马铃薯糖转运蛋白系统进化关系分析和顺式调控元件鉴定
2020-06-01陈军周平王朝海陆燚梁振娟王宗明吴显李晓川
陈军 周平 王朝海 陆燚 梁振娟 王宗明 吴显 李晓川
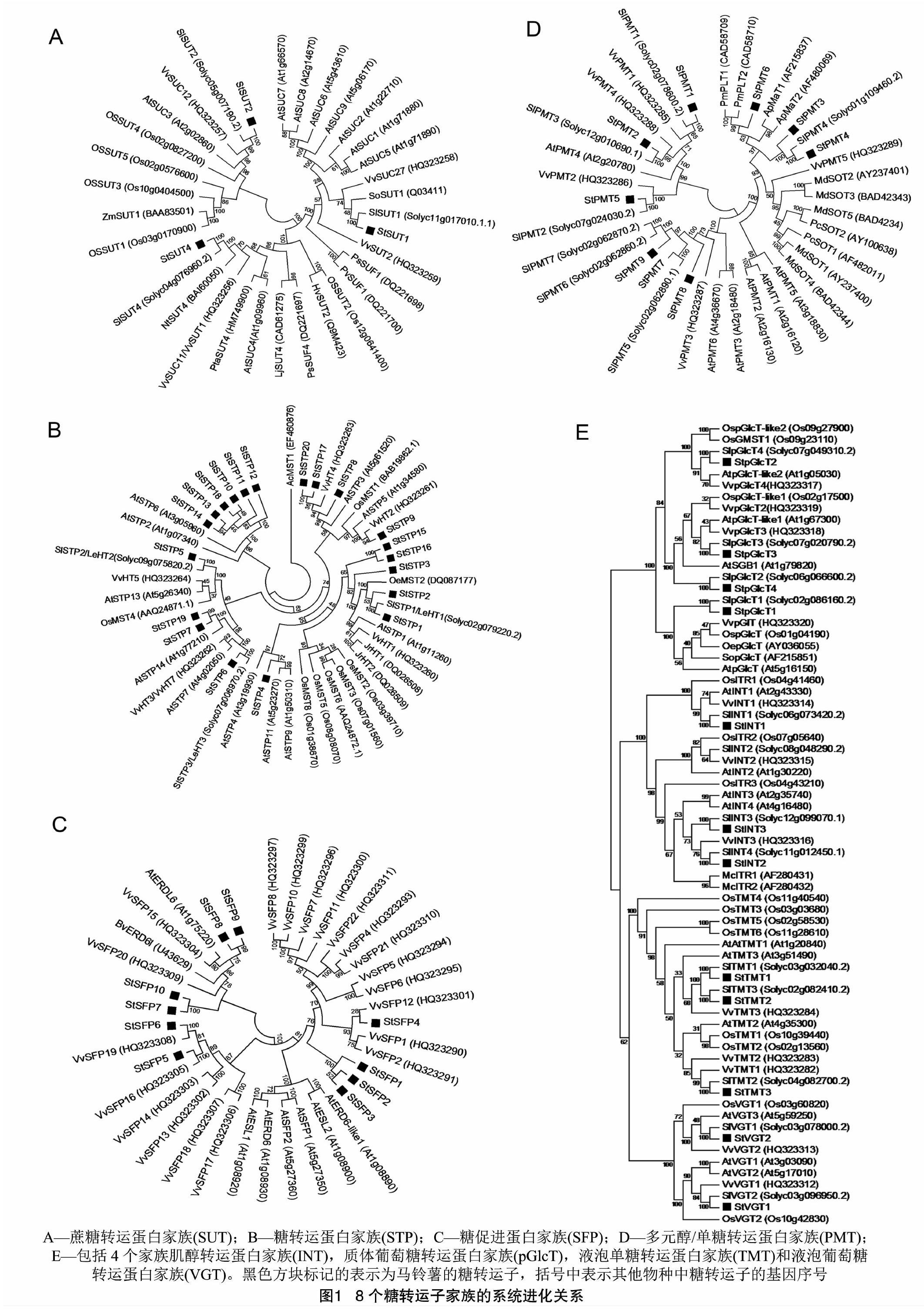
摘要:利用Clustal和MEGA 6程序进行序列分析,建立了54个马铃薯糖转运子之间以及它们与其他物种中的同源蛋白的进化关系。利用PLACE程序鉴定了42个糖转运子的顺式调控元件。此研究结果有利于对马铃薯糖转运子加深理解,从而挑选出提高马铃薯经济性的位点。
关键词:马铃薯;糖转运子;基因组;系统进化关系;顺式调控元件
中图分类号: S532.01文献标志码: A
文章编号:1002-1302(2020)08-0056-07
收稿日期:2019-03-25
基金项目:贵州省科技计划(编号:黔科合基础[2019]1002)、黔科合基础[2016]1003);现代农业产业技术体系建设专项(编号:CARS-10-ES23)。
作者简介:陈 军(1971—),男,研究实习员,研究方向为马铃薯遗传育种。E-mail:jevenlee111@aliyun.com。
通信作者:李晓川,博士,研究实习员,研究方向为马铃薯遗传育种。E-mail:475383510@qq.com。
在先前的研究中,通过序列比对在马铃薯基因组中共鉴定出了54个糖转运蛋白基因[1],它们分别归属于8個基因家族,包括蔗糖转运蛋白(SUC或SUT)家族、糖转运蛋白(STP)家族、糖促进蛋白(SFP)家族、多元醇/单糖转运蛋白(PMT)家族、肌醇转运蛋白(INT)家族、质体葡萄糖转运蛋白(pGlcT)家族、液泡单糖转运蛋白(TMT)家族、液泡葡萄糖转运蛋白(VGT)家族。在本研究中,使用系统进化分析的方法,在每个家族内,将马铃薯与其他植物的糖转运子进行了对比进化关系的分析。同时,为了研究调控糖转运子基因的信号传导,也调查了它们的启动子序列,分析了位于启动子中的顺式调控元件。
1 材料与方法
1.1 糖转运子基因的鉴定
鉴定的糖转运子基因,使用在地址https://blast.ncbi.nlm.nih.gov/Blast.cgi的BLASTp工具,以每个家族中马铃薯糖转运子的蛋白序列作为查询序列,鉴定出各作物物种的糖转运子基因[2]。
1.2 多序列比对及系统进化分析
将鉴定出来的糖转运子的氨基酸序列应用Clustal Omega在线服务器(http://www.ebi.ac.uk/Tools/msa/clustalo/)进行多序列比对[3]。进化树利用MEGA 6软件的Neighbor-Joining参数,经过计算1 000次[4]。根据系统进化分析将糖转运子基因划分为不同的家族。
1.3 启动子序列分析
从马铃薯基因组中截取54个糖转运子基因上游2 kb的基因启动子序列,并利用植物启动子顺式调控元件数据库(PLACE:http://www.dna.affrc.go.jp/PLACE/index.html),搜索糖转运子基因启动子序列上的顺式调控元件。
2 结果与分析
2.1 马铃薯糖转运子成员在其他植物中同源蛋白的系统进化分析
2.1.1 蔗糖转运家族
3个马铃薯蔗糖转运蛋白特性都被较详细地鉴定,它们定位于筛细胞的细胞膜[5-8](图1-A)。SUT家庭成员确定为蔗糖和H+的协同转运子[9-11]。但特性各不相同,StSUT1对蔗糖有高亲和力,但转运力较弱,StSUT4对蔗糖有低亲和力,但转运力强,同时StSUT4受光周期调控,而SUT2位于胞浆区的环状结构是糖的传感器,受糖的诱导[5,12-13]。同时,三者有形成聚合体的能力,三者可能聚合形成的寡聚体具有执行蔗糖运输的功能[8]。
2.1.2 糖转运蛋白家族
除了来源于拟南芥中的STP外,一些茄科植物中的STP也被鉴定[14]。番茄STP命名为LeHT1~LeHT3或SlSTP1~SlSTP3。SlSTP1和SlSTP2在酵母中是能量依赖性的葡萄糖转运蛋白[15-16]。5个马铃薯的STPs(StSTP1、StSTP2、StSTP3、StSTP15和StSTP16)与SlSTP1属同一分支。它们与AtSTP1和VvHT1是在2个亲缘关系很近的单独分支(图1-B)。AtSTP1是在酵母中显示葡萄糖转运活性[17]。基于基因表达分析和原位杂交试验的基础上,VvHT1被认为在早期葡萄果实发育中运输己糖[18-19],而StSTP1在叶子和匍匐茎中表达量较高。
StSTP5同番茄、拟南芥、水稻和葡萄唯一的STP存在于一个单独的分支。在这个分支中,VvHT5可能将碳水化合物供给到生物胁迫下的组织[20]。同样,AtSTP13表达被认为在真菌感染中与细胞程序性死亡是相关的[21]。当AtSTP13过表达时还影响植物的生长和氮含量[22]。而StSTP5在生物胁迫中表达水平显著提高,暗示了它在其中的功能。
StSTP6、StSTP7和StSTP19同在酵母中没有表现出糖运输活动的SlSTP3、AtSTP7和VvHT3在同一分支[14,23-24]。蛋白质StSTP8、StSTP17和StSTP20与VvHT4和AtSTP3形成一个单独的分支。VvHT4在酵母中表达时表现出己糖转运活性[23]。AtSTP3是低亲和力葡萄糖转运蛋白,可能还有其他己糖作为底物[25]。另一个分支是由StSTP9与OsMST1、AtSTP5和VvHT2组成。在酵母表达OsMST1和AtSTP5未见葡萄糖转运活性[26]。StSTP4同AtSTP4、AtSTP9和AtSTP11一起。在这个分支中,AtSTP9和AtSTP11分别在拟南芥花粉中显示己糖转运活性[27-28]。其他拟南芥花粉特异性STP(AtSTP2和AtSTP6)与StSTP10-StSTP14以及StSTP18聚集在一起[29-30]。StSTP4、StSTP10、StSTP11以及StSTP12在RNA-seq数据库中都在雄蕊中显示较高水平的表达。
2.1.3 糖促进蛋白家族
SFP家族的创始成员(AtERD6)在脱水和冷应激过程中表达,但缺乏转运活性的证据[31]。而StSFP6在热胁迫下的相对表达水平有10倍的提高,提示StSFP6可能参与植物抗逆过程。在拟南芥中,AtSFP1和AtSFP2也没有作为转运蛋白活性的证据[32]。最近,研究ERD6-like家族的另一名成员AtESL1,表明ERD6-like家族成员能促进葡萄糖和一系列其他己糖的扩散[33]。然而,没有马铃薯的SFP蛋白(也没有葡萄蛋白及番茄SFP蛋白)与这些拟南芥的SFP聚集在同一分支,暗示这些SFP在这些物种具有不同的功能(图 1-C)。
2.1.4 多元醇/单糖转运蛋白
在拟南芥中,找到了6个PMT转运蛋白。AtPMT5在各种组织转运非特定的多元醇、己糖和戊糖转运(图1-D)[34-35]。AtPMT1和AtPMT2转运木糖醇和果糖并在发育中的木质部和花粉表达[36]。2种定位于韧皮部细胞膜的芹菜PMT蛋白被确定为甘露醇和H+的协同转运子,StPMT3和StPMT4与这两者相近[37-38]。PcSOT1和PcSOT2发现在桃樱(酸樱桃)的储存组织转运山梨醇[39]。在苹果源叶的韧皮部,检测到3种PMT(MdSOT3-MdSOT5)的表达[40]。在大车前(车前草)的韧皮部有2种PMT表达StPMT6与这2种PMT在同一分支[41]。
2.1.5 其他糖转运蛋白家族
肌醇转运蛋白是在冰叶日中花的单倍体中首次表征出来的[42]。随后从拟南芥中鉴定出了3种INT基因。AtINT1编码myo-肌醇转运蛋白并定位到液泡膜[43],而AtINT2和AtINT4则定位到细胞膜[44-45]。
在植物中,质体定位的pGlcT首先在菠菜中被发现,可能的作用是在夜间从叶绿体输出淀粉分解产物[46]。最近也利用拟南芥基因敲除突变体AtpGlcT获得了类似的结果[47]。可以推测,降解的淀粉有利于果实含糖量。从质体导出淀粉分解产物的能力让pGlcT蛋白成为进一步研究果实糖分积累的潜在蛋白[48]。在橄榄树中,1个pGlcT型蛋白在果实发育期间表达[49]。马铃薯StpGlcT1发现与AtpGlcT及OepGlcT在同一分支,而StpGlcT1在成熟的果实中有高水平的表达。StpGlcT2被发现与OsGMST1序列相似度高,OsGMST1的敲除导致对高盐条件的耐受性降低和略有减少葡萄糖和果糖的含量。在水稻中,OsGMST1被证明定位在高尔基体[50]。而AtSGB1作为G蛋白BETA1的抑制子与StpGlcT4有更高的相似性[51]。
到目前为止,已经从拟南芥和水稻中鉴定出TMT。在拟南芥中,AtTMT1和AtTMT2定位在液泡膜,表现出葡萄糖或果糖/H+逆向运输蛋白的活性,将糖输入到液泡[52-54]。类似的结果也在水稻OsTMT1和OsTMT2中获得[55]。但拟南芥TMT能在转录水平对环境刺激作出反应,如冷胁迫,而水稻TMT则没有。
在拟南芥中只有一个VGT经过鉴定[56]。在酵母和拟南芥原生质体中,AtVGT1定位于液泡膜中证明具有向液泡中输入葡萄糖的功能,并且在ATP供能下小程度地输入果糖分到液泡中。
2.2 糖转运子基因转录调控的顺式作用元件
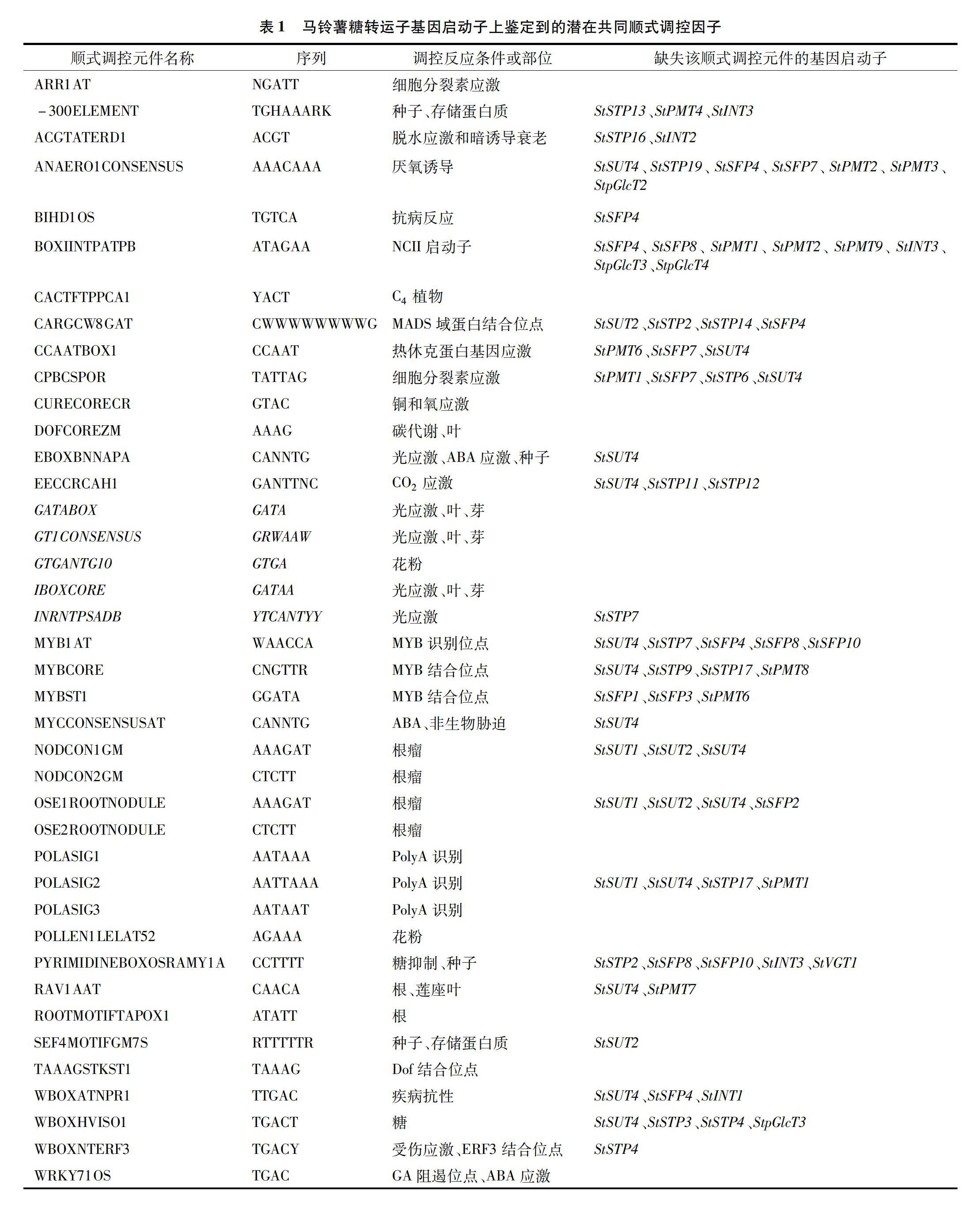
从马铃薯基因组中截取54个糖转运子基因上游2 kb的基因启动子序列,但由于其他基因的ORF出现在糖转运子基因启动子区域内或者由于测序结果的空缺(gap),有3个基因StSUT4(494个碱基对)、StSFP4(756个碱基对)和StINT1(1 417个碱基对),所得到的启动子序列短于2 kb。
经过PLACE程序分析,得到的糖转运子的顺式作用元件经分类并用于比较。42种常見的顺式调控元件,普遍存在于54个糖转运子的启动子区域,其中16种顺式调控元件存在于所有糖转运子的启动子,4种顺式调控元件(BIHD1OS、EBOXBNNAPA、MYCCONSENSUSAT和WBOXATNPR1)只在3个较短的基因启动子(StSUT4、StSFP4和StINT1)中缺失,6种顺式调控元件只在1个基因启动子中缺失(表1)。
此外,有些顺式序列(如DOFCOREZM)高度重复,在54个基因的启动子区域显示多达1 937份。这些共同的顺式作用元件能在不同的植物器官影响基因表达,如叶,芽,根,种子和花(花粉)。它们还响应以不同的植物激素(脱落酸、赤霉素、乙烯、细胞分裂素),以及对许多环境因素(光、二氧化碳,生物和非生物胁迫),主要是存在于叶片和芽的多个顺式作用元件序列(EBOXBNNAPA、GATABOX、GT1CONSENSUS、GTGANTG10、IBOXCORE)需要通过光进行转录调控,这与糖转运子在光合作用器官和储存器官之间转运糖是一致的。
3 结论
在本研究中,利用之前鉴定得到的54个糖转化蛋白,利用系统进化分析的方法,在每个家族内,将马铃薯与其他植物的糖转运子进行了对比进化关系的分析,分析了与糖转化蛋白序列类似的蛋白功能。同时,研究了调控糖转运子基因的信号传导,调查了它们的启动子序列,分析了位于启动子中的顺势调控元件,以期为下一步分析糖转运子的具体功能提供理论基础。
参考文献:
[1]李晓川,周 平,王朝海. 马铃薯糖转运蛋白家族的全基因组鉴定和表达分析[J]. 江苏农业科学,2017,45(12):24-27.
[2]Xu X,Pan S K,Cheng S F,et al. Genome sequence and analysis of the tuber crop potato[J]. Nature,2011,475:189-195
[3]Sievers F,Wilm A,Dineen D,et al. Fast,scalable generation of high-quality protein multiple sequence alignments using Clustal Omega[J]. Molecular Systems Biology,2011,7:539.
[4]Tamura K,Stecher G,Peterson D,et al. MEGA6:molecular evolutionary genetics analysis version 6.0[J]. Molecular Biology and Evolution,2013,30(12):2725-2729.
[5]Barker L,Kühn C,Weise A,et al. SUT2,a putative sucrose sensor in sieve elements[J]. The Plant Cell,2000,12(7):1153-1164.
[6]Kühn C,Franceschi V R,Schulz A,et al. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements[J]. Science,1997,275(534):1298-1300.
[7]Weise A,Barker L,Kühn C,et al. A new subfamily of sucrose transporters,SUT4,with low affinity/high capacity localized in enucleate sieve elements of plants[J]. The Plant Cell,2000,12(8):1345-1355.
[8]Reinders A,Schulze W,Kühn C,et al. Protein-protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element[J]. The Plant Cell,2002,14(7):1567-1577.
[9]Kühn C,Grof C P. Sucrose transporters of higher plants[J]. Current Opinion in Plant Biology,2010,13(3):288-298.
[10]Sauer N. Molecular physiology of higher plant sucrose transporters[J]. FEBS Letters,2007,581(12):2309-2317.
[11]Shiratake K. Genetics of sugar transporters[J]. Genes,Genomes,Genomics,2007,1:73-80
[12]Boorer K J,Loo D D,Frommer W B,et al. Transport mechanism of the cloned potato H+/sucrose cotransporter StSUT1[J]. The Journal of Biological Chemistry,1996,271(41):25139-25144.
[13]Chincinska I,Gier K,Krügel U,et al. Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production[J]. Frontiers in Plant Science,2013,4:26.
[14]Buettner M. The arabidopsis sugar transporter (AtSTP) family:an update[J]. Plant Biology,2010,12(1):35-41.
[15]Reuscher S,Akiyama M,Yasuda T,et al. The sugar transporter inventory of tomato:Genome-Wide identification and expression analysis[J]. Plant and Cell Physiology,2014,55(6):1123-1141.
[16]Gear M L,McPhillips M L,Patrick J W,et al. Hexose transporters of tomato:molecular cloning,expression analysis and functional characterization[J]. Plant Molecular Biology,2000,44:687-697
[17]Sauer N,Friedlnder K,Grml-Wicke U. Primary structure,genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana[J]. The EMBO Journal,1990,9(10):3045-3050.
[18]Fillion L,Ageorges A,Picaud S,et al. Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry[J]. Plant Physiology,1999,120(4):1083-1094.
[19]Vignault C,Vachaud M,Cakir B,et al. VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem[J]. Journal of Experimental Botany,2005,56(415):1409-1418.
[20]Hayes M A,Feechan A,Dry I B. Involvement of abscisic acid in the coordinated regulation of a stress-inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection[J]. Plant Physiology,2010,153(1):211-221.
[21]Norholm M H,Nour-Eldin H H,Brodersen P,et al. Expression of the arabidopsis high-affinity hexose transporter STP13 correlates with programmed cell death[J]. FEBS Letters,2006,580(9):2381-2387.
[22]Schofield R A,Bi Y M,Kant S,et al. Over-expression of STP13,a hexose transporter,improves plant growth and nitrogen use in Arabidopsis thaliana seedlings[J]. Plant Cell and Environment,2009,32(3):271-285.
[23]Hayes M A,Davies C,Dry I B. Isolation,functional characterization,and expression analysis of grapevine (Vitis vinifera L.) hexose transporters:differential roles in sink and source tissues[J]. J Exp Bot,2007,58:1985-1997
[24]McCurdy D W,Dibley S,Cahyanegara R,et al. Functional characterization and RNAi-mediated suppression reveals roles for hexose transporters in sugar accumulation by tomato fruit[J]. Molecular plant,2010,3(6):1049-1063.
[25]Buttner M,Truernit E,Baier K,et al. AtSTP3,a green leaf-specific,low affinity monosaccharide-H+ symporter of Arabidopsis thaliana[J]. Plant Cell and Environment,2000,23(2):175-184.
[26]Toyofuku K,Kasahara M,Yamaguchi J. Characterization and expression of monosaccharide transporters (OsMSTs) in rice[J]. Plant Cell Physiol,2000,41:94-947
[27]Schneidereit A,Scholz-Starke J,Büttner M. Functional characterization and expression analyses of the glucose-specific AtSTP9 monosaccharide transporter in pollen of Arabidopsis[J]. Plant Physiology,2003,133(1):182-190.
[28]Schneidereit A,Scholz-Starke J,Sauer N,et al. AtSTP11,a pollen tube-specific monosaccharide transporter in Arabidopsis[J]. Planta,2005,221(1):48-55.
[29]Truernit E,Stadler R,Baier K,et al. A male gametophyte-specific monosaccharide transporter in Arabidopsis[J]. The Plant Journal,1999,17(2):191-201.
[30]Scholz-Starke J,Büttner M,Sauer N. AtSTP6,a new pollen-specific H+-monosaccharide symporter from Arabidopsis[J]. Plant Physiology,2003,131(1):70-77.
[31]Kiyosue T,Abe H,Yamaguchi-Shinozaki K,et al. ERD6,a cDNA clone for an early dehydration-induced gene of Arabidopsis,encodes a putative sugar transporter[J]. Biochimica et Biophysica Acta,1998,1370(2):187-191.
[32]Quirino B F,Reiter W D,Amasino R D. One of two tandem Arabidopsis genes homologous to monosaccharide transporters is senescence-associated[J]. Plant Molecular Biology,2001,46(4):447-457.
[33]Yamada K,Osakabe Y,Mizoi J,et al. Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides[J]. The Journal of Biological Chemistry,2010,285(2):1138-1146.
[34]Reinders A,Panshyshyn J A,Ward J M. Analysis of transport activity of Arabidopsis sugar alcohol permease homolog AtPLT5[J]. The Journal of Biological Chemistry,2005,280(2):1594-1602.
[35]Klepek Y-S,Geiger D,Stadler R,et al. Arabidopsis POLYOL TRANSPORTER 5,a new member of the monosaccharide transporter-like superfamily,mediates H+-symport of numerous substrates,including myoinositol,glycerol,and ribose[J]. Plant Cell,2005,17:204-218
[36]Klepek Y S,Volke M,Konrad K R,et al. Arabidopsis thaliana POLYOL/MONOSACCHARIDE TRANSPORTERS 1 and 2:fructose and xylitol/H+ symporters in pollen and young xylem cells[J]. Journal of Experimental Botany,2010,61(2):537-550.
[37]Noiraud N,Maurousset L,Lemoine R. Identification of a mannitol transporter,AgMaT1,in celery phloem[J]. The Plant Cell,2001,13(3):695-705.
[38]Juchaux-Cachau M,Landouar-Arsivaud L,Pichaut J P,et al. Characterization of AgMaT2,a plasma membrane mannitol transporter from celery,expressed in phloem cells,including phloem parenchyma cells[J]. Plant Physiology,2007,145(1):62-74.
[39]Gao Z,Maurousset L,Lemoine R,et al. Cloning,expression,and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues[J]. Plant Physiology,2003,131(4):1566-1575.
[40]Watari J,Kobae Y,Yamaki S,et al. Identification of sorbitol transporters expressed in the phloem of apple source leaves[J]. Plant & Cell Physiology,2004,45(8):1032-1041.
[41]Ramsperger-Gleixner M,Geiger D,Hedrich R,et al. Differential expression of sucrose transporter and polyol transportergenes during maturation of common planta in companion cells[J]. Plant Physiol,2004,134:147-160
[42]Chauhan S,Forsthoefel N,Ran Y,et al. Na+/myo-inositol symporters and Na+/H+-antiport in Mesembryanthemum crystallinum[J]. The Plant Journal,2000,24(4):511-522.
[43]Schneider S,Beyhl D,Hedrich R,et al. Functional and physiological characterization of Arabidopsis INOSITOL TRANSPORTER1,a novel tonoplast-localized transporter for myo-inositol[J]. The Plant Cell,2008,20(4):1073-1087.
[44]Schneider S,Schneidereit A,Konrad K R,et al. Arabidopsis INOSITOL TRANSPORTER4 mediates high-affinity H+ symport of myoinositol across the plasma membrane[J]. Plant Physiology,2006,141(2):565-577.
[45]Schneider S,Schneidereit A,Udvardi P,et al. Arabidopsis INOSITOL TRANSPORTER2 mediates H+ symport of different inositol epimers and derivatives across the plasma membrane[J]. Plant Physiology,2007,145(4):1395-1407.
[46]Weber A,Servaites J C,Geiger D R,et al. Identification,purification,and molecular cloning of a putative plastidic glucose translocator[J]. The Plant Cell,2000,12(5):787-802.
[47]Cho M H,Lim H,Shin D H,et al. Role of the plastidic glucose translocator in the export of starch degradation products from the chloroplasts in Arabidopsis thaliana[J]. The New Phytologist,2011,190(1):101-112.
[48]Yin Y G,Kobayashi Y,Sanuki A,et al. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ‘Micro-Tom) fruits in an ABA- and osmotic stress-independent manner[J]. Journal of Experimental Botany,2010,61(2):563-574.
[49]Butowt R,Granot D,Rodríguez-García M I. A putative plastidic glucose translocator is expressed in heterotrophic tissues that do not contain starch,during olive (Olea europea L.) fruit ripening[J]. Plant & Cell Physiology,2003,44(11):1152-1161.
[50]Cao H,Guo S,Xu Y,et al. Reduced expression of a gene encoding a Golgi localized monosaccharide transporter (OsGMST1) confers hypersensitivity to salt in rice (Oryza sativa)[J]. Journal of Experimental Botany,2011,62(13):4595-4604.
[51]Wang H X,Weerasinghe R R,Perdue T D,et al. A golgi-localized hexose transporter is involved in heterotrimeric G protein-mediated early development in Arabidopsis[J]. Molecular Biology of the Cell,2006,17(10):4257-4269.
[52]Wormit A,Trentmann O,Feifer I,et al. Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport[J]. The Plant Cell,2006,18(12):3476-3490.
[53]Wingenter K,Schulz A,Wormit A,et al. Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning,sugar signaling,and seed yield in Arabidopsis[J]. Plant Physiology,2010,154(2):665-677.
[54]Schulz A,Beyhl D,Marten I,et al. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2[J]. Plant Journal,2011,68(1):129-136.
[55]Cho J I,Burla B,Lee D W,et al. Expression analysis and functional characterization of the monosaccharide transporters,OsTMTs,involving vacuolar sugar transport in rice (Oryza sativa)[J]. The New Phytologist,2010,186(3):657-668.
[56]Aluri S,Büttner M. Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering[J]. Proceedings of the National Academy of Sciences of the United States of America,2007,104(7):2537-2542.
