Glargine-300: An updated literature review on randomized controlled trials and real-world studies
2020-05-12SujoyGhoshRomikGhosh
Sujoy Ghosh, Romik Ghosh
Sujoy Ghosh, Department of Endocrinology, IPGME&R, Kolkata 700020, West Bengal, India
Romik Ghosh, Medical Affairs, Sanofi, Mumbai 400072, Maharashtra, India
Abstract
Despite the availability of a variety of insulins, rates of insulinisation and the acceptance of insulin therapy is suboptimal in real-world clinical settings. Patient and physician concerns with hypoglycaemia and weight gain are the two key issues that serve to impede appropriate insulinisation in patients with diabetes.Recently introduced second-generation basal insulin analogues [for e.g., insulin glargine 300 U/mL (Gla-300) and insulin degludec] are designed to have improved pharmacokinetic profiles with an intention to deliver steady insulin levels over a longer period. Several randomised controlled and real-world studies have proven the resultant advantages of second-generations insulin analogues in lowering intra-individual variability in plasma insulin levels, flexibility in dosing,a sustained glucose-lowering effect, and decreasing the risk of hypoglycaemia.Gla-300 is one of the newer second-generation basal insulin analogues to have been approved for both type 1 and 2 diabetes. In this article, we review the currently available clinical and real-world data of Gla-300.
Key words: Insulin; Glargine-300; Type 2 diabetes; Diabetes mellitus; Hypoglycaemia;Glycaemic control
INTRODUCTION
Diabetes is a growing public health problem and imparts significant burden on both healthcare resources and on society. In 2019, approximately 463 million people worldwide had diabetes, and 4.2 million deaths were due to the disease or associated complications. It is estimated that 700 million people will have diabetes by 2045. The total global health expenditure on diabetes is estimated to be 760 billion United States Dollar[1].
Insulin is the cornerstone of therapy for patients with type 1 diabetes (T1DM).Treatment for T1DM consists of multiple daily injections of prandial insulin and basal insulin (BI) or continuous subcutaneous (SC) infusion[2]. Hypoglycaemia risk can be reduced with use of rapid-acting insulin analogues. Prandial insulin doses should be matched with carbohydrate intake, pre-meal blood glucose levels, and anticipated physical activity. ADA recommends that the patients with T1DM, who have been successfully using continuous SC insulin infusion, should have continuous access to this therapy even after 65 years of age[2]. A wide array of pharmacological treatment options is available for patients with T2DM. However, with progressive loss of β-cell function, exogenously administered insulin therapy becomes imperative for many patients with T2DM. In patients who show unstable T2DM or symptoms of acute decompensation despite oral antidiabetic (OAD) treatment, insulin, either alone or in combination with other OADs, is recommended[2].
BI is required to maintain blood glucose at a consistent level during fasting periods.The goal of BI therapy is to sustain physiologic insulin levels between meals, thereby mitigating the risk of hypoglycaemia, particularly at night. When a combination of ≥ 3 OAD agents fails to lower blood glucose levels and insulin therapy needs to be commenced, an effective regimen in the first-line insulinization could be a combination of BI and OADs[3]. Moreover, a short-term intensive insulin therapy in T2DM has been shown to salvage β-cell function[4].
While the currently available insulins are indispensable for management of diabetes, their use in real-world settings is beset by various shortcomings. One of the biggest obstacles in using insulin for the management of diabetes is patients’ fear of hypoglycaemia, which could lead to lack of patient compliance and clinical inertia and ultimately to loss of glycaemic control[5-8]. A majority of both primary care physicians and specialists have indicated that they would treat their patients more aggressively if there was no concern about hypoglycaemia[6]. Moreover, following insulin initiation, most patients fail to achieve glycaemic control in part due to suboptimal titration of the insulin dose[9]. Under-titration could either be due to patient fear of hypoglycaemia or of weight gain. Furthermore, insufficient health care resources could also be responsible for the failure to assist and educate the patient on proper self-titration algorithms.
Current research on insulin therapy focusses on making it safer and more effective for patients. Newer BI formulations have provided advantages of lower intraindividual variability, flexibility in dosing, and a sustained glucose-lowering effect without an increased risk of hypoglycaemia[10,11]. In this review article, we have summarised the clinical and real-world evidence on insulin glargine 300 U/mL (Gla-300, Toujeo®), a second-generation BI analogue approved in 2015 by the USFDA and the EMA for use in patients with T1DM/T2DM.
GLA-300, THE SECOND- GENERATION BASAL INSULIN
Glargine is a human insulin analogue that differs from the endogenous human insulin by a substitution of glycine for asparagine at position A21 and the addition of two arginine residues to the C-terminus of the B-chain. The solution of insulin glargine injection has a pH of 4, which neutralises post-injection to pH 7. The addition of arginine residues increases the isoelectric point of insulin glargine and results in formation of a microprecipitate within an amorphous SC depot, from which slow and protracted release of insulin glargine occurs[12,13]. Gla-300 is a formulation of insulin glargine that delivers the same amount of insulin units as insulin glargine 100 U/mL(Gla-100) in one-third of the injection volume.
Gla-300 comprises the same active glargine molecule as Gla-100 but forms a more compact SC depot with a reduced surface area than Gla-100. It is hypothesized that the size, and hence the surface area, of the SC depot determines the re-dissolution rate(Figure 1)[13,14]. This may allow for a longer SC residence time and degradation by tissue peptidases, resulting in a reduced re-dissolution rate, lower bioavailability, and an increase in daily dose[15]. Accordingly, Gla-300 has a more stable activity profile and a more prolonged and gradual insulin release than Gla-100, resulting in blood glucose control that lasts for up to 36 h[16-19].
Pharmacokinetic and pharmacodynamics profile of Gla-300
Various studies have shown that Gla-300 has a more stable and prolonged pharmacokinetic/pharmacodynamics (PK/PD) profile. In a double-blind randomised study on 50 patients with T1DM, Gla-300 was shown to provide predictable and evenly distributed insulin exposure over 24 h[16]. In another double-blind randomised study comparing Gla-300 to Gla-100 in 30 patients with T1DM, Gla-300 demonstrated a more even steady-state PK/PD profile and a longer duration of action than Gla-100[20]. In this study, Gla-300 maintained tight blood glucose control (≤ 105 mg/dL) for a median of 30 h. In two double-blind, randomised crossover studies in Japanese (n=18) and European (n= 24) patients with T1DM in euglycaemic clamp settings, singledose Gla-300 injections were shown to have a more prolonged and constant PK/PD profile compared with Gla-100[17]. In addition, blood glucose control was maintained for up to 36 h in patients receiving Gla300. An exploratory, open-label, parallel-group,two-period crossover study on 59 patients with T1DM comparing Gla-300 with Gla-100 demonstrated reduced glucose levels (as measured by continuous glucose monitoring) in the last 4 h of the 24-h injection interval, smoother average 24-h glucose profiles regardless of injection time, and reduced nocturnal hypoglycaemia with Gla-300[21].
The pharmacodynamic properties of Gla-300 and degludec (IDeg)-200, both at 0.4 U/kg once-daily fixed dose, were evaluated in 57 patients with T1DM in a twoperiod, two-treatment, two-sequence, crossover study using euglycaemic clamp[22]. In this study, IDeg-200 showed lower day-to-day variability (approximately 4 times lower) and within-day variability (37% lesser) in glucose-lowering effect than Gla-300.In contrast, a second euglycaemic clamp study[23]in 48 patients with T1DM demonstrated that Gla-300 had better steady-state PD profile (20% less within-day variability) and evenly distributed PK profile than IDeg-100 when administered at the same dose (0.4 U/kg/d). Though, there were several differences between both the studies such as morning versus evening injections and use of IDeg-200vsIDeg-100,the difference in results was mainly attributed to parameter used for calculating the within-day variability (fluctuations) of the BIs[24]. The latter study presented fluctuations of the BIs using absolute area under the curve values of the smoothed glucose infusion rate (GIR) curves above and below the average GIR, while, the former study presented percentage of the total glucose-lowering effect (area under the curve-GIR0-24hours).
Reductions in glycated haemoglobin, hypoglycaemia and weight gain
The safety and efficacy of Gla-300 has been assessed in a series of clinical trials comprising the EDITION programme which recruited patients with both T1DM and T2DM[15,25-29]. The patients in the EDITION trials received a range of background therapies and two trials were performed specifically in Japanese populations. All EDITION trials assessed mean glycated haemoglobin (HbA1c) change from baseline to 6 mo and the risk of hypoglycaemia for both Gla-300 and Gla-100. Comparable mean HbA1cchanges between both glargine formulations were observed across all studies(Table 1). Patients treated with Gla-300 consistently experienced a reduced risk of hypoglycaemia, including nocturnal and hypoglycaemia at any time of the day (Table 2). Rates of treatment-emergent adverse events were similar between the glargine formulations (Table 1). Similar to other insulins, the most common adverse event (≥1/10) was hypoglycaemia. Other common adverse events (≥ 1/100 to < 1/10)included lipohypertrophy and injection-site reactions. In the EDITION trials, patients continued the treatment for 6 mo in addition to the initial 6-mo of treatment. At the end of 12 mo, Gla-300 achieved better reductions in HbA1cand the risk of hypoglycemia (at any time) than with Gla100 in EDITION 1 trial and the outcomes were comparable in the other EDITION trials[30].
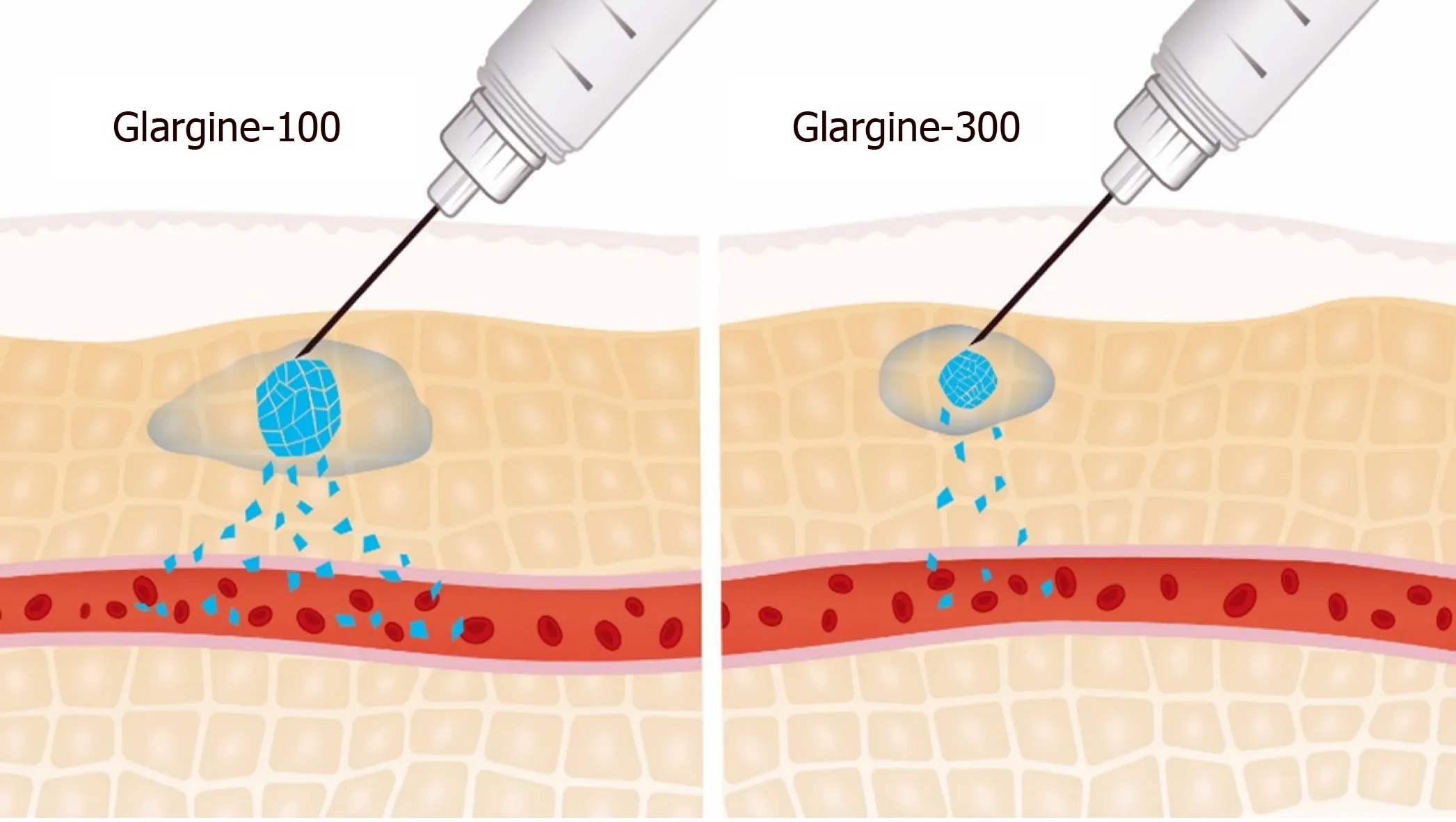
Figure 1 Surface area of subcutaneous depot: Gla-100 and Gla-300. Adapted from[12].
A patient-level meta-analysis of the EDITION 1, 2, and 3 studies revealed comparable glycaemic control between both glargine formulations [reduced HbA1cfor both formulations -1.02% (standard error 0.03, 95%CI: 0.08 to 0.07)] across a large and clinically diverse population with T2DM[31]. When compared with Gla-100, patients treated with Gla-300 had reduced annualised rates of confirmed or severe hypoglycaemia at night (31% difference in rate ratio over 6 mo) and at any time (24 h,14% difference). In addition, there was lower weight gain in patients treated with Gla-300 than in those receiving Gla-100 [LS mean difference 0.28 kg (95%CI: 0.55 to 0.01);P= 0.039]. These efficacy and safety results were further corroborated in a one-year patient-level meta-analysis of the EDITION 1, 2, and 3 studies[32]. When compared with Gla-100, Gla-300 provided more sustained reductions in HbA1cover 12 mo [LS mean difference in change from baseline 0.10% (95%CI: 0.18 to 0.02); 1.09 mmol/mol(2.01 to 0.20);P= 0.0174]. A lower risk of confirmed or severe hypoglycaemia was observed with Gla-300 at night [relative risk (RR): 0.85 (95%CI: 0.77 to 0.92)]; the risk was also lower at any time of day [RR: 0.94 (95%CI: 0.90 to 0.98)]. The rates of nocturnal hypoglycaemia were lower with Gla-300 versus Gla-100 [rate ratio 0.82(95%CI: 0.67 to 0.99)] but were comparable at any time of day. When compared with Gla-100, patients treated with Gla-300 were more likely to achieve HbA1c< 7.0%without nocturnal hypoglycaemia [RR: 1.24 (95%CI: 1.03 to 1.50)]. Another recently reported meta-analysis of the 6-mo pooled data from 2496 patients enrolled in the EDITION 1, 2, and 3 trials aimed at comparing safety and efficacy of Gla-300 and Gla-100 in patients with mild-to-moderate renal impairment[33]. Results from this analysis show that while glycaemic control was comparable between the two groups, there was a reduced overall risk of confirmed or severe hypoglycaemia in both groups.
The safety and efficacy of Gla-300 has been compared in a network meta-analysis with other BI therapies in T2DM[34]. The change in HbA1cprovided by Gla-300 was similar to that of detemir [difference: -0.08; 95% credible interval (CrI): -0.40 to 0.24],neutral protamine Hagedorn (NPH; difference: 0.01; 95%CI: 0.28 to 0.32), IDeg(difference: -0.12; 95%CI: 0.42 to 0.20), and premixed insulin (difference: 0.26; 95%CI:0.04 to 0.58). A significantly lower nocturnal hypoglycaemia rate was observed with Gla-300 when compared with NPH [risk ratio/relative risk (RR): 0.18; 95%CI: 0.05 to 0.55] and premixed insulin (RR: 0.36; 95%CI: 0.14 to 0.94). No significant differences in nocturnal hypoglycaemia rate were observed between Gla-300 and detemir (RR: 0.52;95%CI: 0.19 to 1.36) or IDeg (RR: 0.66; 95%CI: 0.28 to 1.50). There were no significant differences in documented symptomatic hypoglycaemia rates of Gla-300 versus detemir (RR: 0.63; 95%CI: 0.19 to 2.00), NPH (RR: 0.66; 95%CI: 0.27 to 1.49), and IDeg(RR: 0.55; 95%CI: 0.23 to 1.34). While comparable changes in body weight (in kg) were observed between Gla-300 and detemir (difference: 0.69; 95%CI: -0.31 to 1.71), NPH(difference: 0.76; 95%CI: 1.75 to 0.21), and IDeg (difference: 0.63; 95%CI: 1.63 to 0.35);weight gain was significantly lower compared with premixed insulin (difference: 1.83;95%CI: 2.85 to 0.75). Another systematic review and network meta-analysis comparing 10 BIs evaluated data from > 26000 patients from 39 randomised trials lasting ≥ 12 wk in duration and reported a favourable outcome for Gla-300 in terms of HbA1creduction, change in body weight, and any hypoglycaemia[35].
The safety and efficacy of Gla-300 has also been studied in older people with T2DM. SENIOR was an open-label, two-arm, parallel-group, multicentre phase 3b trial that compared Gla-300 with Gla-100 in 1014 randomised participants (mean age 71 years)[36]. In the overall population, similar reductions in HbA1cwere observed from baseline to week 26 for Gla-300 (0.89%) and Gla-100 (0.91%) (LS mean difference:0.02%; 95%CI: 0.092 to 0.129). The rates and incidence of confirmed or severe hypoglycaemia events were low and comparable between both treatment groups.Lower rates of documented symptomatic hypoglycaemia were observed with Gla-300.Significantly lower annualised rates of documented symptomatic hypoglycaemia were also observed [Gla-300: 1.12; Gla-100: 2.71; rate ratio: 0.45 (95%CI: 0.25 to 0.83)].In patients aged ≥ 75 years, reductions in HbA1cfrom baseline to week 26 were comparable in both groups (LS mean difference: -0.11%; 95%CI: -0.330 to 0.106);while, the hypoglycaemia risk was lower with Gla-300 than Gla-100 [documented symptomatic hypoglycaemia (< 54 mg/dL): 1.5%vs10.4%; relative risk: 0.33; 95%CI:0.12 to 0.88][33].
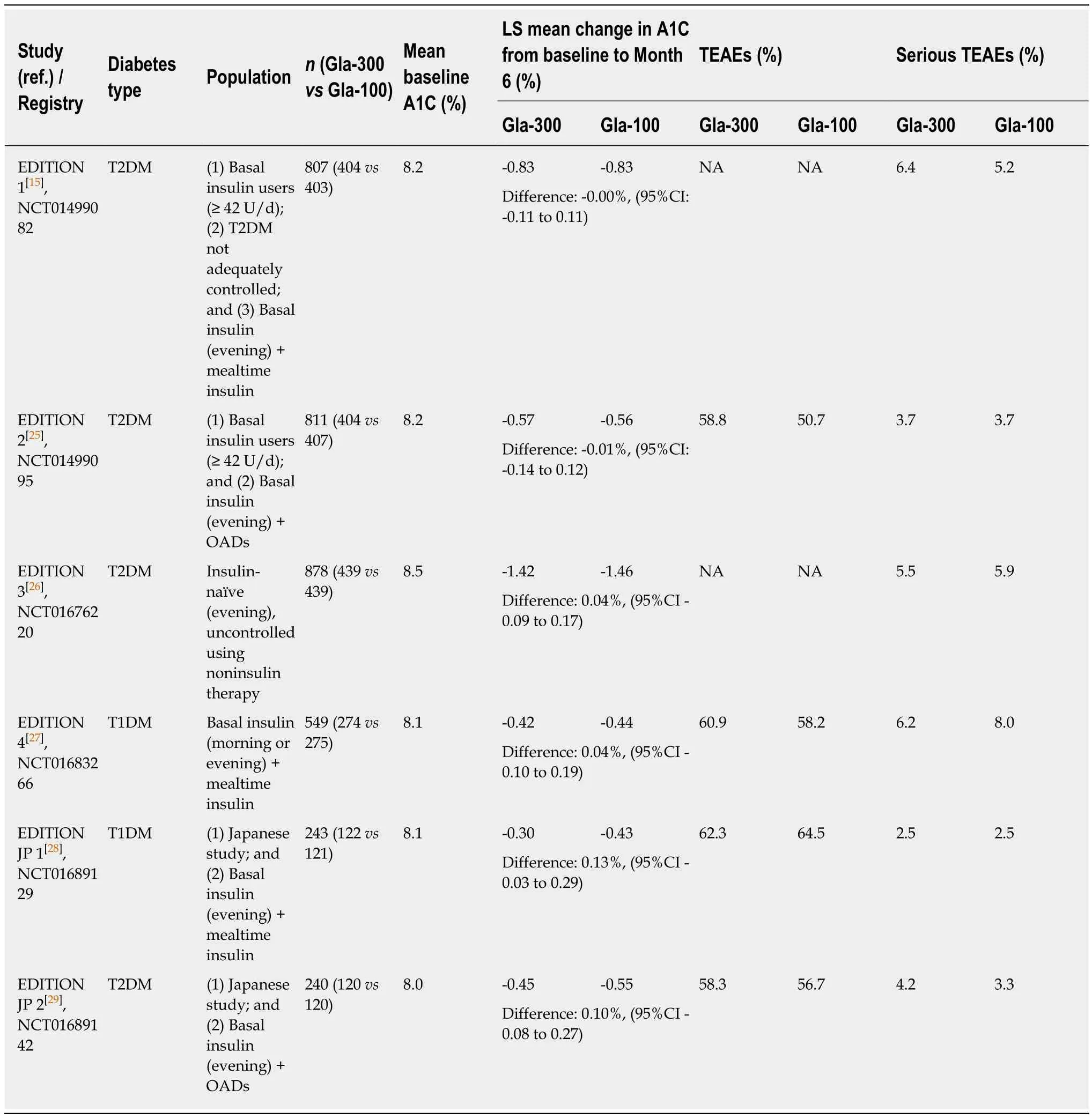
Table 1 Efficacy and safety of Gla-300 vs Gla-100 across the EDITION Phase 3 Clinical Trial Program
Apost-hocanalysis of patient-level meta-analysis of EDITION 1, 2, and 3 studies examined the extent of glycaemic control and risk of hypoglycaemia with Gla-300vsGla-100 in patients aged ≥ 65 years and with T2DM. The analysis showed comparable glycaemic control with Gla-300 and Gla-100 (LS mean difference in HbA1cchange from baseline to month 6: 0.00; 95%CI: -0.14% to 0.15%). A reduction in risk of hypoglycaemia was observed for Gla-300vsGla-100 (RR: 0.70; 95%CI: 0.57 to 0.85)[37].
Gla-300: Real world evidences
Oriotet al[38]evaluated the glycaemic control in patients with T1DM after switching from Gla-100 to Gla-300 in a real-world clinical practice. Patients were first treated with Gla-100, either once or twice daily, and then switched to Gla-300. Glycaemic
control was evaluated at two-time intervals - the first (period 1) during a 2-wk period before and after switching to Gla-300 and the second (period 2) at 12 and 24 wk following the switch. Results showed that HbA1cremained at pre-switch levels during period 1 but decreased from 8.0% ± 1.0% (65.5 ± 10.5 mmol/mol) to 7.9% ± 1.0% (62.8± 10 mmol/mol) by the end of period 2. The number of nocturnal hypoglycaemic events significantly reduced following the switch (22.2% before switchvs12.2% after switch; RR: 0.46; 95%CI: 0.30 to 0.68;P< 0.0001) as was the proportion of patients with nocturnal hypoglycaemia per period (30.0% before switch vs. 16.0% after switch; RR:0.53; 95%CI: 0.31 to 0.86). Moreover, no perceptible weight gain was reported in study patients.

Table 2 Risk of hypoglycaemia for Gla-300 vs Gla-100 across the EDITION Phase 3 Clinical Trial Program(Safety population)
Usage patterns and clinical outcomes have been assessed before and after Gla-300 initiation in patients with T2DM starting or switching to Gla-300. A retrospective observational study using data acquired from physician survey medical records revealed a similar final titrated dose among insulin-naive patients starting BI treatment [LS mean 0.43 units/kg (Gla-300)vs0.44 units/kg (Gla-100);P= 0.77][39].Significant reductions in HbA1clevels were observed for both glargine formulations[LS mean 1.21% (Gla-300) and 1.12% (Gla-100); bothP< 0.001]. Compared with Gla-100, Gla-300 was associated with a lower rate of hypoglycaemic events after treatment initiation (RR: 0.31; 95%CI: 0.12 to 0.81;P= 0.018) at similar daily doses. Significantly lower daily doses of BI were observed after switching to treatment with Gla-300 from treatment with another BI (0.73 units/kg before switchvs0.58 units/kg after switch;P= 0.02). Mean HbA1cwas significantly lower after the switch to Gla-300 than before switching (adjusted difference 0.95%; 95%CI: 1.13 to 0.78;P< 0.0001). In addition,hypoglycaemic events per patient-year (PPY) were significantly lower in patients receiving Gla-300 (RR: 0.17; 95%CI: 0.11 to 0.26;P< 0.0001).
In another retrospective study conducted in routine clinical settings in Japan, 20 patients with T1DM and 62 patients with T2DM who had switched from Gla-100 to Gla-300 were evaluated for the safety and efficacy of Gla-300, 3 mo following the switch[40]. HbA1c level substantially decreased in patients with T2DM (P< 0.01) and while these values were lowered in T1DM patients, the magnitude of reduction was not statistically significant. Decreases in body-mass-index, an indicator of weight gain,were observed in patients with T1DM (P= 0.06) as well as T2DM (P< 0.05). Rates of hypoglycaemia were similar across all groups. These findings hint that switching the BI regimen to Gla-300 is effective in achieving glycaemic control as well as avoiding weight gain.
DELIVER 2 was a retrospective cohort study that compared real-world clinical and healthcare-resource utilisation data in patients with T2DM who were on BI treatment and who switched to Gla-300 or to another BI[41]. Eligible patients aged ≥ 18 years and were receiving BI. Data were collected from the Predictive Health Intelligence Environment database of electronic medical records, here representing 39 integrated healthcare-delivery networks in the United States. A comparable change in HbA1cfrom baseline was observed in both matched cohorts (n= 1819 in each) (0.51%, Gla-300; 0.51%, other BI;P= 0.928) (Figure 2). Patients in both cohorts were also equally likely to achieve HbA1c< 7.0% (16.8%, Gla-300; other BI, 18.4%;P= 0.223) and < 8.0%(44.0% Gla-300; 44.2%, other BI;P= 0.920) during follow up. Significantly fewer patients treated with Gla-300 experienced hypoglycaemia during the same period(15.4%, Gla-300; 18.1%, other BI;P= 0.015) (Figure 3). Patients who switched to Gla-300 had a lower risk of requiring hypoglycaemia-related hospitalisation, emergency department (ED) services, and outpatient visits when compared to those who switched to other BIs [adjusted odds ratio (aOR): 0.67, hospitalization,P= 0.037; 0.62,ED services,P= 0.007; 0.77, outpatient visits,P= 0.011). Considering all hypoglycaemia-related healthcare-resource utilisation data together, switching to Gla-300 resulted in an overall savings of $1439 per patient per year.
The DELIVER 3 was a retrospective study that assessed in real-world clinical settings the glycaemic control and risk of hypoglycaemia with Gla-300 in older patients with T2DM[42]. Patients with T2DM aged ≥ 65 years already on BI therapy,who switched to either Gla-300 or other BI (Gla-100 or detemir), were identified from the Predictive Health Intelligence Environment database (representing 39 integrated healthcare delivery networks). In this study, 1176 older adults with T2DM who switched from BI to Gla-300 were propensity score-matched to 1176 older adults who switched to a first-generation BI (Gla-100 or detemir). When compared with other BIs,switching to Gla-300 led to greater or similar changes in HbA1c(Gla-300vsother BI mean ± SD - variable follow-up: -0.45% ± 1.40%vs-0.29% ± 1.57%,P= 0.021; fixed follow-up: -0.48% ± 1.49%vs-0.38% ± 1.59%,P= 0.114). Similar proportions of patients in each cohort (Gla-300 and other BI) achieved HbA1c< 7.0% (variable followup: 18.5vs19.7, respectively,P= 0.514; fixed follow-up: 19.3%vs21.3%, respectively,P= 0.292) and < 8.0% (variable follow-up: 49.1%vs49.1%, respectively,P= 1.000;fixed follow-up: 50.9%vs51.8%, respectively,P= 0.773). Patients who switched to Gla-300 were less likely to have hypoglycaemia [Gla-300vsother BI: 0.52vs0.80 events rate PPY; adjusted rate ratio: 0.63 (95%CI: 0.53 to 0.75);P< 0.001] and inpatient/ED-associated hypoglycaemia [Gla-300vsother BI: 0.12vs0.27 events rate PPY; adjusted rate ratio: 0.43 (95%CI: 0.31 to 0.60);P< 0.001 based on variable followup]. The incidence of hypoglycaemia was significantly or numerically lower with Gla-300 during the fixed follow-up, Patient aged ≥ 75 years were more prone to hypoglycaemia compared with overall population[42].
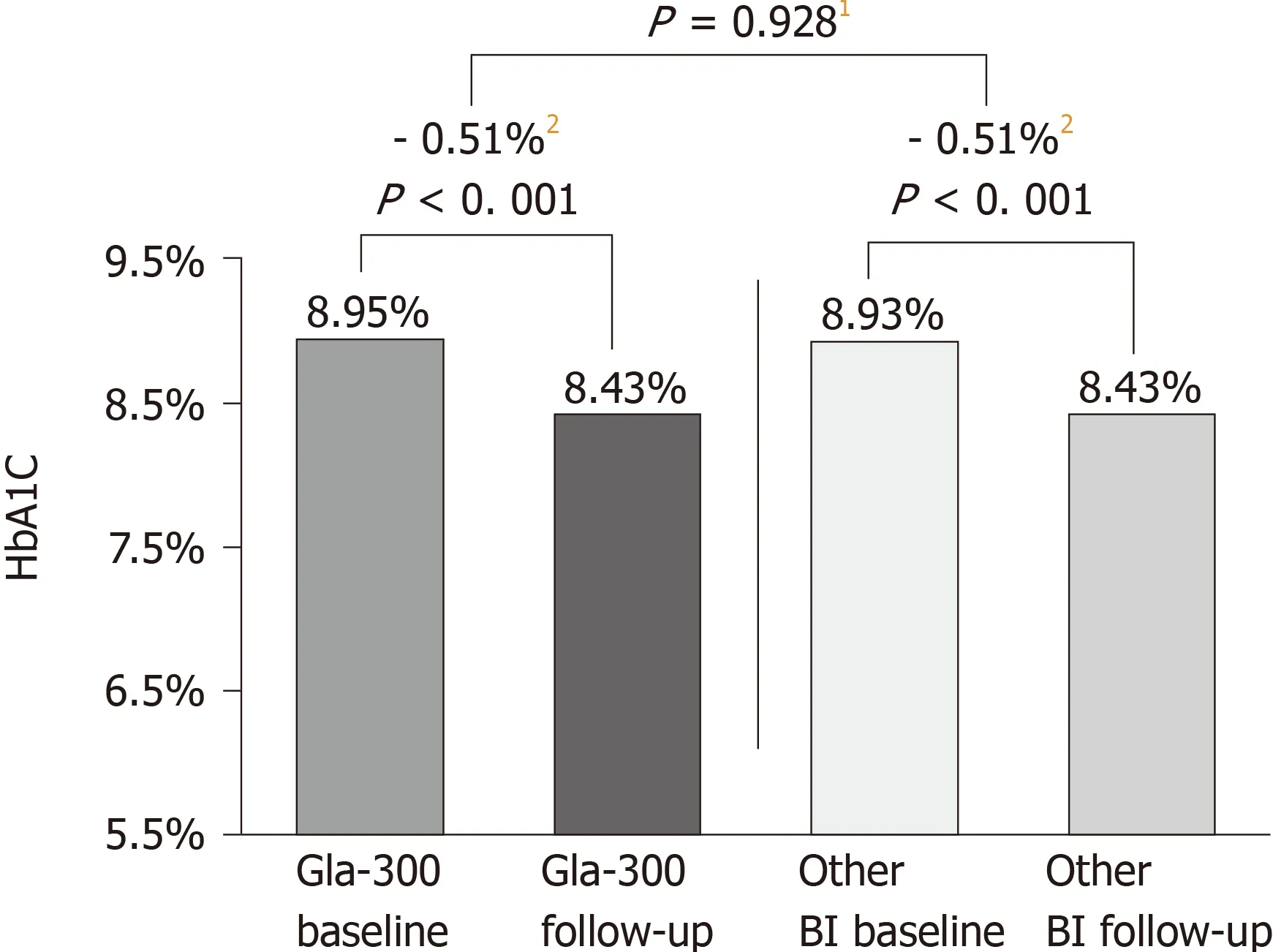
Figure 2 Glycated haemoglobin change during the 6mo followup period (DELIVER 2 Study)[41]. DELIVER 2, a retrospective analysis of electronic medical records from the Predictive Health Intelligence Environment database.1Comparison of mean reduction in Gla300 vs other basal insulin. 2Magnitude of HbA1c change. BI: Basal insulin; Gla-300: Insulin glargine 300 U/mL; HbA1c: Glycated haemoglobin.
DELIVER Naïve was a retrospective study in insulin-naïve patients with T2DM who initiated Glar-300 or Glar-100. During 6-mo follow-up, HbA1creduction was significantly higher in patients who initiated with Gla300 compared with those who initiated Gla-100 (mean ± SD: -1.52 ± 2.08%vs-1.30 ± 2.12%;P= 0.003). More number of patients who initiated Gla-300 achieved target HbA1c< 7% than Gla-100 (25.0%vs21.5%;P= 0.029) and HbA1c< 8% (55.0%vs49.2%;P= 0.002). Gla-300 initiators had lower inpatient/ED-associated hypoglycaemia incidence (OR: 0.35;P= 0.009) during 3-mo follow-up, while, during 6-mo followup, numerically lower all hypoglycaemia incidence (OR 0.77;P= 0.057) and the inpatient/ED incidence (OR: 0.61;P= 0.051)[43].
DELIVER Naïve D was a real world, retrospective, observational study in insulin naïve adults with T2DM who started receiving Gla-300 or IDeg. In matched cohorts (n= 638 each), the decreases in HbA1cfrom baseline to follow-up (between 3 to 6mo from baseline) were significant in both the groups (P< 0.001 for both) and comparable between the groups (mean ± SD: Gla-300 -1.67 ± 2.22%; IDeg 1.58 ± 2.20%;P= 0.51). In addition, incidence of hypoglycaemia (overall and inpatient/EDassociated) was similar between the treatment groups. Results from study suggest that second generation BI analogues offer advantages over firstgeneration BI analogues[44]. The results from this study highlight the real-world impact of Gla-300 were aligned with BRIGHT study, a randomized, controlled trial, that demonstrated a comparable improvement in HBA1c with Gla-300 and IDeg.
The realworld effectiveness of insulin IDeg and Gla-300was compared in insulin naïve adult patients with type 2 diabetes in a retrospective, non-interventional(CONFIRM) study[45]. This study revealed significantly improved effects on both HbA1c and hypoglycaemia with IDeg versus Gla-300; however, the propensity score matching in this study had critical flaws[46]. At baseline, the matched cohorts were not well-balanced in the number of hypoglycaemia episodes prior to insulin initiation(IDegvsGlar-300: 6.7%vs5.6%) as well as the rate of hypoglycaemia per patient years of exposure (PYE) differed (0.301 events/PYE for IDegvs0.210 events/PYE for Gla-300). Both these discrepancies have led to confounding interpretation of hypoglycaemia results (0.391 events/PYE for IDegvs0.389 events/PYE for Gla-300 post-initiation at 180 d of follow-up)[45]. Imbalance in the cohort in terms of hypoglycaemia, before initiation of insulin treatment, may be the reason for dissimilarity of the results reported rather than the effects of treatment[47].
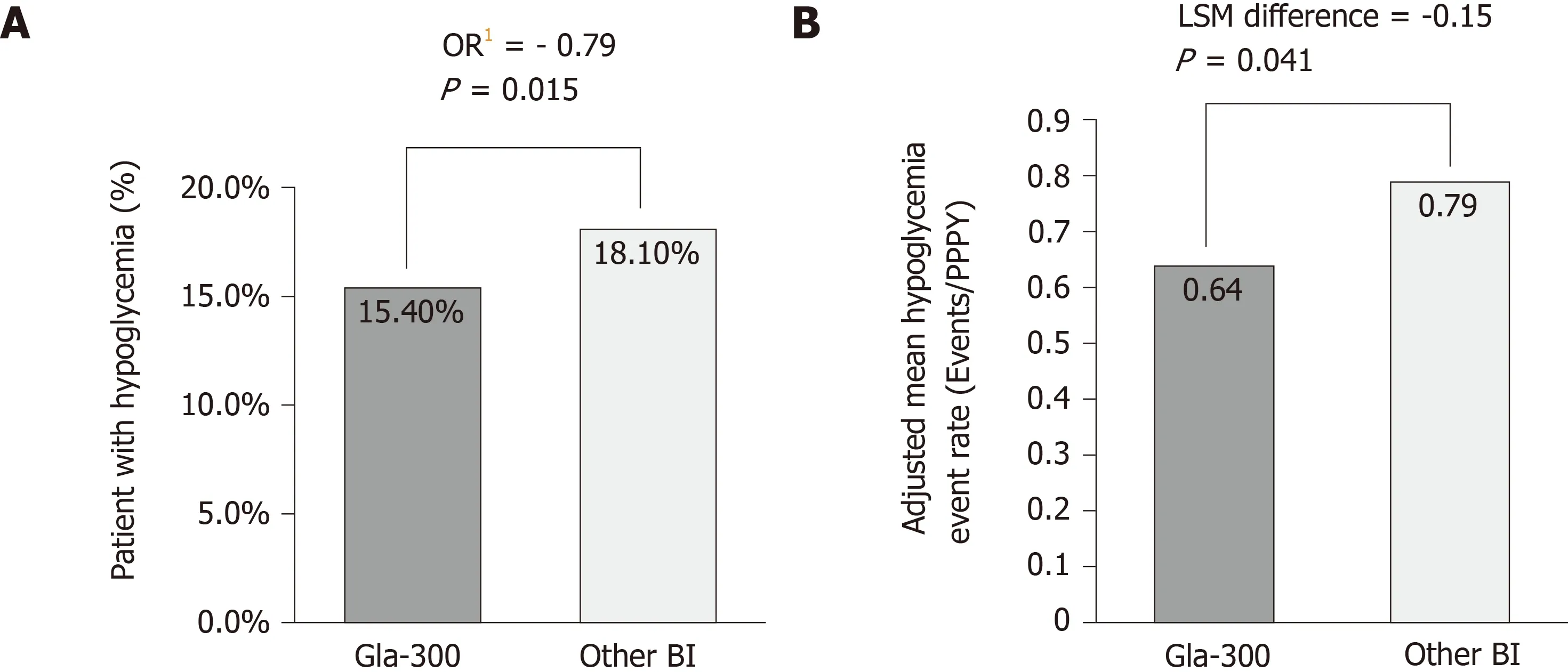
Figure 3 Hypoglycaemia incidence and event rate (DELIVER 2 study)[41]. A: Percentage of patients with hypoglycemia1 at 6-mo after basal insulin switch by insulin type; B: Adjusted mean hypoglycemia event rate2 (Events/Per patient per year) during 6-mo follow-up. DELIVER 2, a retrospective analysis of electronic medical records from the Predictive Health Intelligence Environment database. 1Adjusted for baseline hypoglycaemia incidence; 2Adjusted for baseline hypoglycaemia event rate. BI: Basal insulin; Gla-300: Insulin glargine 300 U/mL; HbA1c: Glycated haemoglobin; OR: Odds ratio; PPPY: Per patient per year.
The LIGHTNING study applied predictive modelling to real-world data and observed similar rates of severe hypoglycemia after switching from another BI to either Gla-300 or IDeg, in clinically vulnerable subgroups of patients with T2DM at high risk of hypoglycemia[47]. The LIGHTNING study compared hypoglycemia rates with Gla-300vsthe first and second-generation BI analogues. Predictive modelling (n= 198198 patient-treatments) showed that rate of severe hypoglycaemia was approximately 50% lower with Gla-300 compared with Gla-100 or insulin detemir in insulin-naïve patients, and 30% lower than insulin detemir in BI switchers (for all,P<0.05). Data analysed using propensity score matching (n= 157573 patient-treatments)showed analogous HbA1creductions with Gla-300 compared with BI analogues (mean± SD in insulin naïve: Gla-300vsIDeg, -1.44 ± 2.32%vs-1.56 ± 2.26%; Gla-300vsGla-100, -1.28 ± 2.16%vs- 1.42 ± 2.09%; Gla-300vsinsulin determir, -1.27 ± 2.15%vs-1.12± 2.06%; mean ± SD in BI switchers: Gla-300vsIDeg, -0.66 ± 1.81%vs-0.60 ± 1.73%;Gla-300vsGla-100, -0.59 ± 1.84%vs-0.52 ± 1.87%; Gla-300vsinsulin determir, -0.59 ±1.84%vs-0.50 ± 2.14%). Rate of severe hypoglycaemia was lower with Gla-300vsGla-100 or insulin detemir (both,P< 0.05) and similar rates versus IDeg were observed in both, insulin-naïve and BI-switcher cohorts[48].
Clinical outcomes in the real-world scenario in T2DM patients switching from the first-generation BI analogues (Gla-100 and detemir) to the second-generation analogues (Gla-300 and IDeg) were assessed in a cohort analysis of the DELIVER D+study[49]. Patients who switched to Gla-300 (n= 1592) or IDeg (n= 1592) were propensity score matched for baseline characteristics and evaluated for incidence of hypoglycaemia during a 12-mo period. The HbA1cchange and target attainment were analysed in patients with HbA1cvalues at baseline and during 3 to 6mo’ followup (742 and 727 in matched Gla300 and IDeg cohorts, respectively). Average decreases in HbA1cand target achievement rates were similar between the Gla-300 and the IDeg groups. Using an intent-to-treat approach revealed that incidence of hypoglycaemia decreased substantially in the Gla-300 group (all hypoglycaemia: 15.6% to 12.7%;P=0.006; hypoglycaemia associated with inpatient/emergency room (ER) visit: 5.3% to 3.5%;P= 0.007) but not in the IDeg group. However, after adjusting for baseline hypoglycaemia, no difference was evident between the Gla-300 and the IDeg groups.Using the on-treatment approach replicated this finding; albeit patients receiving Gla-300 had a lower inpatient/ER visit rate (adjusted rate ratio: 0.56;P= 0.016).
Gla-300 vs insulin degludec - Data from randomized controlled trials
Trial-level meta-analyses of the EDITION (vsGla-100) and BEGIN (vsIDeg)programmes compared the glycaemic control and risk of hypoglycaemia between Gla-300 and Gla-100 or IDeg. In BEGIN, while IDeg achieved a greater fasting plasma glucose reduction than Gla-100, Gla-100 offered a greater reduction in HbA1c(mean difference: 0.09%; 95%CI: 0.01 to 0.18). In EDITION, no difference was observed in fasting plasma glucose and HbA1creduction between both glargine formulations. Risk of nocturnal confirmed or severe hypoglycaemia was lower with IDeg than Gla-100(RR: 0.79; 95%CI: 0.66 to 0.94). When compared with Gla-100, Gla-300 was associated with reduced risk of both nocturnal (RR: 0.75; 95%CI: 0.61 to 0.92) and anytime (24 h)(RR: 0.81; 95%CI: 0.69 to 0.94) confirmed or severe hypoglycaemia[50].
The BRIGHT study is the first head-to-head clinical trial that directly compared the safety and efficacy of second-generation BIs (Gla-300 and IDeg-100) in adults with T2DM who were uncontrolled on OADs (with/without GLP-1 receptor agonist at stable dose for ≥ 3 mo) and insulin naïve[51]. In this study, patients were excluded if found with HbA1c < 7.5 % or > 10.5 % (at screening), BMI < 25 kg/m2or > 40 kg/m2,currently or previously using insulin except for a maximum of 8 consecutive days or totally 15 d (e.g., acute illness, surgery) during the last year prior to screening.BRIGHT was a phase 4, 24-wk, multinational, multicentre, open-label, two-arm,parallel-group trial that enrolled 929 insulin-naïve adults with T2DM inadequately controlled with OADs with or without a GLP-1 receptor agonist. Patients were randomised in 1:1 ratio to receive Gla-300 (0.2 U/kg) or IDeg (10 U) administered once daily using similar treat-to-target titration protocols. Non-inferiority of Gla-300vsIDeg was demonstrated for HbA1cchange from baseline to week 24. Both BIs provided a similar reduction in fasting self-monitored plasma glucose.Hypoglycaemia incidence and rates were comparable with both insulins during the full study period but lower in favour of Gla-300 during the titration period. During the titration period of first 12-wk, the event rates of hypoglycaemia (≤ 70 mg/dL)were lower with Gla-300vsIDeg by 23% (RR: 0.77; 95%CI: 0.62 to 0.96) at any time of day (24 h) and 35% (RR: 0.69; 95%CI: 0.43 to 0.98) at night[52-54].
In a small-scale randomized cross-over study designed to compare the efficacy and safety of Gla-300 and IDeg using continuous glucose monitoring, patients were assessed for average percentage of time with sustained blood glucose levels of 70-180 mg/dL (efficacy) and incidence of hypoglycaemia defined as blood glucose level < 70 mg/dL (safety)[55]. There was no statistically significant difference in mean percentage of time within target glucose range between the Gla-300 and IDeg groups (77.8 ±19.2%vs76.9 ± 18.3%, respectively;P= 0.848). However, the mean percentage of time of hypoglycaemia was substantially lower in the Gla-300 group (1.3 ± 2.7%vs5.5 ±6.4% for IDeg;P= 0.002). This observation held true even for duration of severe or nocturnal hypoglycaemia. Another study also reiterated the comparable efficacy of Gla-300 and IDeg in maintaining blood glucose levels and the better safety profile of Gla-300, especially with regard to nocturnal hypoglycaemia (P= 0.021).[56]
In BRIGHT study, patients with impaired renal function (eGFR < 60 mL/min/1.73 m2) demonstrated greater HbA1creduction with Gla-300 than IDeg (LS mean difference: -0.43; 95%CI: -0.74 to -0.12) and no difference in incidence of hypoglycemia[57]. In addition, HbA1creduction in both treatment arms was similar in patients aged < 70 years but greater with Gla-300 than IDeg-100 in those ≥ 70 years(LS mean difference: -0.34; 95%CI: -0.59 to -0.10) with no difference in incidence of hypoglycaemia[58].
The CONCLUDE study[59](an open-label randomized, active-controlled, 2-arm parallel-group, multicentre, phase 3 study) compared safety and efficacy of IDeg-200vsGla-300, in European and North American adults (n= 1609) with T2DM, who were already taking BI (Glar-100, detemir, or NPH) with or without OAD. In this study,adults (aged > 18 years) with T2DM were included if they met the following criteria:HbA1c ≤ 80 mmol/mol (9.5%), BMI ≤ 45 kg/m2and treated with BI (once or twice daily; NPH insulin, insulin detemir, glargine U100) with/without OADs at stable doses for at least 90 d. Major exclusion criteria were treatment with bolus or premixed insulin or with sulfonylureas/glinides within 90 d before the screening visit, severe renal impairment (eGFR < 30 mL/min·1.73 m), or impaired liver function (alanine aminotransferase or aspartate aminotransferase ≥ 2.5 times the upper limit of normal).The primary end point was to assess the rate of overall symptomatic hypoglycaemia in maintenance period of 36 wk. The results showed that during the maintenance period of 36 wk, the rate of overall symptomatic hypoglycemia in patients treated with insulin degludec was not statistically significant compared to Gla-300. Since the study did not meet the primary endpoint, the secondary endpoints were considered exploratory and not conclusive[60-62].
Role of Gla-300 in diabetes management
The ideal insulin therapy would offer the possibility of once-daily injection with flexible timing accompanied with a low risk of hypoglycaemia and ease of titration.As discussed above, Gla-300 provides a stable PK/PD profile requiring less aggressive titration not more frequently than every 3 to 4 d to achieve a steady state over the dosing period[63,64]. Moreover, Gla-300 was associated with lower incidence of hypoglycaemia in the initial titration (the first 8 wk of treatment) and maintenance phases that would help to mitigate the fear of hypoglycaemia[32,55]. The EDITION trials revealed that Gla-300 achieved comparable HbA1creductions as Gla-100 with a lower risk of confirmed or severe hypoglycaemia. These studies also demonstrated a reduced risk of nocturnal hypoglycaemia even during the titration phase[15,26,27].During the active titration period (0-12-wk) of the BRIGHT study, Gla-300 was associated with reduced risk of anytime confirmed hypoglycaemia (≤ 70 and < 54 mg/dL) than IDeg-100 and a comparable risk of nocturnal confirmed hypoglycaemia(≤ 70 mg/dL)[55]. The safety and efficacy of Gla-300 has also been demonstrated in older populations. Gla-300 can be injected in the morning or evening and the injection device is convenient and easy-to-use[65]. The results from the EDITION development program indicate that a range of patients with both T1DM and T2DM may benefit from Gla-300. Patients at high risk of hypoglycaemia or hypoglycaemia-related events(such as falls) may derive significant benefit. The prolonged duration of action of Gla-300 may benefit those requiring twice-daily insulin, while the flexibility in time of dosing may improve adherence in those with rigid dosing schedules or complex regimens[66].
Gla-300 is administered in a pre-filled, disposable injector. Each pen delivers a maximum dose of 80 units/injection. Due to the pH of the diluent, Gla-300 should not be mixed with other insulins[10]. Patients switching from other once-daily BIs can initiate Gla-300 at the same unit-for-unit dose[67]. Switching from Gla-100 may require dose adjustments as the two glargine formulations are not comparable. For patients switching from twice-daily BI, the recommended starting dose of Gla-300 is 80% of the previous total daily dose of BI. Gla-300 is recommended for once-daily dosing at the same time each day. Follow-on pre-defined evaluation of EDITION 1 and 2 indicate that occasional dosing flexibility (dosing interval 24 ± 3 h) is possible[68]. In addition, patients who self-titrated Gla-300 achieved similar rates of target glucose levels without hypoglycaemia as those with clinician-titrated Gla-300[68]. It has also been reported that the effectiveness of Gla-300 is not dependent on the duration of prior BI therapy or other concomitant anti-hyperglycaemic treatments[58,69].
While the efficacy and safety of Gla-300 have been demonstrated in the EDITION clinical trials, a comprehensive series of both prospective (observational and interventional) and retrospective real-world evidence studies will provide further evidence on the clinical and economic benefits provided by Gla-300 in a range of diabetes populations.
In sulin glargine as active component: Other considerations
Gla-100 is a widely used BI and has been studied extensively, pre- and post-licensure,and its safety has been well-established. One of the seminal studies to investigate the outcomes of BI use in > 12000 people with type 2 diabetes presenting with cardiovascular risk factors, the ORIGIN trial, concluded that after a median follow-up of 6.2 years, Gla-100 had no discernible association with cardiovascular outcomes or cancers[70,71]. Gla-300 comprises the same glargine molecule as Gla-100. The advantage offered by Gla-300 over Gla-100 is one of improved PK of insulin glargine release from the injected site thereby offering a smoother plasma insulin plateau for a longer duration. Following dose titration in the EDITION and BRIGHT trials, it was observed that a higher dose of Gla-300 was required to attain target HbA1c[31,55].Importantly, despite of slight dose difference, Gla-300 demonstrated lower (vsGla-100 and IDeg during titration period) or similar hypoglycemia (vsIDeg during the whole study and maintenance period) with similar changes in weight gain. One explanation for this could be differences in degradation of insulin glargine at the injection site due to longer residence time of the Gla-300 SC depot in comparison to Gla-100 or insulin IDeg[15-19]. However, despite this increase in dose and comparable glycaemic control,Gla-300 was associated with a lower incidence of hypoglycaemia and lesser weight gain, the most common clinical concerns with insulinisation in people with diabetes.
Real-world study evaluating medical records of patients with T2DM from an US database showed that switching to Gla-300 from another BI lowers HbA1c,hypoglycaemic events and frequency of dosing, with numerically lower daily insulin dose. This suggests that Gla-300 in the real-life settings did not lead to dose increases and was associated with less frequent daily dosing[40].
A real-world study in Europe (France, Spain, and Germany)[72]evaluated clinical outcomes in patients with T2DM who switched from another BI to Gla-300 or Gla-100.Switching to Gla-300vsGla-100 showed similar changes in glycaemic control and weight from baseline, a significantly greater reduction in the number of hypoglycaemia events, and no differences in weight-adjusted insulin dose change.
There is no international standard established for ascertaining the potency of insulin analogues. Moreover, “units” used to describe insulin analogues are not equivalent to ‘International Units’ that are used to describe the potency of insulins for which an international standard existse.g., human insulin. Hence, the units used to express the potency of Gla-300 are unique to insulin analogues manufactured by Sanofi (Lantus®, Apidra®, Toujeo®).
CONCLUSION
Data from clinical trials and real-world experience have shown that Gla-300 offers a number of benefits in patients with T1DM/T2DM in comparison to other available insulins and insulin analogues. Individual results from the EDITION programme and the meta-analyses of these show that Gla-300 is non-inferior to Gla-100 in attaining HbA1c targets in both insulin-naïve and insulinised patients. Additionally, the EDITION trials demonstrated a reduced risk of hypoglycaemia during the titration phase that could help to build patient confidence to initiate and properly titrate their BI with less fear of hypoglycaemia. These results were recapitulated in older people in the SENIOR trial. Furthermore, the BRIGHT study found Gla-300 comparable to insulin IDeg, another second-generation BI analogue, in reduction of HbA1c levels.The most notable advantage with Gla-300 observed in these trials was the reduction in incidence of hypoglycaemia (especially in the titration period) as well as the risk of weight gain. Evidence from the DELIVER and LIGHTNING studies indicate that these findings with Gla-300 have been translated in real-world settings. At the time of this review, various other real-world studies are either ongoing or have just been completed. When these data are collectively considered, we can conclude that Gla-300 addresses the critical issues of hypoglycaemia and weight gain and has the potential to improve rates of insulinisation in people with T1DM/T2DM.
ACKNOWLEDGEMENTS
Drafting and editorial support in the preparation of this publication was provided by Satyendra Shenoy of Describe Scientific Writing & Communications. Satyendra Shenoy of Describe Scientific Writing & Communications would like to acknowledge Irene Farré of IF Medical Writing and Derek Ho. Drafting and editorial support in preparation of this publication was also provided by Sonal More of Tata Consultancy Services and was paid for by Sanofi. Editorial support was provided by Anahita Gouri and Rohan Mitra of Sanofi India. The authors individually and collectively are responsible for all content and editorial decisions and did not receive any payment from Sanofi directly or indirectly (through a third party) related to the development or presentation of this publication.
杂志排行
World Journal of Diabetes的其它文章
- Age of onset of diabetes and all-cause mortality
- Hydrocortisone, ascorbic acid and thiamine for sepsis: Is the jury out?
- Long-term effect of clopidogrel in patients with and without diabetes: A systematic review and metaanalysis of randomized controlled trials
- Novel insight into perirenal adipose tissue: A neglected adipose depot linking cardiovascular and chronic kidney disease
- Lack of Syndecan-1 produces significant alterations in whole-body composition, metabolism and glucose homeostasis in mice
