Design of Mg–Zn–Si–Ca casting magnesium alloy with high thermal conductivity
2020-04-29BzhenovKoltyginSungPrkYuTitovButinMtveevBelovBelovMlyutin
V.E. Bzhenov, A.V. Koltygin, M.C. Sung, S.H. Prk, A.Yu. Titov, V.A. Butin,S.V. Mtveev, M.V. Belov, V.D. Belov, K.V. Mlyutin
a National University of Science and Technology “MISiS”, Leninskiy pr. 4, Moscow 119049, Russia
b LG Electronics Inc., Yeoui-daero, Yeongdeungpo-gu. 128, LG Twin Tower, Seoul 07336 Republic of Korea
Abstract Magnesium alloys are commonly used to produce lightweight parts. While most magnesium alloys exhibit low thermal conductivities,high thermal conductivities are needed for electronic devices. In this study, we attempted to develop new magnesium casting alloys with high thermal conductivities. The Mg–Zn–Si–Ca alloy compositions were chosen using CALPHAD (CALculation of PHAse Diagrams) calculations,and alloy samples were prepared. The fluidity and hot-tearing resistance were measured. The results indicated that these properties were similar to those of AZ91 alloy. Tensile tests showed that high-pressure die casting could produce Mg–Zn–Si–Ca alloys possessing mechanical properties 1.5–3 times higher than those produced via sand casting. The alloy thermal conductivity was 126W/mK at room temperature. The corrosion rates of the as-cast samples in NaCl/water solutions were two times higher than that of AZ91.
Keywords: Magnesium alloy; Thermal conductivity; Phase composition; Corrosion rate; Sand casting; High-pressure die casting.
1. Introduction
Magnesium alloys are commonly used as aircraft and aerospace engineering construction materials because they are lightweight and have high strength-to-weight ratios. However,they are not commonly considered for automobile electronics,household appliances, and other electronic devices because of their low thermal conductivities. Magnesium alloys can also be expensive due to the use of cerium, neodymium, yttrium,thorium, zirconium, and silver as alloying elements to improve strength,and these high costs can limit their application[1–4].
Pan et al. investigated the thermal conductivities of magnesium binary alloys and found that alloying elements can be arranged in the following order of decreasing thermal conductivity: Zn, Al, Ca, Sn, Mn, and Zr [5]. Thus, Zn can be used in high-thermal-conductivity magnesium alloys. Si and Ca have no influence on the thermal conductivity due to their low solid solubilities in Mg [6,7].
The following magnesium casting alloys have high thermal conductivities (>100W/mK): EQ21, QE22A(Mg–RE–Ag–Zr); EZ33A, ZE63A, ZE41A (Mg–Zn–RE–Zr);ZH62A, HZ32A, HK31A (Mg–Zn–Th–Zr); ZK51A(Mg–Zn–Zr); and ZC63 (Mg–Zn–Cu–Mn) [4]. Only ZC63 has a thermal conductivity greater than 120W/mK. Copper is known to have a detrimental effect on the Mg corrosion rate. Because of this, it must be used with care [8].
Mg–Al–Si alloys are currently used in the high-pressure die casting (HPDC) of parts. For example, AS21, AS21X,and AS42 alloys are well known [9–12]. The strengthening of AS-series alloys is achieved primarily via Mg2Si phase particles [13]. However, the aluminum in these alloys decreases their thermal conductivities. For example, the thermal conductivity of AS21 is 68W/mK at room temperature [14].Mg–Si–(Ca,Zn) and Mg–Zn–Si alloys have been synthesized for biomedical applications due to the biological function of Si in the human body [15,16]; however, no information about their thermal conductivity is available.
The Mg–Si–Zn–Ca system was chosen for the development of high-thermal-conductivity alloys. All the components in this system are low cost, and the alloys of this system are known to exhibit high thermal conductivities. Zinc acts to strengthen the solid solution [9,10], while calcium improves ductility and creep resistance, promotes grain refinement, and increases the ignition temperature of the melt[9,10,17].While silicon improves creep resistance and affects grain size[9,10],a high Si content can cause the formation of primary Mg2Si and reduce alloy ductility [18]. The goal of this study was to develop an Mg–Si–Zn–Ca magnesium alloy with high thermal conductivity.
2. Materials and methods
2.1. Sample production via gravity casting
Magnesium (99.9 wt%), silicon (99.99 wt%), zinc(99.98 wt%), and calcium (99.9 wt%) were used as raw materials. In addition, Mg-30 wt% Ca and Mg-10 wt% Si master alloys were prepared to aid in the solvation of alloying elements in the melt and to decrease elemental losses. The alloys were melted in a steel crucible using a high-frequency induction furnace. The melt was protected from oxidation by a carnallite flux cover.
The spiral test was used for fluidity measurements. A nobake sand mold was produced, and the test was performed at 740°C [19]. The hot-tearing resistances of the alloys were determined by “dog bone” testing [20] after pouring the sample into a steel mold at 740°C. The alloy hot-tearing resistance was evaluated based on the dog-bone section length achieved without cracking. The fluidities and hot-tearing resistances of our experimental alloys were compared to those of AZ91 magnesium alloy. Cylindrical samples with diameters of 12mm were cast into no-bake sand molds to measure the mechanical properties of the alloys.
2.2. Sample production via HPDC
Samples were produced via HPDC using a TOYO Mg-125 cold-chamber machine.The holding furnace capacity was 500kg of magnesium alloy. To prevent melt ignition, the furnace and die were protected using a mixture of N2and SF6.The die temperature was 200°С, and the temperature of the injected alloy was 680°С.
The HDPC casting scheme is shown in Fig. 1. The die used allowed us to produce samples for fluidity measurements(left) and tensile tests (center) along with bar samples (right).
2.3. Methods for the determination of alloy properties
Chemical compositions were determined via energydispersive X-ray spectroscopy (EDS). Areas with dimensions of 1×1mm were analyzed. The alloy chemical compositions and preparation details are presented in Table 1.
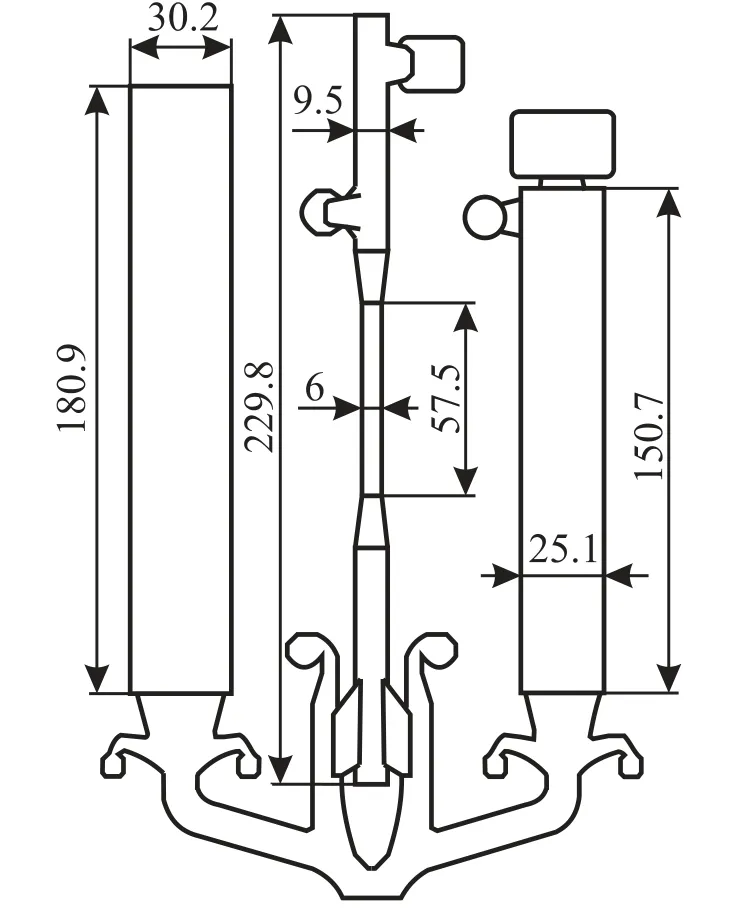
Fig. 1. Schematic showing the probe produced by HDPC (dimensions in mm).
The alloy microstructures were investigated using scanning electron microscopy (SEM; Tescan Vega SBH3) and an optical microscope (Carl Zeiss Axio Observer D1m). The SEM microscope was equipped with an EDS system (Oxford Instruments AZtecEnergy). To reveal the sample microstructure,an etchant composed of picric acid (4g), acetic acid (0.5ml),nitric acid (0.5ml), and ethanol (100ml) was used.
Polythermal sections, liquidus projections, and solidification pathways were calculated using the CALPHAD (CALculation of PHAse Diagrams) method in Thermo-Calc Software [21] with version 4 of the TCMG4 magnesium-based alloy database [22]. Tensile tests on the samples described above were performed using an Instron 5569 universal testing machine.
Room-temperature (25°C) density (ρ) measurements were carried out via hydrostatic weighing.A NETZSCH DIL 402 C dilatometer was used to obtain the thermal expansion coefficients. Next, the temperature dependence of ρ was calculated.The thermal diffusivity (a) was measured via the laser flash method using a NETZSCH LFA 447 instrument. The heat capacity (Cp) was determined using a NETZSCH DSC 204F1 Phoenix calorimeter.The temperature dependences of the density, thermal diffusivity, and heat capacity were implemented as third-order polynomials, and the thermal conductivity (λ)was calculated using the following euqation:

Electrochemical investigations of the Mg–Si–Ca–Zn and AZ91 alloys were performed in 3 wt% NaCl solutions in water. The experiments were performed using an IPC Pro-MF potentiostat/galvanostat/FRA corrosion system at 25°C.A three-electrode system was employed in which the alloy samples served as working electrodes with an exposure areaof nearly 1 cm2. Platinum and saturated calomel electrodes were used as the counter and reference electrodes, respectively. Potentiodynamic polarization experiments were performed from the cathodic region at −2300mV to the anodic region at −1000mV with a scan rate of 1mV/s. The corrosion current density (icorr) and corrosion potential (Ecorr) were determined from Tafel fitting, and the corrosion rate was calculated [23].

Table 1 Alloy chemical compositions and preparation details.
Immersion corrosion tests were performed using rectangular Mg–Si–Ca–Zn and AZ91 alloy specimens with sizes of approximately 30×20×10mm and a mean surface area of 20 cm2. The corrosion media were the same as those used in the electrochemical corrosion tests. The specimens were weighed prior to the tests. The test duration was 6 d. Once each test was finished, the sample was extracted, dried in air,and then weighed again to calculate the mass gain per unit of surface area. Finally, the mass loss and corrosion rate in mm per year were calculated [24].
3. Results and discussion
3.1. Mg–Zn–Si–Ca system phase equilibrium calculations
The liquidus surface projection and primary solidification regions of various phases in the Mg–Zn–Si–Ca system at 0.7 wt% Zn and various Si and Ca contents are shown in Fig. 2.Three regions of primary solidification are observed: magnesium solid solution (Mg), Mg2Si, and CaMgSi. Coarse Mg2Si primary crystals are formed when the Ca and Si contents are low and high, respectively. Primary CaMgSi crystal formation is observed when both the silicon and calcium contents are high. CaMgSi primary crystals are preferable for the alloy microstructure since their crystals are finer than those of Mg2Si [25,26].
Fig.3(a)shows the Mg–Zn–Si–Ca polythermal section that exists when the Ca and Si contents are held at 0.5 wt% and 1 wt%, respectively, and the Zn content is allowed to vary. The liquidus line in Fig. 3(a) is marked in red. Increases in Zn content do not significantly influence the liquidus temperature. However, the influence of Zn on the solidus temperature[the solidus line is shown in blue in Fig. 3(a)] is significant.If the Zn content exceeds 6 wt%,the solidification range exceeds 300°C. This means that the hot-tearing susceptibility of the Mg–Zn–Si–Ca alloy increases with increasing Zn content. Thus, alloys with Zn contents near 1 wt% are preferable because their solidification range is near 100°C.
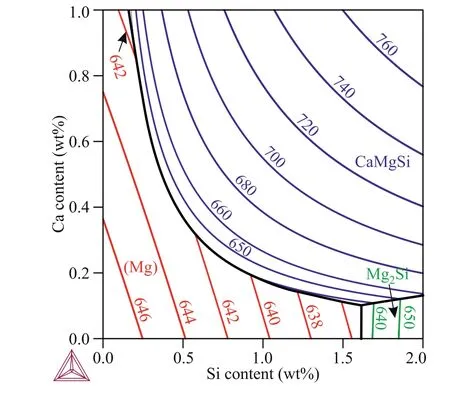
Fig. 2. Liquidus surface projection and primary solidified phase regions in Mg–0.7 wt% Zn–(0–2) wt% Si–(0–1) wt% Ca alloys.
The influence of the Si content on Mg–Zn–Si–Ca system equilibrium at constant Zn and Ca contents of 0.7 wt% and 0.5 wt%, respectively, is shown in the polythermal section in Fig. 3(b). At Si contents less than 0.35 wt%, solidification starts with the formation of (Mg) primary crystals and ends with formation of the (Mg)+CaMgSi eutectic. The solidification range is large for these alloys. In alloys with Si contents greater than 0.35 wt%, solidification starts with CaMgSi primary crystal formation. The solidification of these alloys ends with formation of the ternary(Mg)+CaMgSi+Mg2Si eutectic.At the same time, alloys with Si contents (>1.1 wt%) exhibit liquidus temperatures exceeding 700°C. Thus, the Si content in the desired Mg–Zn–Si–Ca alloy must be near 1 wt%.
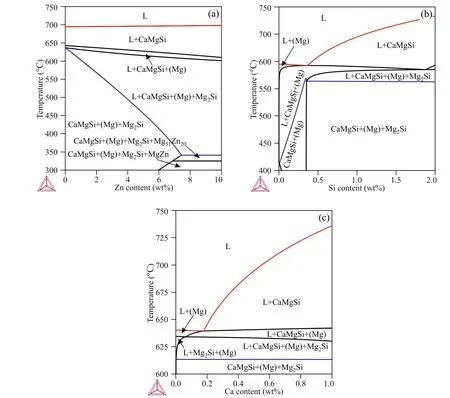
Fig. 3. Polythermal sections: (a) Mg–0.5 wt% Ca–1 wt% Si–(0–10) wt% Zn; (b) Mg–0.7 wt% Zn–0.5 wt% Ca–(0–2) wt% Si; and (c) Mg–0.7 wt% Zn–1 wt%Si–(0–1) wt% Ca.
The Mg–Zn–Si–Ca polythermal section based on constant Zn and Si contents of 0.7 wt% and 1 wt%, respectively, and varying Ca content is shown in Fig. 3(c). The solidus temperature does not change as the Ca content increases because solidification ends with the formation of the(Mg)+CaMgSi+Mg2Si eutectic for all alloys. If the Ca content exceeds 0.2 wt%, the primary solid phase is CaMgSi rather than (Mg). Further increases in Ca content promote an increase in the liquidus temperature. Thus, the Ca content of the Mg–Zn–Si–Ca alloy must be in the range of 0.3–0.6 wt%.
The solidification pathways calculated using the Sheil–Gulliver model [27] for the alloys designated A, B, and C in Table 1 are shown in Fig. 4. These alloys were chosen to clarify the influences of the Si and Ca contents on the Mg–Zn–Si–Ca alloy phase composition and solidification range in conditions far from the typical equilibrium conditions associated with this casting technique.
All the considered alloys have nearly the same solidification pathway. CaMgSi primary crystals solidify first. However, the liquidus temperature of alloy C is higher than those of alloys A and B because of its higher Ca content. Next,the solidification of the(Mg)+CaMgSi binary eutectic begins.Alloy solidification ends via the solidification of a ternary eutectic that consists of (Mg), CaMgSi, and Mg2Si phases. As a result, non-equilibrium conditions double the solidification range. However, the solidification temperature does not exceed 200°C for any of the investigated alloys.
In the Mg–Zn–Si–Ca alloys, the (Mg)+CaMgSi binary eutectic fraction decreases with increasing silicon content, while the fraction of (Mg)+CaMgSi+Mg2Si ternary eutectic increases. In accordance with the liquidus surface projection shown in Fig. 2, alloys with higher Si contents (e.g., Mg-1.22 wt% Si-0.54 wt% Zn-0.27 wt% Ca alloy) are closer to the ternary eutectic point and have higher amounts of ternary eutectic.
3.2. Mg–Zn–Si–Ca alloy microstructures
The as-cast microstructure of the Mg–Zn–Si–Ca alloy obtained via sand mold casting (alloy A in Table 1) is presented in Fig. 5(a). The EDS maps showing the Si and Ca distributions within the alloy microstructure are shown in Fig. 5(b) and (c), respectively. The bright phase contains both Si and Ca, and the mean composition determined by EDS analysis is Mg-28.3 at% Si-26.0 at% Ca. The dark phase contains Si but not Ca, and its composition is Mg-28.7 at% Si. In accordance with the EDS analysis, CALPHAD calculations, and previously reported data [15,26], the bright and dark phases can be designated as CaMgSi and Mg2Si,respectively. The as-cast microstructure of alloy A consists of (Mg) dendrites along with CaMgSi and Mg2Si eutectic phases at the dendritic cell boundaries. The calculated solidification pathway suggests that some of the bright particles may be CaMgSi primary crystals.
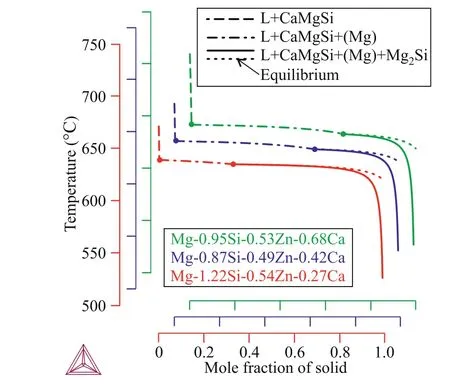
Fig. 4. Alloy solidification pathways calculated using the Scheil–Gulliver model. The equilibrium solidification pathway is also shown.
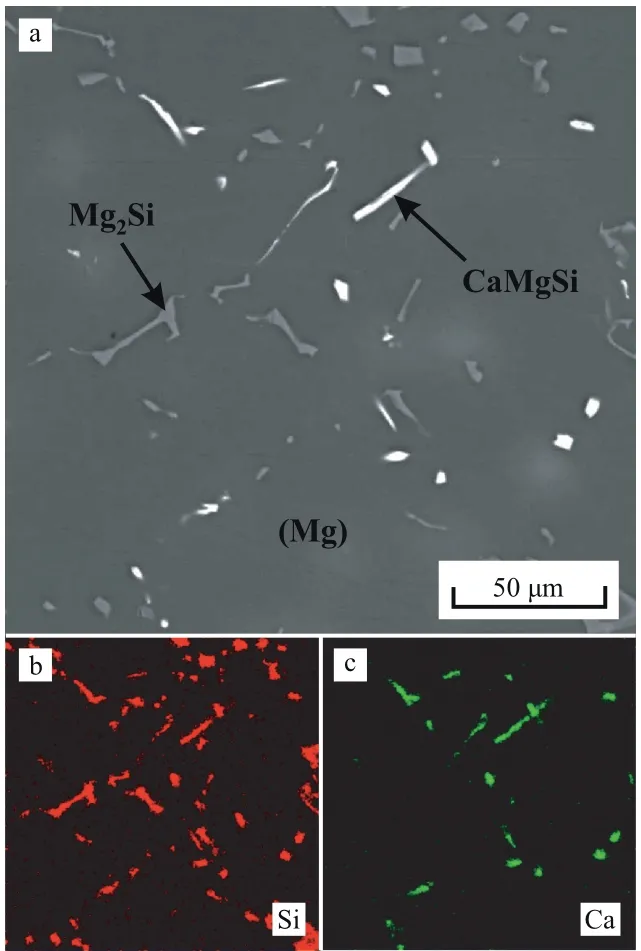
Fig. 5. As-cast (sand mold casting) Mg–Zn–Si–Ca alloy microstructures (alloy A in Table 1): (a) SEM image and EDS maps showing the (b) Si and(c) Ca distributions.
It is known that Ca, La, Y, KBF4, etc. are used for morphological refinement, and decreasing the Mg2Si particle size improves the mechanical properties of the alloy [15,28–33].However, no information is available about the influence of these additions on CaMgSi morphology and size refinement.Heat treatment can also favor the spheroidization of the Mg2Si eutectic phase [34]; however, the morphology of the CaMgSi phase does not change significantly after 200h of heat treatment at 550°C [26]. Thus, we cannot use additions or heat treatments to improve the mechanical properties of the alloys.
The microstructures of Mg–Zn–Si–Ca alloys B and C(Table 1) obtained via HPDC are shown in Fig. 6(a) and (b),respectively. The phases in these microstructures are much finer than those in the alloy casted using a sand mold. This is attributed to the high cooling rate associated with HPDC.Only (Mg) primary crystals are observed in the alloy B microstructure containing 0.42 wt% Ca. The solidification pathway calculated for alloy B indicates that the CaMgSi phase must solidify first; however, the volume fraction of this phase is too low. Unlike the previously analyzed microstructures,that of alloy C containing 0.68 wt% Ca [Fig. 6(b)] contains CaMgSi primary crystals with lengths that do not exceed 20μm.
The grain sizes of the Mg–Zn–Si–Ca alloys obtained via sand mold casting (alloy A) and HPDC (alloy C) were analyzed using the linear intercept method on the etched samples.The grain sizes of the alloys obtained via sand mold casting and HPDC are 390 ± 150 and 42 ± 13μm, respectively.
The (Mg) solid solution composition was estimated via EDS analysis. The Si and Ca contents are lower than the EDS determination limit. These results support the observed high thermal conductivities of Mg–Zn–Si–Ca alloys.
3.3. Comparison of Mg–Zn–Si–Ca and AZ91 alloy castabilities
Alloys must have sufficient castability to be suitable for part production. The fluidities and hot-tearing resistances of the Mg–Zn–Si–Ca and AZ91 alloys are presented in Fig. 7.The hot-tearing test results show that the maximum length of an un-cracked Mg–Zn–Si–Ca alloy dog-bone section (alloy A in Table 1) is 72mm. For comparison, the same parameter for AZ91 is only 50mm. This indicates that the hot-tearing resistance of the Mg–Zn–Si–Ca alloy is higher than that of AZ91.
The fluidities determined by sand mold spiral testing are presented in Fig. 7. The spiral lengths of Mg–Zn–Si–Ca alloy A and AZ91 are similar (430 and 450mm, respectively). The fluidity of AZ91 was also measured in Ref. [19] at the same pouring temperature. In this case, the mean spiral length was 446mm. The fluidity (probe length) obtained via the HPDC of Mg–Zn–Si–Ca alloy D and AZ91 are 139mm and 140mm,respectively. The equal fluidities of these alloys may be attributed to the high pressures applied to the alloys during injection. High pressures can mask differences in alloy fluidities when HPDC is used. We can conclude that the fluidities of Mg–Zn–Si–Ca and AZ91 alloys are nearly the same.
3.4. Mg–Zn–Si–Ca alloy mechanical properties
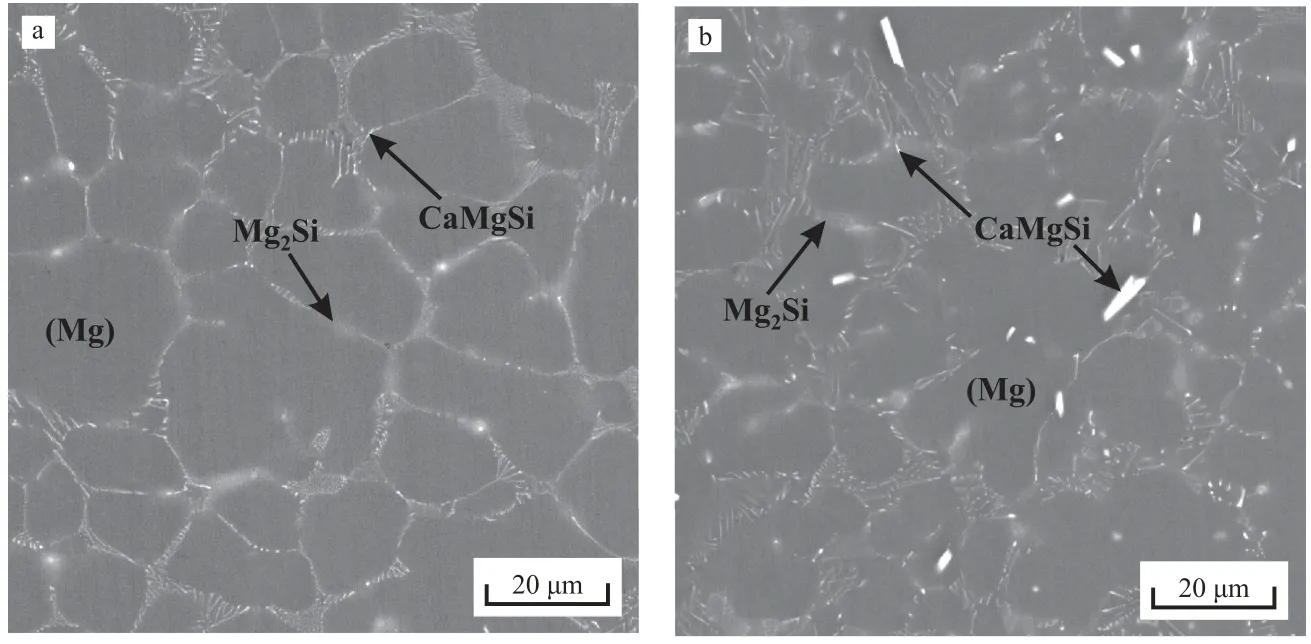
Fig. 6. Microstructures of as-cast (HPDC) Mg–Zn–Si–Ca alloys (a) B and (b) C. The alloy compositions are presented in Table 1.

Fig. 7. Hot-tearing resistances and fluidities of Mg–Si–Zn–Ca and AZ91 alloys.
The tensile properties of the Mg–Zn–Si–Ca alloys obtained via sand mold casting (alloy A) and HPDC (alloy D) are shown in Fig. 8. The alloy yield strength (YS) is 57MPa after sand casting but 117MPa after HPDC. The ultimate tensile strength (UTS) of the sand mold-casted sample is 133MPa, while that of the sample produced via HPDC is 215MPa. The elongation of the sample formed by HPDC(12%)was also greater than that of the sand mold-casted alloy(4.5%). Thus, when sand mold casting is replaced by HPDC,the Mg–Zn–Si–Ca alloy mechanical properties are enhanced by 1.5–3 times. The mechanical properties of the alloy with a composition close to that of alloy D but obtained by sand mold casting were also investigated. The mechanical properties of this alloy were a little worse than those of alloy A. This result is attributed to the formation of large primary CaMgSi crystals. To achieve good mechanical properties via sand mold casting, the Ca content in Mg–Si–Ca–Zn alloy must be lower.
3.5. Mg–Zn–Si–Ca alloy thermal conductivities
The thermal properties and density of the Mg–Zn–Si–Ca alloy produced via sand mold casting (alloy A) are shown in Fig. 9. The alloy density and thermal diffusivity decrease as the temperature increases, while the heat capacity increases.
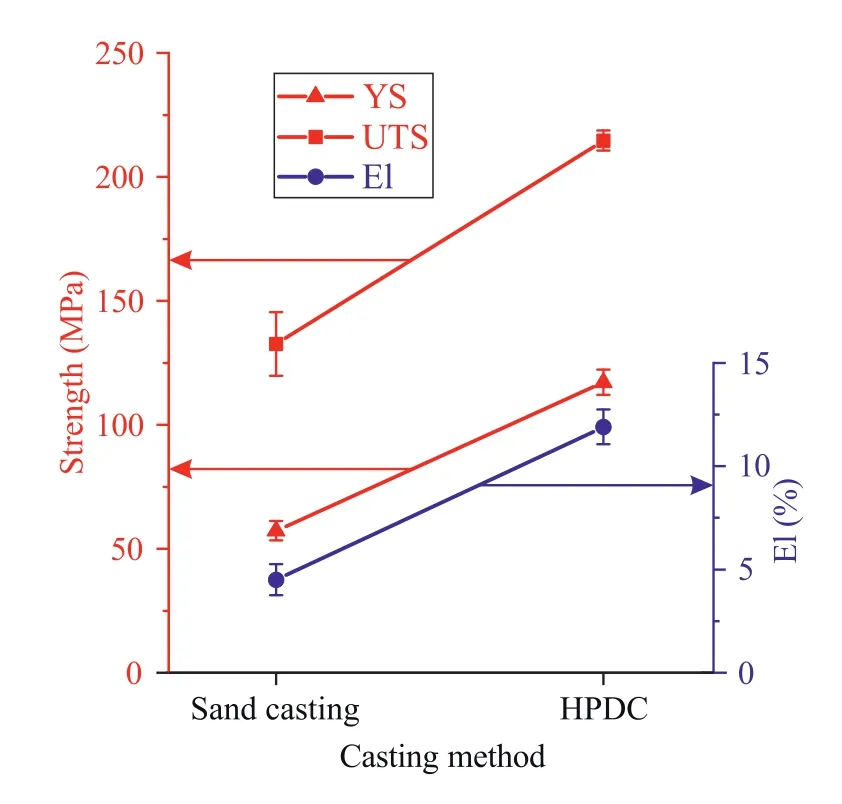
Fig. 8. Mechanical properties of Mg–Zn–Si–Ca alloys after sand casting (alloy A) and HPDC (alloy D).
The thermal conductivity [Eq. (1)] increases with increasing temperature from room temperature to 125°C and then decreases. The room-temperature thermal properties and density of Mg–Zn–Si–Ca alloy A are as follows: ρ = 1.76g/cm3,a=69 mm2/s, Cp= 1.03J/gK, and λ = 126W/mK. For comparison, the thermal conductivity of pure Mg at 27°C is 156W/mK [35]. Thus, the thermal conductivity of the designed alloy is 81% that of pure magnesium.
3.6. Comparison of Mg–Si–Ca–Zn and AZ91 alloy corrosion behaviors
The results of electrochemical investigations of Mg–Zn–Si–Ca and AZ91 alloys performed in 3 wt% NaCl solution are shown in Fig. 10. For each sample, 2–3 curves were constructed. Two types of samples were used to clarify the influence of the cooling rate on the corrosion resistance of Mg–Zn–Si–Ca alloy. The first sample was cast into a sand mold (alloy E in Table 1), while the second was cast into a graphite mold (alloy F in Table 1). For comparison, a sample of AZ91 alloy was obtained via graphite mold casting. No heat treatment was applied to these alloys.

Fig. 9. Properties of Mg–Zn–Si–Ca alloy A: heat capacity (Cp), density (ρ),thermal diffusivity (a), and thermal conductivity (λ).
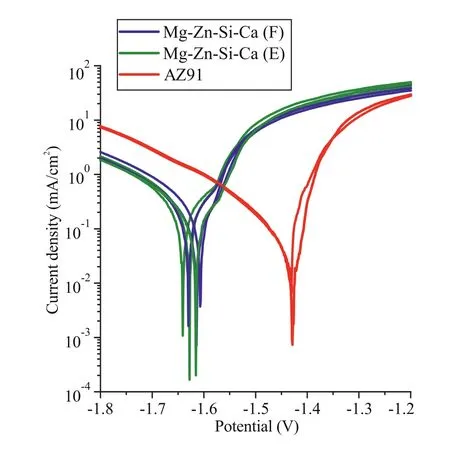
Fig. 10. Polarization curves of Mg–Zn–Si–Ca alloys produced via sand mold casting (alloy E) and graphite mold casting (alloy F). The curves for graphite mold-casted AZ91 are also shown.
As shown in Fig. 10, the polarization curves of alloys E and F are nearly the same. The mean corrosion potential and current density of alloy E (sand mold casting) are −1.61V and 0.17mA/cm2, respectively. For alloy F (graphite mold casting), the mean corrosion potential and current density are−1.60V and 0.19mA/cm2, respectively. Thus, we can conclude that the higher cooling rate during solidification using graphite mold casting does not significantly change the corrosion behavior. The corrosion potential of AZ91 is more positive (−1.42V) than those of its counterparts, and the current density (0.12mA/cm2) is nearly two times lower than those of the Mg–Zn–Si–Ca alloys.

Fig. 11. Corrosion rates of Mg–Zn–Si–Ca alloys produced via sand mold casting (alloy E) and graphite mold casting (alloy F) obtained by electrochemical and immersion corrosion testing. The results for graphite moldcasted AZ91 are also shown.
The calculated corrosion rates of alloys E and F along with AZ91 obtained via electrochemical and immersion corrosion tests are shown in Fig. 11. The corrosion rates for E and F alloys obtained via electrochemical testing (immersion testing)are 3.9 (3.6) mm/year and 4.2 (4.4) mm/year, respectively.Thus, the corrosion rates of Mg–Zn–Si–Ca alloys obtained via sand mold and graphite mold casting are the same. In addition, the results of both immersion and electrochemical testing are the same. The corrosion rates of AZ91 alloy determined by electrochemical and immersion testing are 2.5 and 2.1mm/year,respectively.Thus,we can conclude that the corrosion resistances of the as-cast Mg–Zn–Si–Ca alloys are near two times lower than that of AZ91. However, this is not a serious problem because of the use of coatings on magnesium alloy parts.
4. Conclusions
Mg–Zn–Si–Ca alloys with compositions of Mg(0.5–0.8)%–Zn(0.8–1.3)%–Si(0.2–0.8)%–Ca(wt%) were designed and can be recommended as high-thermal-conductivity alloys for part production via sand casting or HPDC. The conclusions regarding the properties of these alloys are summarized as follows:
(1) The calculated equilibrium solidification ranges of these alloys is near 100°C.In non-equilibrium conditions,the solidification range doubled based on calculations using the Scheil–Gulliver solidification model.
(2) The as-cast alloy microstructure consisted of primary (Mg) dendrites and eutectics that included (Mg),CaMgSi,and Mg2Si phases.Small quantities of primary CaMgSi crystals with sizes near 20μm were also observed in the high-Ca-content alloy microstructures.
(3) Compared to AZ91 magnesium alloy, the Mg–Zn–Si–Ca alloys exhibited the same fluidities and higher hot-tearing resistances.
(4) Tensile testing of the Mg–Zn–Si–Ca alloy obtained via sand mold casting indicated a YS of 57MPa, UTS of 133MPa, and elongation of 4.5%. When HPDC was used, the tensile properties were 1.5–3 times higher. In this case, YS was 117MPa, UTS was 215MPa, and elongation was 12%.
(5) The room-temperature thermal conductivity of Mg–Zn–Si–Ca alloy was 126W/mK, 81% that of pure magnesium.
(6) The corrosion rates of the as-cast alloys in 3 wt%NaCl solution were approximately 4mm/year for samples obtained using sand and graphite mold casting.For comparison, the corrosion rate of AZ91 was two times lower (approximately 2mm/year) than those of the Mg–Zn–Si–Ca alloys.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Latest research advances on magnesium and magnesium alloys worldwide
- Advances in coatings on biodegradable magnesium alloys
- Stability of twins in Mg alloys – A short review
- A review on thermal conductivity of magnesium and its alloys
- Effect of Ca addition on the microstructure and the mechanical properties of asymmetric double-sided friction stir welded AZ61 magnesium alloy
- Effect of pre-deformation on microstructure and mechanical properties of WE43 magnesium alloy II: Aging at 250 and 300 °C
