Long-term effect of clopidogrel in patients with and without diabetes: A systematic review and metaanalysis of randomized controlled trials
2020-05-12LiRongLiangQianMaLinFengQiQiuWenZhengWuXiangXie
Li-Rong Liang, Qian Ma, Lin Feng, Qi Qiu, Wen Zheng, Wu-Xiang Xie
Li-Rong Liang, Department of Clinical Epidemiology & Tobacco Dependence Treatment Research, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China
Qian Ma, Department of Cardiology, Beijing Anzhen Hospital, Capital Medical University,Beijing 100029, China
Lin Feng, Wu-Xiang Xie, Peking University Clinical Research Institute, Peking University First Hospital, Beijing 100034, China
Qi Qiu, Institute of Clinical Pharmacology, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, China
Wen Zheng, Emergency Critical Care Center, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, China
Abstract
BACKGROUND
Previous studies have shown that patients with diabetes mellitus (DM) respond poorly to clopidogrel treatment.
AIM
To systematically evaluate the efficacy of clopidogrel for the treatment of acute coronary syndromes or ischemic stroke in patients with or without DM.
METHODS
PubMed, the Cochrane Central Register of Controlled Trials, and EMBASE were searched from 1980 on 27 June 2019 to identify relevant randomized controlled trials that compared the effect of a combination of clopidogrel and aspirin with aspirin alone. A random-effects meta-analysis was used to estimate the hazard ratio (HR) and its 95% confidence interval (CI). Sensitivity analysis was performed using a fixed-effect model. The I2 statistic was used to evaluate the heterogeneity of the study data.
RESULTS
Six randomized controlled trials, comprising 43352 participants (13491 with and 29861 without DM) who had received antiplatelet therapy for ≥ 3 mo, were included in the meta-analysis. Compared with aspirin alone, a combination of clopidogrel and aspirin significantly reduced the risk of any cardiovascular event in patients without DM (HR = 0.78, 95%CI: 0.71-0.86, P < 0.001; I2 = 23%, P =0.26). Clopidogrel plus aspirin also significantly reduced cardiovascular risk in patients with DM, although the effect was smaller (HR = 0.89, 95%CI: 0.81-0.99, P= 0.030; I2 = 0%, P = 0.74). Nevertheless, there was no significant difference in the efficacy of clopidogrel at reducing the risk of cardiovascular events in patients with DM vs those without (P for interaction = 0.062).
CONCLUSION
Thus, the present study shows that the addition of clopidogrel to aspirin significantly lowers cardiovascular risk in patients with or without DM who have experienced ischemic cardiovascular disease. The beneficial effect of the addition of clopidogrel to aspirin for patients with DM was lower than that in patients without DM, although the modifying effect of DM did not reach significance.
Key words: Clopidogrel; Diabetes; Aspirin; Meta-analysis; Randomized controlled trial
INTRODUCTION
Ischemic cardiovascular disease remains the leading cause of mortality globally[1,2].Early initiation of dual antiplatelet therapy with a P2Y12receptor antagonist plus aspirin is recommended for patients with ischemic cardiovascular disease in the current guidelines[3,4]. Clopidogrel remains the most widely prescribed P2Y12receptor antagonist and is recommended by the latest guidelines for the management of ischemic stroke and acute coronary syndromes (ACS)[5,6]. Studies have shown that patients with diabetes mellitus (DM) exhibit a poorer response to clopidogrel than patients without DM[7,8], leading to a significant difference in the incidence of recurrence of cardiovascular events and mortality associated with DM[9].
Previous research has shown that hyperglycemia increases platelet reactivity, and the underlying mechanisms include higher platelet receptor expression, intracellular downstream signaling abnormalities, and P2Y12mutation and expression level[7,10]. The findings of the OPTIMUS trial, which compared the efficacy of a 150-mg maintenance dose of clopidogrel with 75 mg in 40 patients with both DM and coronary artery disease, showed that the higher maintenance dose of clopidogrel is associated with greater antiplatelet effects[11]. Hence, it is reasonable to hypothesize that clopidogrel treatment of ischemic vascular disease would be associated with a less substantial reduction in the risk of cardiovascular events in patients with DM than in those without.
Subgroup analyses of data from several randomized controlled trials (RCTs)comparing the efficacy of a combination of clopidogrel and aspirin with aspirin alone have shown that DM reduces the effect of clopidogrel on the incidence of recurrence of cardiovascular events and all-cause mortality, although the interactions were not significant[12-14]. A large-scale cohort study also showed that DM was associated with a lower efficacy of clopidogrel for a reduction in 1-year cardiovascular mortality in patients with myocardial infarction[9]. However, the long-term effects of clopidogrel in patients with and without DM have not been systematically reviewed. Therefore, we hypothesized that the presence of DM modifies the long-term efficacy of clopidogrel for a reduction in cardiovascular risk, and we performed a systematic review and meta-analysis to test this hypothesis.
MATERIALS AND METHODS
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[15].
Data sources
The literature search was conducted on 27 June 2019 using the Cochrane Library database for trials, EMBASE, and PubMed. The search strategy was: (1) PubMed(from 1980) 2019/06/27, (clopidogrel[Title/Abstract]) AND aspirin[Title/Abstract],article types: Clinical Trial; (2) EMBASE (from 1980) 2019/06/27, #2 #1 AND'randomized controlled trial'/de, #1 'clopidogrel':ab,ti AND 'aspirin':ab,ti; and (3)Cochrane Central Register of Controlled Trials (CENTRAL, from 1980) 2019/06/27,keywords: (“clopidogrel” AND “aspirin”); search limits: Trials.
Study selection
The inclusion criteria for RCTs were double-blind design, clopidogrel and aspirinvsaspirin for the treatment of ischemic cardiovascular disease, age ≥ 18 years, subgroup analysis according to the presence of diabetes (if not reported, the corresponding author was contacted to obtain the data)[16], and a duration of treatment ≥ 3 mo[17]. We also included the CHARISMA trial, because the percentage of patients with documented vascular disease in the trial was high (78%)[14].
Outcome
The outcomes assessed in the meta-analysis were fatal and non-fatal cardiovascular events. Table 1 summarizes the definitions of the primary outcomes of the included RCTs.
Quality assessment and data extraction
Two researchers (Liang LR and Ma Q) used a specially designed questionnaire to extract data independently. Consensus was reached by discussion. The Cochrane Collaboration’s risk-of-bias table was used to assess the quality of the included RCTs independently by two authors (Feng L and Xie WX)[18]. Figure 1 shows the risk of bias in the RCTs.
Statistical analysis
Review Manager 5.3 (Nordic Cochrane Center, Copenhagen, Denmark) was used to conduct the meta-analysis within random-effect models to estimate the pooled hazard ratio (HR) and its 95% confidence interval (CI). Sensitivity analysis was also performed using fixed-effect models. To evaluate the modifying effect of DM on the efficacy of clopidogrel, we used theZ-test to compare the difference between the two pooled HRs from the subgroup analyses, using the method proposed by Altman and Bland[19]. We used theI2statistic to estimate the heterogeneity of the included RCTs,and the Egger test and funnel plots to estimate the level of publication bias in STATA(version 11; Stata Corp, College Station, TX). All analyses were two-sided, and an alpha value of 0.05 was used as the threshold for statistical significance.
RESULTS
Study characteristics
Figure 2 shows the study selection process, which was conducted according to the PRISMA statement. Two thousand and sixty-four publications were identified in the initial search, from which 598 duplicates were excluded, and a further 1432 articles were removed after reviewing their title and abstract. The full text of the remaining 34 articles was then reviewed independently by two researchers, and 28 of these were excluded because of a treatment duration < 3 mo, a lack of comparison between clopidogrel plus aspirin and aspirin alone, a lack of subgroup analysis according tothe presence of DM, a long-termvsshort-term analysis, a high-dosevslow-dose analysis, ACS or ischemic stroke patients were not studied, or duplication (Table 2).Therefore, six RCTs, comprising 43352 participants (13491 with and 29861 without DM), were eligible for the present analysis[12-14,16,20,21]. Table 3 presents the characteristics of the included RCTs, of which three had recruited patients who had experienced an ischemic stroke or transient ischemic attack, two had recruited ACS patients, and one had recruited patients with cardiovascular disease or multiple risk factors. Table 4 summarizes the antiplatelet treatments and daily doses used in these trials.
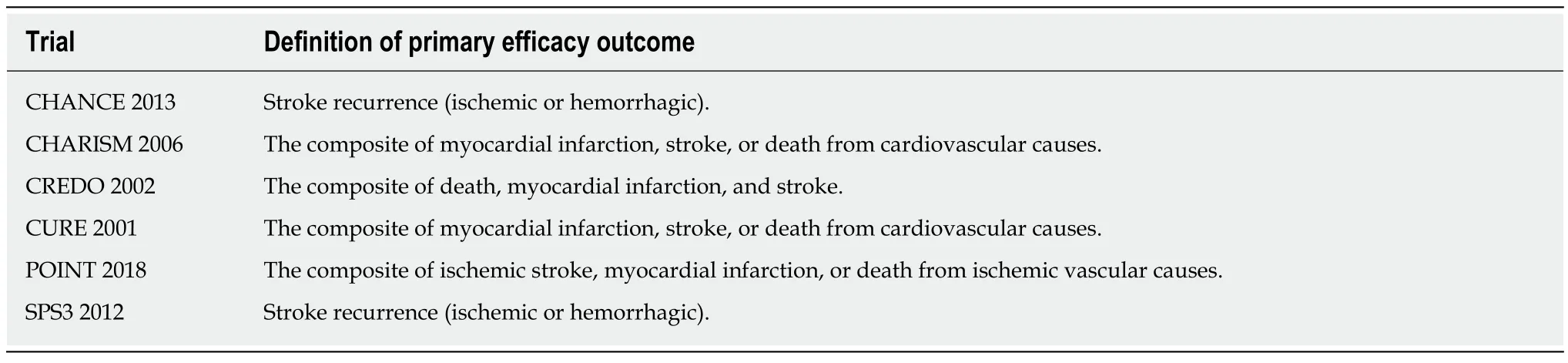
Table 1 Definitions of the primary efficacy outcome in each of the included trials
Effect of clopidogrel, according to diabetes status
As shown in Figure 3, compared with aspirin alone, the addition of clopidogrel to aspirin significantly reduced the risk of any cardiovascular event in patients without DM (HR = 0.78, 95%CI: 0.71-0.86,P< 0.001;I2= 23%,P= 0.26) and also in patients with DM, although the benefit was less (HR = 0.89, 95%CI: 0.81-0.99,P= 0.030;I2=0%,P= 0.74). The presence of DM was associated with an 11% higher HR, although the difference in the efficacy of clopidogrel in patients with and without DM did not reach significance (Pfor interaction = 0.062).
Publication bias
Both funnel plots (Figure 4) and Egger tests (P= 0.592 for patients without DM, andP= 0.296 for patients with DM) showed no evidence of publication bias.
Sensitivity analysis
The pooled results of a meta-analysis using fixed-effect models was consistent with the principal findings of random-effect models (Figure 5). Clopidogrel treatment was associated with relative risk reductions of 11% (HR = 0.89, 95%CI: 0.81-0.99,P= 0.030)and 21% (HR = 0.79, 95%CI: 0.73-0.85,P< 0.001) for any cardiovascular event in patients with and without DM, respectively. However, the modifying effect of DM on the incidence of cardiovascular events was not significant (Pfor interaction = 0.064).
DISCUSSION
Our systematic review and meta-analysis found that the addition of clopidogrel to aspirin significantly reduced the risk of any cardiovascular event in patients with or without DM. In addition, the results show that the presence of DM is associated with an increase of 11% in the HR, although this difference was not significant. To our knowledge, this is the first meta-analysis to evaluate the efficacy of long-term clopidogrel treatment in patients with ischemic cardiovascular disease, according to the presence or absence of DM.
The latest evidence provided by head-to-head RCTs has led to the current guidelines for the management of ACS stating that clopidogrel is inferior to ticagrelor and prasugrel as a P2Y12inhibitor for patients with ST-segment elevation myocardial infarction or non-STE ACS[3,4]. Recently, subgroup analyses of these RCTs have shown that the effects of ticagrelor and prasugrel are not modified by the presence of DM[22,23]. Evidence from previous trials and the present meta-analysis indicate that it is reasonable to use ticagrelor or prasugrel in preference to clopidogrel for the treatment of ACS patients, and especially those with DM. However, it should be noted that both ticagrelor and prasugrel are associated with higher risks of bleeding than clopidogrel;therefore, clopidogrel can still be used if ticagrelor and prasugrel are not available or are contraindicated[3,4]. Furthermore, data regarding the clinical efficacy and safety of ticagrelor or prasugrel for the treatment of ischemic stroke are limited. The SOCRATES trial, which compared the efficacy and safety of ticagrelor and aspirin in 13199 patients who had experienced a mild ischemic stroke or high-risk transient ischemic attack, found that ticagrelor was no more effective than aspirin at lowering cardiovascular risk over 90 days[24]. Therefore, clopidogrel remains the first choice of P2Y12inhibitor for the treatment of ischemic stroke[6]. In the present study, a weaker effect of clopidogrel was identified in patients with DM, which implies that a new antiplatelet agent is needed for ischemic stroke patients with DM.
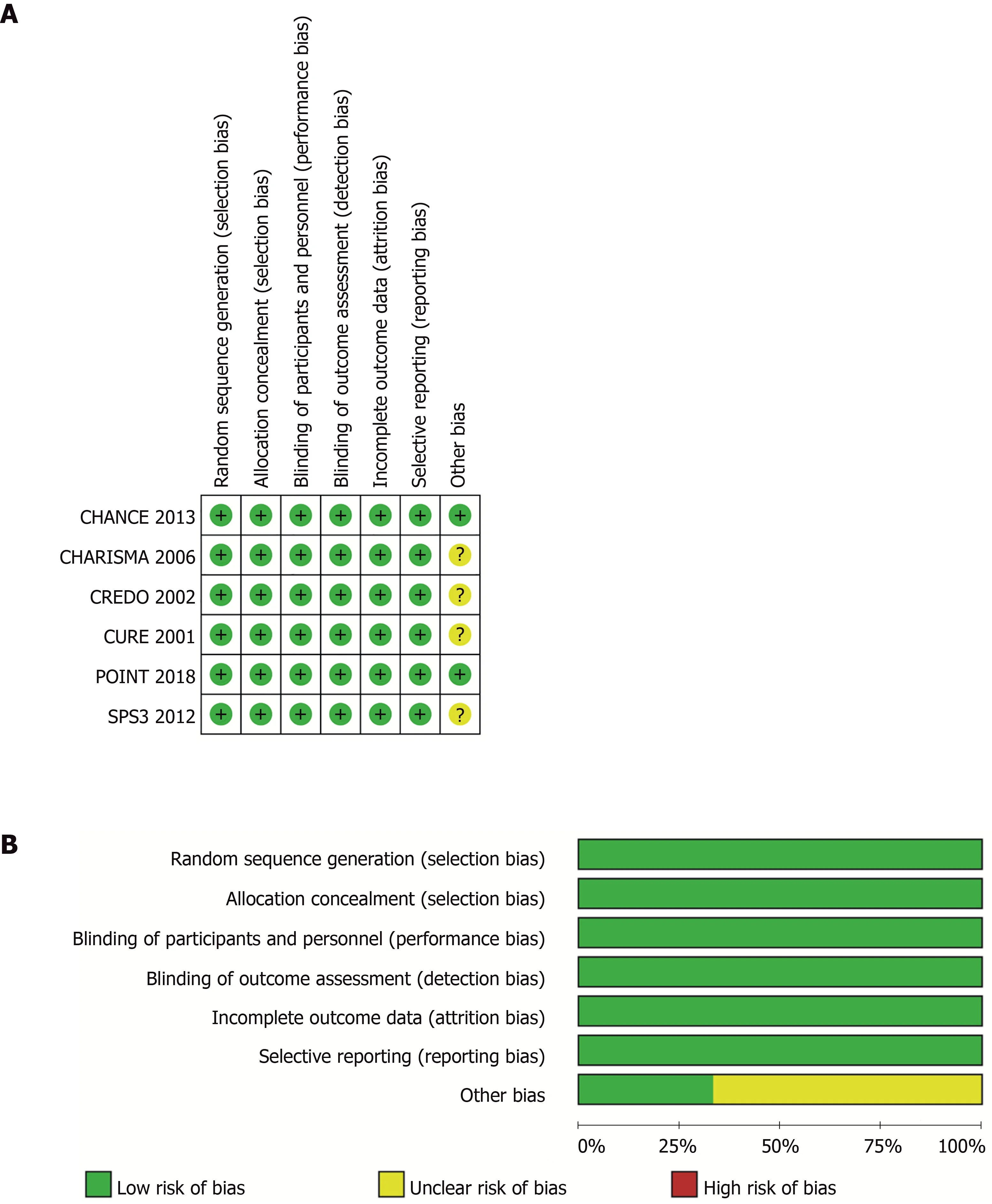
Figure 1 Risk-of-bias of included trials. A: Judgements about each source of bias in each study; B: Review authors’ judgements regarding the risk of each source of bias. The data are presented as percentages across all the included studies and are classified as Low, Unclear, or High. We followed the recommended approach for assessing the risk of bias in studies included in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.3.0. This addresses seven specific domains: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data,selective reporting, and other bias. Each domain includes one or more specific entries in a “Risk of bias” table. The tool involves assigning a judgement relating to the risk of bias for that entry. This is achieved by answering a pre-specified question about the adequacy of the study in relation to the entry, such that a judgement of“Yes” indicates a low risk of bias, “No” indicates a high risk of bias, and ‘Unclear’ indicates an unclear or unknown risk of bias.
A prospective cohort study of 58851 patients who had experienced a myocardial infarction confirmed this hypothesis[9]. Andersson and colleagues reported that clopidogrel treatment was associated with lesser reductions in 1-year all-cause mortality and cardiovascular mortality in patients with DM, compared to those without[9]. The results of the large-scale cohort study were consistent with the present findings, and we consider that one of the most important reasons why the present meta-analysis did not demonstrate a modifying effect of DM was the relatively small number of RCTs included. Therefore, a second meta-analysis should be performed when data from additional RCTs become available.
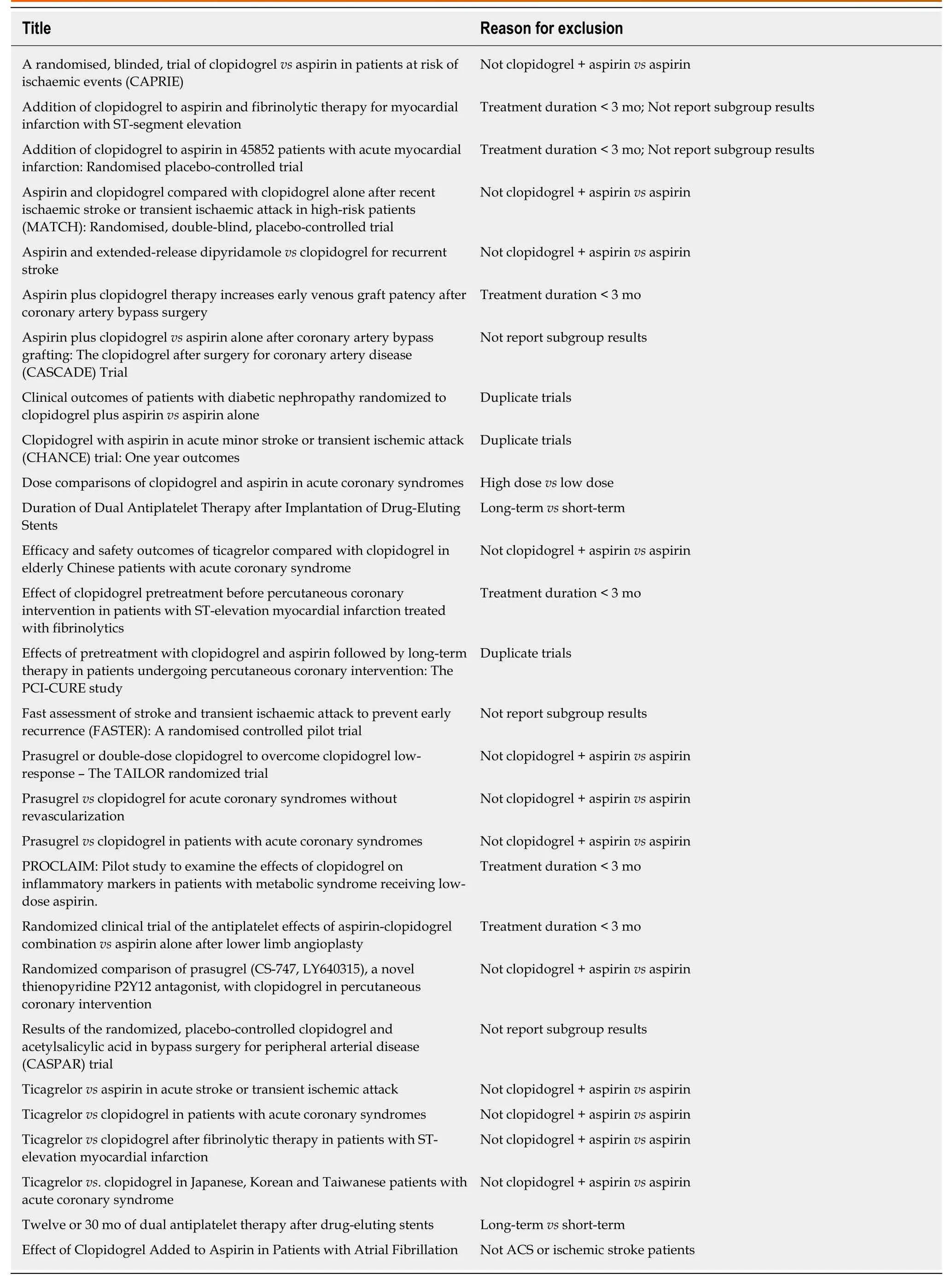
Table 2 Characteristics of the excluded trials
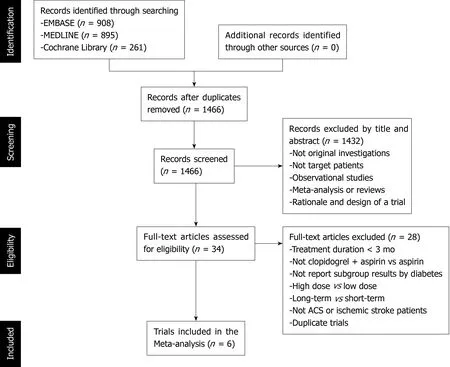
Figure 2 Study selection flow chart.
Several potential mechanisms that might explain the variability in the response to clopidogrel have been suggested. Firstly, a pharmacokinetic study showed that variability in intestinal absorption is the principal cause[25]. Second, polymorphisms in hepatic cytochrome (CYP) P450 3A, including in CYP3A4 and CYP3A5, may also be important: An inverse correlation has been shown between the percentage platelet aggregation and CYP3A4 activity in clopidogrel users[26]. In particular, of the multiple polymorphisms of CYP3A4, the IVS10 + 12G>A variant has been shown to regulate clopidogrel responsiveness[27]. Furthermore, a CYP3A5 polymorphism has been shown to be associated with atherothrombosis after clopidogrel treatment[28], although there is new evidence that challenges the existence of the association between CYP3A5 genotype and clopidogrel response[29]. Third, genetic platelet receptor polymorphisms have been implicated. Single-nucleotide polymorphisms of platelet receptors, such as GP IIIa L33P (= PlA1/2) and GP Ia 807 C/T have been suggested to be risk factors for thrombosis[30]. Fourth, the use of concurrent medication, such as calcium channel blockers, has recently been demonstrated to be a predictor of atherothrombotic events after clopidogrel treatment[29]. These mechanisms might also explain the effect of DM on the clopidogrel response.
Our study had several strengths: The inclusion of high-quality RCTs, large samples,no significant heterogeneity among the studies, and no evidence of publication bias.However, the study also had some limitations. First, the primary outcomes of the included RCTs were not entirely consistent, and secondary outcome data cannot be collected, because publications describing RCTs only show subgroup data for the primary outcomes. The present study only pooled the results of the primary outcomes of the included trials, which may have caused bias. Second, we estimated the pooled HRs on the basis of trial-level data, whereas pooled HRs derived from individuallevel data would be more accurate. Third, although we conducted a thorough literature search to identify relevant RCTs, it is possible that some appropriate RCTs were not included in this study, implying the possibility of selection bias.
In conclusion, the present study found that the addition of clopidogrel to aspirin significantly reduced cardiovascular risk in patients with and without DM who had experienced ischemic cardiovascular disease. The beneficial effect of a combination of clopidogrel and aspirin for patients with DM appeared to be lower than that for patients without DM, although this difference did not reach significance.
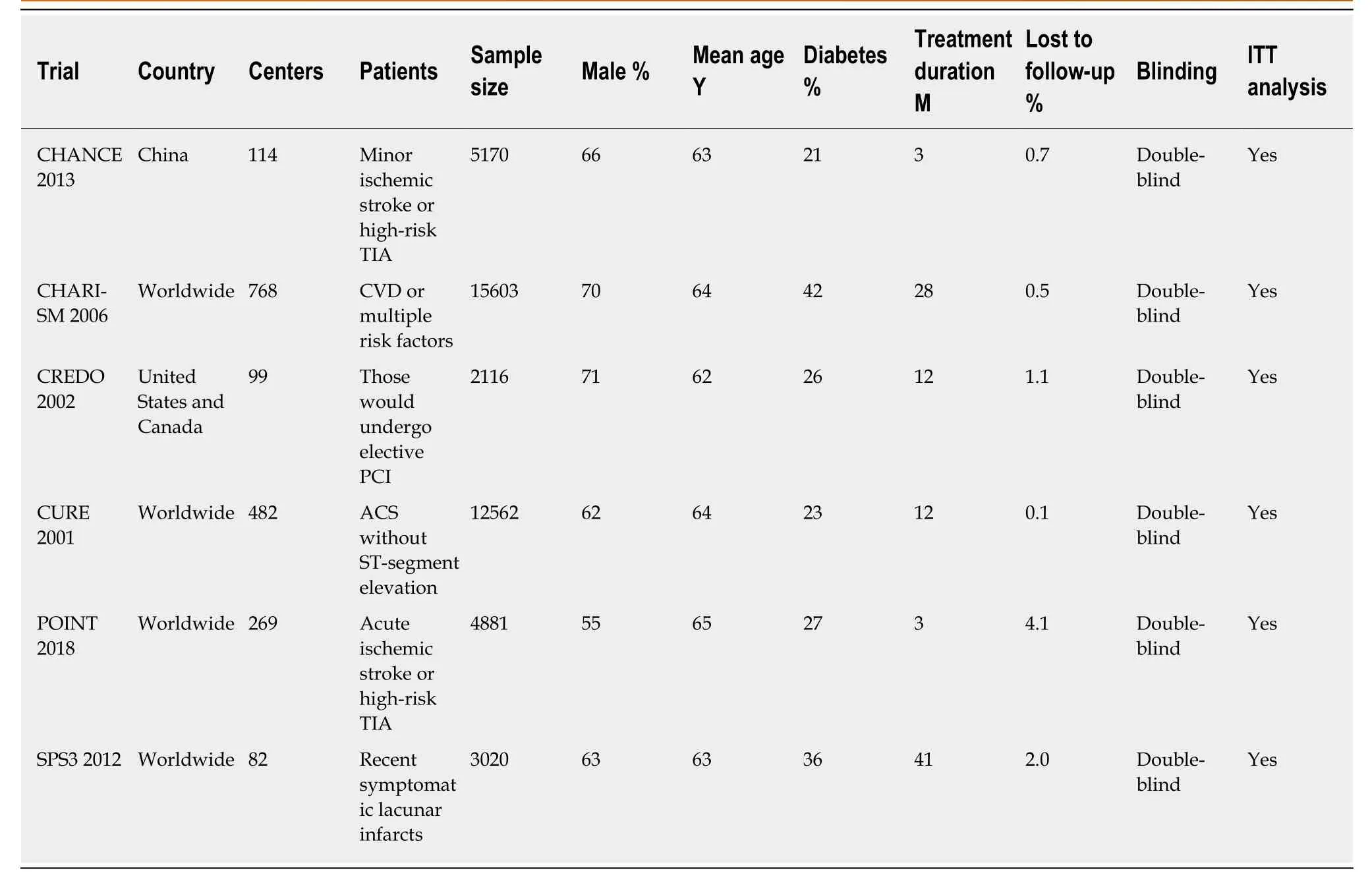
Table 3 Baseline characteristics of participants in the included trials
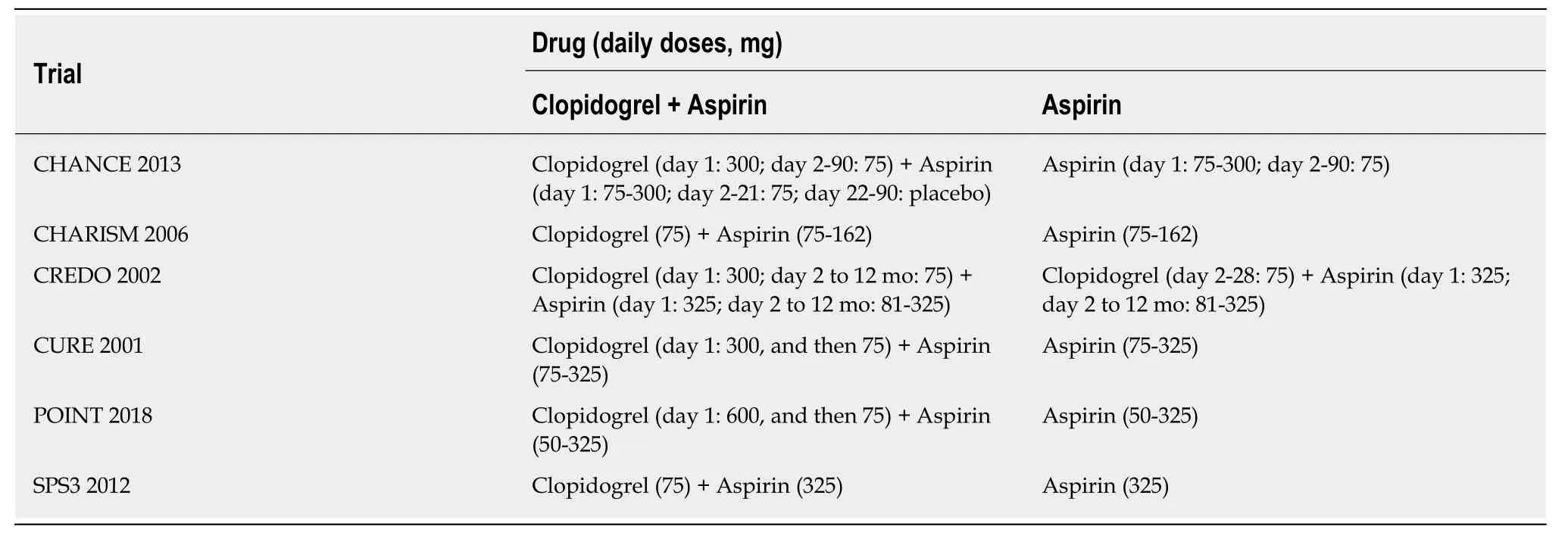
Table 4 Antiplatelet treatments and the daily doses used in the included trials
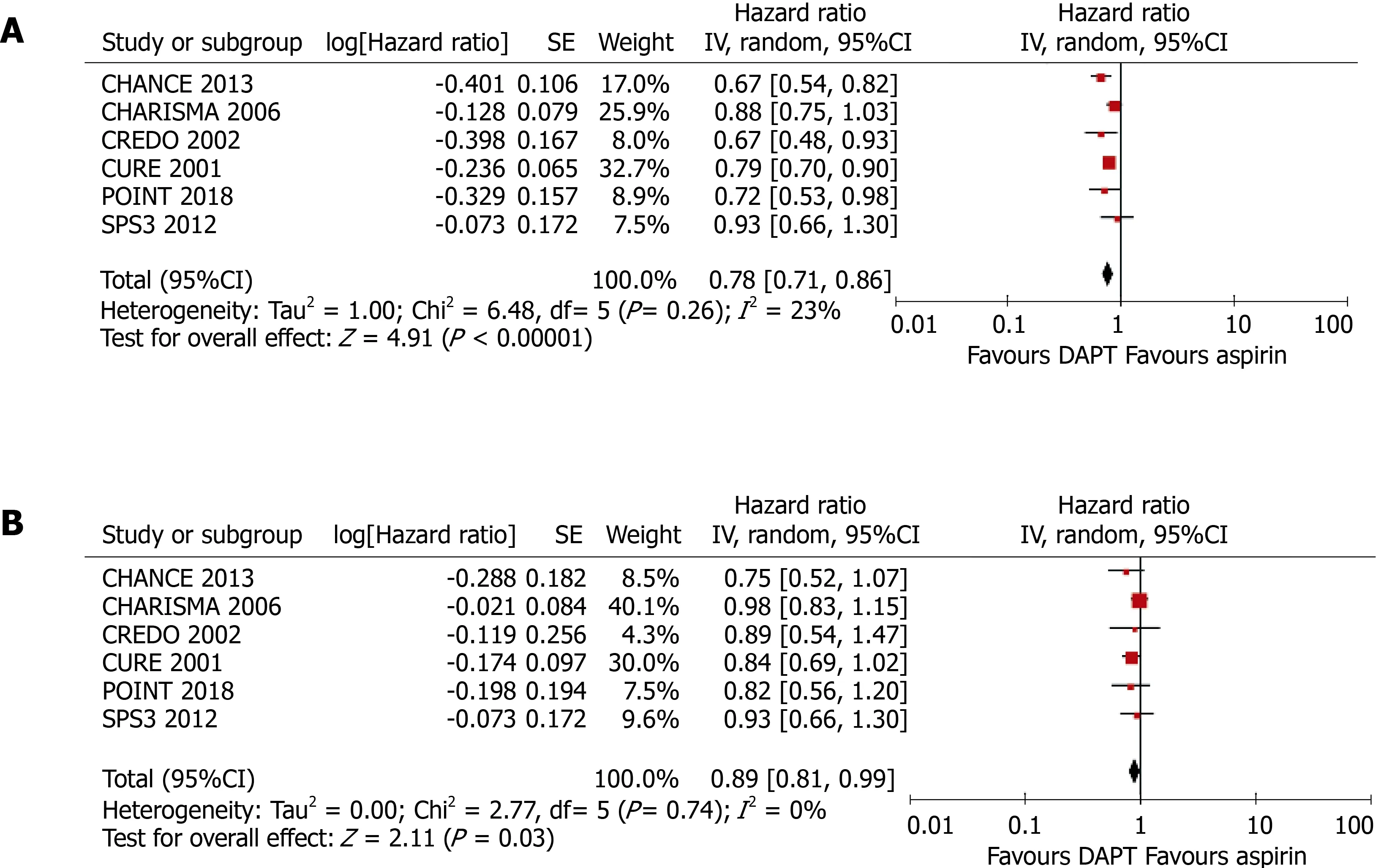
Figure 3 Meta-analyses using random-effect models conducted on the pooled data describing the effect of clopidogrel treatment on the incidence of any cardiovascular event. A: In patients without diabetes mellitus; B: In patients with diabetes mellitus. DAPT: Dual antiplatelet therapy.
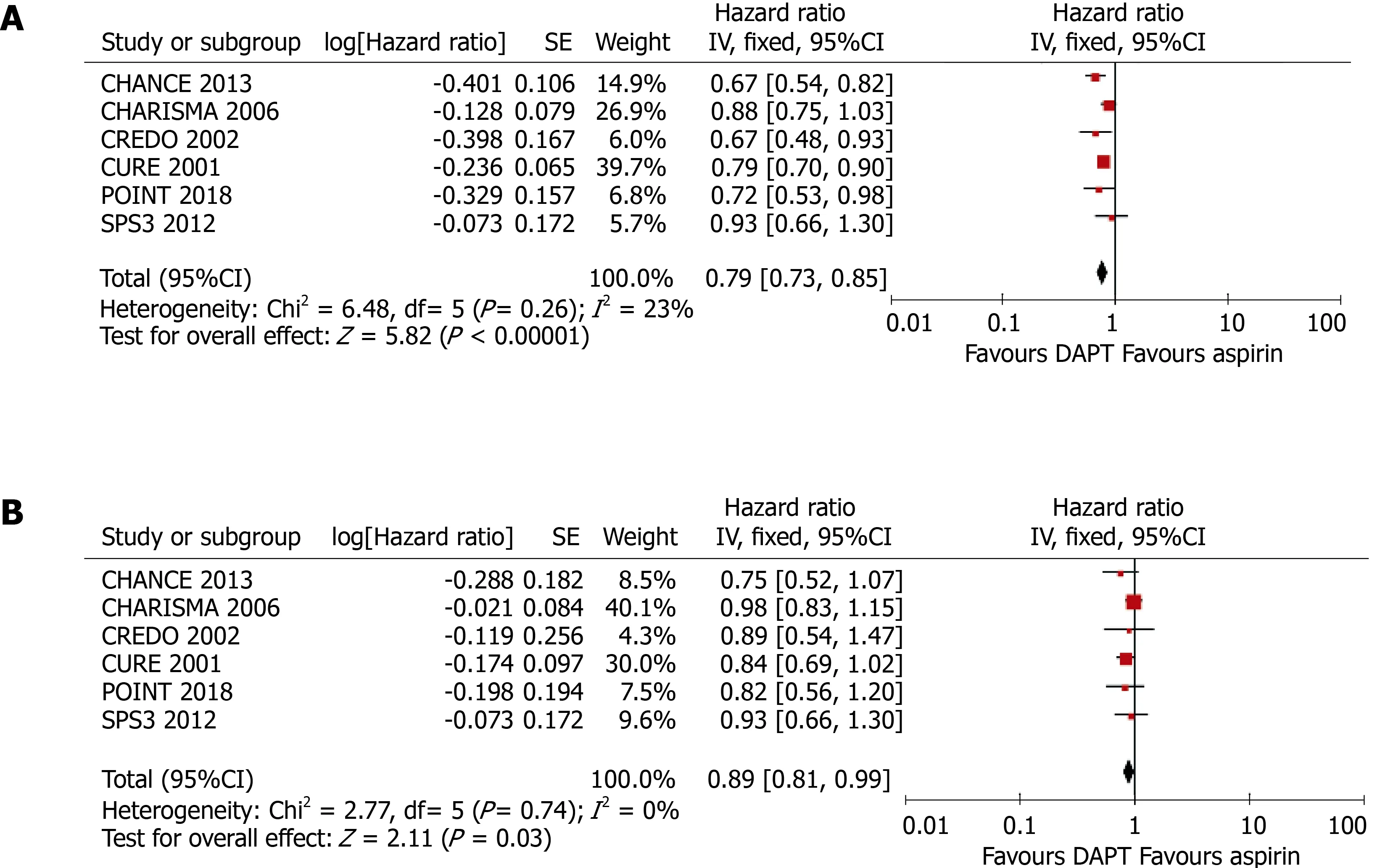
Figure 5 Meta-analyses using fixed-effect models conducted on the pooled data describing the effect of clopidogrel treatment on the incidence of any cardiovascular event. A: In patients without diabetes mellitus; B: In patients with diabetes mellitus. DAPT: Dual antiplatelet therapy.
ARTICLE HIGHLIGHTS
Research background
Clopidogrel remains the most widely prescribed P2Y12 receptor antagonist and is recommended by the latest guidelines for the management of ischemic stroke and acute coronary syndromes. Studies have shown that patients with diabetes mellitus (DM) exhibit a poorer response to clopidogrel than patients without DM, leading to a significant difference in the incidence of recurrence of cardiovascular events and mortality associated with DM. However, the long-term effects of clopidogrel in patients with and without DM have not been systematically reviewed.
Research motivation
We hypothesized that the presence of DM modifies the long-term efficacy of clopidogrel for a reduction in cardiovascular risk. To our knowledge, this is the first meta-analysis to evaluate the efficacy of long-term clopidogrel treatment in patients with ischemic cardiovascular disease, according to the presence or absence of DM.
Research objectives
This study aimed to systematically evaluate the efficacy of clopidogrel for the treatment of acute coronary syndromes or ischemic stroke in patients with or without DM.
Research methods
A systematic review and meta-analysis of randomized controlled trials.
Research results
Six randomized controlled trials, comprising 43,352 participants (13,491 with and 29,861 without DM) who had received antiplatelet therapy for ≥ 3 mo, were included in the meta-analysis. Compared with aspirin alone, a combination of clopidogrel and aspirin significantly reduced the risk of any cardiovascular event in patients without DM (HR = 0.78, 95%CI: 0.71-0.86, P < 0.001; I2 = 23%, P = 0.26). Clopidogrel plus aspirin also significantly reduced cardiovascular risk in patients with DM, although the effect was smaller (HR = 0.89, 95%CI: 0.81-0.99, P = 0.030; I2 =0%, P = 0.74). Nevertheless, there was no significant difference in the efficacy of clopidogrel at reducing the risk of cardiovascular events in patients with DM vs those without (P for interaction = 0.062).
Research conclusions
The present study found that the addition of clopidogrel to aspirin significantly reduced cardiovascular risk in patients with and without DM who had experienced ischemic cardiovascular disease. The beneficial effect of the addition of clopidogrel to aspirin for patients with DM was lower than that in patients without DM, although the modifying effect of DM did not reach significance. In the present study, a weaker effect of clopidogrel was identified in patients with DM, which implies that a new antiplatelet agent is needed for ischemic stroke patients with DM. We hypothesized that the presence of DM modifies the long-term efficacy of clopidogrel for a reduction in cardiovascular risk. Cardiologist and neurologist should pay attention to the presence of DM when provide clopidogrel for patients with ischemic cardiovascular disease.
Research perspectives
A systematic review and meta-analysis of randomized controlled trials to summarize the evidence on this topic is important. A new antiplatelet agent is needed for ischemic stroke patients with DM.
ACKNOWLEDGEMENTS
We thank Prof. S. Claiborne Johnston, MD, PhD, from the Dean’s Office, Dell Medical School, University of Texas at Austin, Austin, US, for providing the subgroup data according to diabetes status from the POINT trial. We also thank Mark Cleasby, PhD,from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
杂志排行
World Journal of Diabetes的其它文章
- Age of onset of diabetes and all-cause mortality
- Hydrocortisone, ascorbic acid and thiamine for sepsis: Is the jury out?
- Novel insight into perirenal adipose tissue: A neglected adipose depot linking cardiovascular and chronic kidney disease
- Lack of Syndecan-1 produces significant alterations in whole-body composition, metabolism and glucose homeostasis in mice
- Glargine-300: An updated literature review on randomized controlled trials and real-world studies
