. Several studies contributed to establish efficient prophylactic protocols for HBV recurrence and with the advent of more potent anti-viral drugs an increased control of infection was achieved in transplanted patients as well as in the general immunecompetent HBV population. Success obtained in the last decade led some authors to the conclusion that HBV is now to consider just as a “mere nuisance”.However, with regard to HBV and LT, outstanding issues are still on the table: (1)A standard HBV prophylaxis protocol after transplant has not yet been clearly defined; (2) The evidence of HBV resistant strains to the most potent antiviral agents is claiming for a new generation of drugs; and (3) The possibility of prophylaxis withdrawal in some patients has been demonstrated, but reliable methods for their selection are still lacking. The evolution of LT for HBV is examined in detail in this review together with the description of the strategies adopted to prevent HBV recurrence and their pros and cons.
Key words: Liver transplant; Hepatitis B virus; Viral recurrence; Prophylaxis;Minimization; Antiviral drug
INTRODUCTION
The hepatitis B virus (HBV) is a small DNA virus belonging to theHepadnaviridaefamily[1]. Despite the adoption, in several countries, of an extended vaccination campaign starting in 1992, HBV infection still represents an important health problem with 350-400 million people infected in the world[2]. Without treatment, at least one third of patients are estimated to progress to significant liver disease, including endstage liver cirrhosis and tumors. In fact, the natural history of HBV liver disease includes a spectrum of clinical conditions ranging from a non-frequent fulminant hepatitis to HBV-related hepatocellular carcinoma and/or end-stage liver disease[3,4].While vaccination and the new antiviral drugs are effective, respectively, in avoiding HBV infection and preventing the most severe sequelae of HBV disease, liver transplantation (LT) remains the main therapeutic option in patients with more severe forms of HBV liver injury[5]. However, in the early 90's the possibility to offer LT to HBV candidates was an argument of debate. In fact, it was evident that HBV disease recurrence in the graft was severe in a significant proportion of patients[6]. Moreover,an aggressive clinical form of viral reactivation, named Fibrosing Cholestatic Hepatitis, was also described in nearly 25% of HBV transplanted patients, leading to a dramatic and rapidly progressive course[7]. Therapeutic advancement and prophylactic strategies against HBV radically changed this picture in the last three decades, allowing the consideration of HBV recurrence after LT to no longer be of concern. In this review we will describe HBV viral features, its natural history, and current outcome of HBV after LT.
NATURAL HISTORY OF HBV
HBV, a double stranded small DNA virus, replicating by reverse transcription, is able to convert its DNA in a covalently closed circular (ccc) form when reaching the hepatocyte's nucleus. cccDNA represents a mini-chromosome containing information for antigens (HBsAg, HBeAg, and HBcAg), X protein, and polymerase production[8].The infection route is mainly represented by vertical transmission in endemic areas.The estimated risk of acquiring the infection from an HBeAg+ mother is around 80%[9]. On the other hand, sexual or needle transmission are important paths in nonvaccinated adult patients of western countries[10]. Evolution of infection is dependent on the host, the viral genetics and virus/host interaction[3,11,12]. Vertical transmission at birth is associated (without peri-natal treatment) with a lifetime infection, usually with an immune-tolerant state[11]. This clinical situation is characterized by HBeAg positivity, high levels of HBV-DNA and normal liver function tests. Conversely, in adult normal subjects, immuno-tolerance usually lasts for 2-4 wk, the time span corresponding to the HBV incubation phase. Activation of the immune system against HBV determines: (1) Decreased HBV-DNA levels; (2) Increased liver inflammation;and (3) Elevation of serum levels of liver function tests. These features characterize the immune-active phase. This stage may evolve into: (1) Infection resolution with production of high titers of HBsAb (this target is reached by more than 90% of healthy adult individuals within 6 mo of initial HBV contact); (2) Fulminant hepatitis (rarely,≤ 0.5%); or (3) HBsAg persistence and evolution to chronic hepatitis[4]. During chronic hepatitis, the seroconversion to the HBeAg negative state (with development of HBeAb titer) represents an important achievement as it corresponds to decreased levels of HBV-DNA, liver inflammation and injury[13-15]. Moreover, HBeAg seroconversion with the consequent drop in HBV-DNA serum levels has been related to reduced fibrosis progression, histological staging, and onset of cirrhosis and hepatocellular carcinoma[16-18]. A subject with an acquired HBeAg negative state is usually defined as an inactive HBV carrier, referring to a remission state of the liver disease. Unfortunately, seroreversion to an HBeAg positive condition may occur over time (in approximately 20% of patients), also transiently, leading to a “de novo”immune-active inflammatory stage. Moreover, HBeAg loss (both spontaneous and drug induced) may determine selection in the host of pre-core mutants of HBV (not producing HBeAg). These strains are not affected, during their replicative phases, by anti-HBe antibodies, thus they determine progression of liver injury in approximately 10 to 30% of patients obtaining HBeAg loss[3,5,19]. The main clinical and virological features, in the different phases of HBV chronic infection, are reported in Table 1.From the above, it is evident that host-immune-system/virus interaction is a major determinant of the presence and severity of liver injury. This result is far more evident in subjects undergoing immune system changes related to biological or immunosuppressive therapies, including the majority of transplanted patients. In this setting severe reactivation of HBV is an element of concern[20].
HBV DURING IMMUNE SYSTEM SUPPRESSION OR MODIFICATION (DRUG-INDUCED IMPAIRMENT OF THE IMMUNE SYSTEM AS A RISK FACTOR FOR HBV REACTIVATION)
Reactivation of HBV is represented by sudden reappearance or increase of viral DNA in the serum of a patient with a resolved or clinically silent HBV infection[20]. This condition, which may also occur spontaneously, has been reported more frequently in patients undergoing immunosuppressive therapy for malignant or non-malignant disease[21]. In an early study in non-Hodgkin lymphoma patients under chemotherapy,HBV reactivation accounted for 72% of cases in HBsAg positive subjects[22]. More importantly, viral reappearance was also observed in HBsAb/HBcAb or only HBcAb positive subjects, thus suggesting the possibility of HBV reactivation also in conditions in which the infection was considered resolved in the past. Further studies also demonstrated HBV reappearance in non-neoplastic clinical settings such as Crohn's disease[23]or rheumatologic affections[24]. Treatment with biological agents,such as B-cell depleting (i.e., rituximab) or anti-tumor necrosis factor drugs(infliximab), carries a significant risk of HBV reactivation[25]. However, standard steroid treatment may also be responsible for HBV reappearance[26]. Evolution of viral reactivation is generally thought to occur in three separate phases[20,21]. At the beginning, a rise in HBV-DNA (at least ten-fold in comparison with baseline values) is observed during immunosuppressant treatment. In the second phase, when drugs are tapered or discontinued, the inflammatory damage begins, being triggered by the host immune defense that is also, in part, restored. In the last phase, the liver damage is repaired or may progress to end-stage liver failure. The evidence of a possible dramatic evolution of HBV reactivation in liver failure prompted the adoption of strategies to counteract this preventable occurrence. First of all, an adequate screening for HBV virus, including HBcAb, is proposed in individuals undergoing chronic therapy with immunologic modifiers. Secondly, antiviral agents able to prevent or cure this clinical condition are administered according to both viral and patient's features[27-29]. However, the clinical strategies commonly employed to prevent HBV reinfection are still lacking significant scientific evidence[27]. Therefore, the question of the best approach in different clinical scenarios remains open.
Finally, the most important clinical setting in which HBV reappearance is a relevant issue is that of transplant. Transplanted patients usually require long-term high-dose immunosuppression to prevent rejection. In HBsAg positive patients undergoing bone marrow transplantation, HBV reactivation accounts for nearly the totality of cases[30]. Even in HBsAb/HBcAb+ subjects, reappearance of active HBV is not rare,accounting for nearly 20% of cases[31]. Starting from the early eighties, HBV reactivation was reported to be very frequent in the setting of kidney and heart transplantation, and it was characterized by the insurgence of HBV chronic hepatitis[20]. Indeed, HBV reactivation or recurrence also represents an important issue in liver transplanted patients. These subjects, in fact, share the same immunosuppressive need as other transplanted patients but, at the same time, are suffering the most important sequelae of HBV before surgery. This setting probably represents the most important clinical scenario in which dramatic HBV resurgence was observed
Phase 1: lmmune tolerant Phase 2: lmmune active Phase 3: Asymptomatic carrier Phase 4: HBV reactivationand prophylactic measures were firstly pursued. At the same time, LT was the setting in which the risk of transplant with anti-core-HBV positive liver graft was identified.
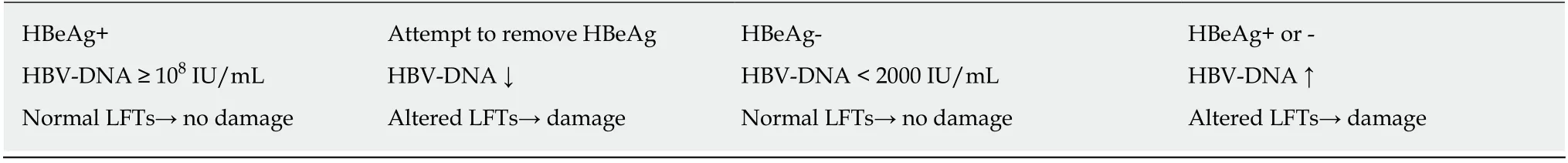
Table 1 Main virological and biochemical features in the different clinical phases of chronic hepatitis B in HBsAg+ patients
HBV RECURRENCE IN THE EARLY TIMES OF LIVER TRANSPLANTATION (THE PURSUIT OF AN EFFECTIVE PROPHYLACTIC STRATEGY)
HBV recurrence/reactivation after LT was already recognized almost 50 years ago[32].In the early 90's the feasibility of LT in HBV patients remained a crucial question since several reports observed viral recurrence in nearly all transplanted subjects, with an aggressive course in the larger part of them. While graft replacement was able to transiently reduce viral load, viral resurgence in the course of immunosuppression was related to significant liver damage and cirrhosis development[6,33]. So, at that time,LT in HBsAg positive patients was considered a high risk procedure for graft and patient loss, with an unacceptable hazard in particular in HBeAg+ subjects[34]. The disappointing results, and the need to pursue a solution for HBV patients with endstage liver disease, stimulated the research for a possible prophylactic therapy after LT. In a pioneering study conducted at Paul Brousse Hospital (Villejuif, France) in the eighties, an extended passive immune-prophylaxis was tested in HBsAg positive patients after LT[35]. Despite the monthly HBsAb immunoglobulin (HBIG)administration, 29% of patients experienced HBsAg and HBV-DNA reappearance in serum, however these data demonstrated the possibility to reduce HBV recurrence after LT. In a further European retrospective study on 372 HBV liver transplanted patients (between 1977 and 1990), a reduced rate of HBV reactivation was statistically associated with the absence of HBV-DNA before transplant and again to long-term passive immune prophylaxis with HBIG[36]. The exact mechanisms of the beneficial effects of immunoglobulin in this setting are not completely clear at present. Both reduced deletion of infected hepatocytes and prevention of viral aggression of liver cells have been suggested as possible effects[37]. Starting from the mid 90's, evidence was gathered on the role of lamivudine (Lam) treatment in repressing HBV replication[38,39]. Since, at that time, only HBIG-based prophylaxis was available after LT, and this therapy was a life-long, suboptimal, expensive treatment, the evaluation of the Lam effect in this clinical setting began. In an English study, 17 HBsAg positive patients were enrolled to receive Lam 4 wk before liver transplant and to continue 1 year thereafter[40]. Twelve out of seventeen patients were transplanted. In them, Lam induced a loss of HBsAg and undetectable HBV-DNA serum levels within 4 wk of treatment and after transplant. Moreover, liver histology did not show features suggesting HBV recurrence after LT, and these results were obtained without concomitant HBIG immune prophylaxis. Unfortunately, in the same study, selection of a resistant strain to Lam was observed in one patient after 20 wk of treatment. This occurrence was characterized by reactivation of HBV and evidence of chronic hepatitis on liver tissue after 1 year. Similar to that observed in HIV therapy[41], HBV strains not-responding to Lam were characterized by mutation of polymerase at the highly conserved YMDD motif[42-44]. With regard to liver transplanted HBV patients,extended follow up of Lam resistant patients was lately reported. Resistance to Lam began to occur, typically, six months after its introduction and was sometimes characterized by severe disease recurrence[45,46]. A combination of Lam therapy with HBIG was then attempted in order to further reduce HBV recurrence after LT. In a study, fourteen HBsAg positive LT patients were treated with Lam plus HBIG[47]. In a median follow-up of one year, all patients were HBV-DNA negative in serum, thus demonstrating the superiority of combination therapy in comparison with monotherapy with either Lam or HBIG. These data were also confirmed in a study with an extended (average 31 mo) follow-up[48]. Thus, the past millennium ended with the positive perspective that prevention of HBV recurrence/reactivation in HBsAg transplanted subjects was feasible. On the basis of these results, the possible exclusion of HBV subjects from transplant lists was largely reexamined.
HBV RECURRENCE/REACTIVATION AFTER LIVER TRANSPLANTATION IN THE THIRD MILLENNIUM (TESTING NEW THERAPEUTIC APPROACHES AND DRUGS)
The efficacy of passive immunization, in association with Lam, was again demonstrated in retrospective studies after the year 2000[49,50]. However, since this strategy was flawed by the relevant cost of HBIG and the need of life-long administration, the possibility to induce active immunization in HBV liver transplanted patients was examined.
HBV vaccination
In a study on 17 HBsAg+, HBeAg and HBV-DNA negative liver transplanted patients(after at least 18 mo of HBIG treatment), the double dose administration of HBV vaccine at baseline, 1 and 6 mo was tested[51]. After vaccination 84% of patients developed an HBsAb titer. During a further follow-up of 14 mo, HBsAg reappearance was not observed. These positive results were not replicated in a following study in which three reinforced and sequential cycles of HBV vaccination determined only a 17.6% HBsAb seroconversion in HBV transplanted patients[52]. In an editorial in the same journal, the limits of this strategy in transplanted patients were discussed,underscoring the scarce vaccine efficacy during immunosuppression and the long time needed to reconstitute the immune system after its depression[53]. In conclusion, it was confirmed that HBIG and antiviral therapy were regarded as the most appropriate measures against HBV recurrence after LT[54].
Adefovir dipivoxil
With regard to antiviral agents, in those years, a new drug implemented the armamentarium for the therapy of HBV. Adefovir dipivoxil (ADV), a nucleotide analog inhibiting viral reverse transcriptase that was abandoned for treatment of HIV because of kidney damage when used at high dose, was licensed for HBV treatment since it was active at lower, non-toxic levels for this virus (10 mg/d). ADV treatment in the majority of immune-competent HBsAg patients (both HBeAg positive or negative) determined a clear reduction of HBV-DNA, improvement of liver histology,and normalization of liver enzymes after a 48 wk course[55,56]. Moreover, emergence of ADV resistant mutants was not observed during these trials. Despite the fact that possible long-term viral resistance to ADV remained to be assessed, the efficacy of this new antiviral drug allowed hope for a new era in which HBV could be regarded as just a “mere nuisance”[57]. Soon, ADV was employed for the treatment of Lam resistant HBV after transplant[58]. Again, a significant improvement of liver function was recorded in nearly 90% of patients, and no resistant HBV strains were selected after 48 wk of therapy. However, ADV viral resistance was then observed with prolonged follow-up[59,60]. This was characterized by a novelN236Tmutation of HBV polymerase.In spite of this, the clinical evolution in patients was not worrisome since these ADV resistant strains were easily suppressed by Lam concomitant therapy. On the base of these findings, a possible Lam + ADV concomitant treatment for HBV was suggested[61]. Data from a systematic review including 2162 HBV LT patients[62]identified the following as possible risk factors for HBV recurrence: (1) Being HBVDNA positive at transplant (8.5%vs4%); (2) Administration of low dose HBIG in the first week after LT (6.1%vs3.5%); and (3) Combination therapy with HBIG + LamvsHBIG + ADV (6.1%vs2%). This picture was destined to undergo further changes with the advent of new nucleos(t)ide analogues with high genetic barriers.
New high genetic barrier nucleos(t)ide analogues.
Starting from 2012, entecavir (ETV) and tenofovir dipivoxyl (TDF) were proposed by several guidelines as a first line of treatment for chronic HBV hepatitis[5,63]. In fact, both drugs were demonstrated to be very effective in clinical studies, to have an excellent safety profile, and to be affected by a minimal or absent emergence of resistant HBV strains[64-68]. In a systematic review[69]on nucleos(t)ide analogues for HBV prophylaxis after LT, the comparison between Lam + HBIGvsthe association of ETV or TDF with HBIG demonstrated the superiority of the latter treatments (HBV recurrence rate 6.1%vs1%,P< 0.001). Moreover, in the same analysis, preliminary data evidenced slightly better results with either ETV or TDF monotherapy (after HBIG discontinuation) in comparison with the canonical Lam + HBIG prophylaxis (HBV recurrence rate 3.9%vs6.1%, difference not statistically significant). These findings introduced the concept of a possible minimization of HBV prophylaxis after LT, stimulating research with this target.
TOWARD HBV PROPHYLAXIS MINIMIZATION AFTER LT
Several strategies have been designed to minimize HBV prophylaxis after LT. The most relevant are described in the following subparagraphs with the corresponding results. Main studies on this issue are also summarized in Table 2.
HBIG dose reduction
Since long-term administration of HBIG was a critical point for its high cost, that would easily reach $100.000/pts/year[70], several attempts were carried out to reduce HBIG administration and acceptable results obtained. In a 2004 study conducted in our Unit (Liver Transplant Center, University of Rome Tor Vergata), we evaluated the possibility to prevent HBV recurrence after LT by administering HBIG on demand(when HBsAb serum levels were ≤ 70 IU/L) instead of the standard monthly administration[71]. Moreover, in the same study, two different HBIG doses (5000 IU or 2000 IU) were employed. In eleven HBV patients, at low risk for reactivation (HBsAg,HBV-DNA negative) and under concomitant Lam therapy, this strategy did not determine any HBV reactivation for 1 year follow up. On the other hand, the treatment based on administration of 2000 IU HBIG on demand reduced the cost of passive immune-prophylaxis by more than 50%. In 2007, the Australasian Liver Transplant Study Group assessed the association of very-low HBIG doses (400-800 IU)+ Lam on HBV recurrence after LT[72]. This strategy accounted for a modest HBV recurrence risk of 4% in 5 years, and the results were considered highly satisfactory since the majority of patients (85%) were HBV-DNA positive at transplant.
High-genetic barrier nucleos(t)ide analogues monotherapy
The advent of high-genetic barrier nucleos(t)ide analogues ETV and TDF, allowed speculation on a possible prophylaxis without HBIG. ETV monotherapy, tested on 80 patients undergoing LT for HBV, was able to suppress HBV-DNA (under the lower detection limit) in nearly 99% of cases after 24 mo[73]. Extended follow up of this study(8 years) demonstrated a 92% loss of HBsAg, while HBV-DNA was undetectable in all[74]. On the other hand, discontinuation of HBIG in transplanted patients treated with TDF + HBIG did not change any viral or patient profile in a 72 wk follow-up[75].Good results with either ETV and TDF were also replicated in other studies[76,77]. In a 5-year follow up in patients discontinuing HBIG and commencing either ETV or TDF after LT, HBsAg+ seroconversion occurred in 8% of cases, while HBV-DNA reappearance was not observed[78]. On the basis of these results, the most authoritative guidelines in the field now contemplate ETV or TDF monotherapy as an efficient prophylactic measure in subjects at low risk of HBV recurrence after LT[78,79].
Complete withdrawal of HBV prophylaxis
In the past years, our group examined a more radical approach to HBV prophylaxis minimization. This was characterized by the complete withdrawal of antiviral drugs in well selected HBV transplanted patients. We started with the assumption that reappearance of HBV after transplantation was dependent on the presence of cccDNA in the graft. Contrary to a North American study, (including several HBeAg/HBVDNA+ patients at LT) in which total HBV-DNA and cccDNA were detected in liver tissue in 83% and 18% of cases, respectively[80], in a preliminary evaluation of HBsAg patients transplanted in our center, only 1 out of 44 was found to be positive for cccDNA[81]. Among those that were negative for liver ccc-DNA, 30 were selected and underwent sequential withdrawal of HBIG and Lam. The majority of patients (83%,n= 25) did not experience any HBV recurrence in a median follow up longer than 2 years. Five patients came back to an HBsAg positive status. Prompt resumption of HBV prophylaxis allowed infection control, avoiding any significant clinical impairment[82]. From this study, we concluded that complete withdrawal of HBV prophylaxis after LT was feasible in patients with negative serum HBV-DNA and tissue cccDNA at transplant. An editorial, in the same journal, wisely observed that the time had come for an individualized prophylaxis in HBV transplanted patients[83].In fact, recurrence of HBV was mainly reported in patients who were HBV-DNA positive at transplant (> 100.000 copies/mL) and/or HBeAg+[36,72,84]. On the other hand, those not falling in the above category were considered at low risk for HBV recurrence. In this perspective, the target of HBV-DNA negativity was to be pursued before transplant in order to perhaps minimize prophylaxis after grafting. Conversely,for high-risk patients (HBeAg, HBV-DNA positive), more robust antiviral protocols were to be considered.

Table 2 Main studies examining prophylaxis minimization in hepatitis B virus liver transplanted patients, employing different approaches
More recently, data on longer (6-year) follow up of this original cohort were published by our group[85]. Only 3 patients needed prophylaxis resumption (10%). Of the whole cohort, 93% remained HBsAg negative and 100% had undetectable HBVDNA. More interestingly, 60% of patients spontaneously developed an HBsAb titer >10 IU/L. This was probably related to the minimization or withdrawal of antirejection therapy that is routinely pursued in our center in patients transplanted for several years. Comment on this study appeared in a new editorial[86]. While these data were encouraging, it underscored that limits remained in the identification of low risk patients. cccDNA techniques, in fact, needed to be standardized to be widely and consistently applicable in clinical settings, but on the other hand, extra-hepatic HBV reservoirs were still a possible issue of concern.
CONCLUSION
Several important achievements were obtained in the last fifty years with regard to HBV liver transplanted patients. The original exclusion of these subjects from LT waiting lists, due to poor outcome, was counteracted by the adoption of effective measures to prevent HBV recurrence. At present, high genetic barrier anti-viral drugs are giving an important contribution in transplanted patients, as well as in the HBV immune-competent population. Recently, tenofovir alafenamide, a TDF analog with improved renal safety and increased ability to reduce alanine aminotransferase, was employed in LT patients with good results[87]. However, of concern, HBV mutants with resistance to TDF (the drug with the highest genetic barrier) were recently identified, underscoring the need of a new generation of HBV agents to be employed,at least, as a rescue therapy[88]. The future of LT for HBV is not completely predictable at this stage. It will, however, depend on the global burden of HBV and the possible discovery of HBV eradicating drugs.
杂志排行
World Journal of Gastroenterology的其它文章
- Folic acid attenuates high-fat diet-induced steatohepatitis via deacetylase SlRT1-dependent restoration of PPARα
- Genetic association analysis of CLEC5A and CLEC7A gene singlenucleotide polymorphisms and Crohn's disease
- Natural products that target macrophages in treating non-alcoholic steatohepatitis
- Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor:A case report
- Annexin A2 promotion of hepatocellular carcinoma tumorigenesis via the immune microenvironment
- Computed tomography vs liver stiffness measurement and magnetic resonance imaging in evaluating esophageal varices in cirrhotic patients: A systematic review and meta-analysis
