Latest research advances on magnesium and magnesium alloys worldwide
2020-04-29JiangfengSongJiaSheDaolunChenFushengPan
Jiangfeng Song, Jia She,∗, Daolun Chen, Fusheng Pan,∗
a National Engineering Research Center for Magnesium Alloys, Chongqing University, Chongqing 400044, China
b State Key Laboratory of Mechanical Transmissions, College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
c Department of Mechanical and Industrial Engineering, Ryerson University, Toronto, Ontario M5B 2K3, Canada
Abstract In the past two years, significant progresses have been achieved in high-performance cast and wrought magnesium and magnesium alloys,magnesium-based composites, advanced cast technologies, advanced processing technologies, and functional magnesium materials, such as Mg ion batteries, hydrogen storage Mg materials, bio-magnesium alloys, etc. Great contributions to the development of new magnesium alloys and their processing technologies have been made by Chongqing University, Shanghai Jiaotong University, Chinese Academy of Sciences,Helmholtz Zentrum Geesthacht, Queensland University, Brunel University, etc. This review paper is aimed to summarize the latest important advances in cast magnesium alloys, wrought magnesium alloys and functional magnesium materials worldwide in 2018–2019, including both the development of new materials and the innovation of their processing technologies. Based on the issues and challenges identified here,some future research directions are suggested, including further development of high-performance magnesium alloys having high strength and superior plasticity together with high corrosion resistance and low cost, and fundamental research on the phase diagram, diffusion,precipitation, etc., as well as the development of advanced welding and joining technology.
Keywords: Cast magnesium alloys; Wrought magnesium alloys; Functional magnesium materials; Cast and processing technologies; Corrosion and surface treatment; International standard.
1. Introduction
Magnesium and its alloys, due to their excellent physical and chemical properties such as low density, good damping performance, biocompatibility, recyclability, large hydrogen storage capacity, and high theoretical specific capacity for battery, are considered to have great application prospects in the fields of aerospace, transportation, electronic 3C,biomedical and energy sectors [1–3]. However, there are still many difficulties to be overcome for these advantages of magnesium and its alloys to play a major role in the large-scale industrial applications. In terms of structural materials [4,5], the low strength, relatively poor plasticity and corrosion resistance of magnesium alloys hinder the large-scale applications of magnesium and its alloys; in terms of functional materials [6,7], problems such as too fast degradation rate of Mg alloys and narrow hydrogen charging and discharging window still need to be solved.
In the past decade or so, many scholars have carried out a lot of research on the above deficiencies. The ultimate tensile strength(UTS)of magnesium alloys has reached 500MPa[8–10], and the elongation (EL) has attained more than 30%[11,12]. Through surface treatment, the corrosion resistance of magnesium alloys has been effectively improved [13,14].After years of research, the hydrogen storage capacity of Mg-based materials has also been improved [15,16]. Other Mg-based functional materials have also made a remarkable progress. For example, magnesium alloy has an excellent thermal conductivity, which has been widely used in LEDand other fields [17,18]; the electromagnetic shielding performance of magnesium is superior, which has been used in electromagnetic shielding field [19,20]. However, magnesium and its alloys with excellent properties are still rare, the processing technology is still very harsh, and the strength and plasticity of magnesium alloys still cannot be enhanced simultaneously. These shortcomings also restrict the large-scale applications of magnesium alloys.
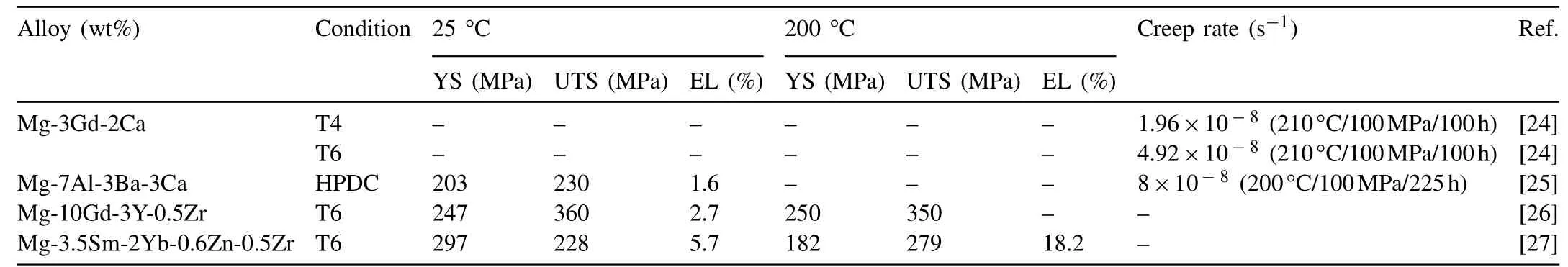
Table 1 Mechanical properties of heat resistant cast magnesium alloys.
In the past two years, many scholars have done a lot of research on magnesium and its alloys to promote its increasing applications, including composition optimization, processing technology improvement, characterization and understanding of microstructure, etc. [21–23]. The present work seeks to review the important advances of magnesium and its alloys worldwide in 2018–2019.
2. Cast magnesium alloys
2.1. Heat resistant cast Mg alloys
In 2018–2019,heat resistant cast magnesium alloys focuses on RE-containing alloys, especially Gd-containing alloys, the mechanical properties of some new types of heat resistant cast magnesium alloys developed worldwide are listed in Table 1.The ultimate tensile strength of a sand cast Mg–Gd–Y–Zr reaches 350MPa at 200°C and 368MPa at 125°C. However,the high content of Gd results in high cost which restricts its automotive applications. The alloy with excellent high temperature performance with low cost is still in need.
Mingxing Zhang’s group developed a Mg–3Gd–2Ca(wt%)alloy with a good creep resistance [24]. They found that when tested at 210°C/100MPa for 100h, the as solid solution treated (T4) alloy showed a better creep resistance than the peak aged alloy(T6).At the steady-state creep stage,the minimum creep rates were measured to be 1.96×10−8s−1in the T4 alloy and 4.92×10−8s−1in the T6 alloy. Microstructural analysis revealed that this is attributed to the synergistical effects of solid solution strengthening by the Gd–Ca co-clusters(particularly at the early stage of creep) and dynamic precipitation hardening (particularly at the late stage of creep).
Gavras et al. [25] developed a high-pressure-die-cast(HPDC) DieMag633 (Mg–6Al–3Ba–3Ca) alloy with good creep property. They examined the compressive creep resistance of HPDC alloys, including commercial AE42,AE44-2, AE44-4, MRI230D, DieMag211, DieMag422, and DieMag633 alloys. The density measurements reveal that DieMag211 and DieMag633 have the lowest porosities with only 1.8% and 1.5%, respectively. MRI230D and two highconcentrated DieMag422, and DieMag633 alloys have the best creep resistance at 200°C. Similar trends are also shown in the tensile properties at room temperature and 150°C. DieMag633 alloy exhibits an outstanding strength.Microstructural observations revealed various phases in the high-pressure-die-cast alloys. Such thermal stable phases help to improve the creep resistance by suppressing the formation of thermal unstable phase Mg17Al12.
A low pressure sand cast Mg–10Gd–3Y–0.5Zr (wt%)alloy with high strength at both room and high temperatures was developed by Wencai Liu et al. [26], which has excellent heat resistant properties. The UTS, YS (yield strength) and EL of the alloy peak-aged at 250°C for 12h tested at room temperature are 360MPa, 247MPa and 2.7%, respectively. Both UTS and YS of 225°C×14h aged alloy increase with increasing test temperature from room temperature to 200°C and reach the peaks of 350MPa and 250MPa at 200°C(Fig.1(a)),while UTS and YS of 250°C×12h aged alloy increase from room temperature to 125°C and reach the peaks of 368MPa and 255MPa at 125°C (Fig. 1(b)).
A cast Mg–3.5Sm–2Yb–0.6Zn–0.5Zr (wt%) alloy with improved strength and ductility at elevated temperatures was successfully developed by Jian Meng’s group [27]. The alloy prepared with permanent mold cast exhibited a YS of 182±4MPa, a UTS of 279±6MPa, and an elongation of 18.2±2.4% at 200°C and a YS of 127±4MPa, a UTS of 186±6MPa, and an elongation of 15.0±3.2% at 300°C.It is found that the improved mechanical properties at both RT and high temperatures are mainly attributed to fine grains, precipitation of both basal and prismatic precipitates,and particle-free zones near grain boundaries and around tridentate precipitates.
2.2. High performance cast Mg alloys
A lot of high strength magnesium alloys were developed in the past decades. High strength of as-cast Mg–Gd and Mg–Y based alloys developed before 2018 were summarized by Fusheng Pan et al. [28] and Jinghuai Zhang et al. [29].The work before 2018 mainly focused the improvement of tensile strength, but the elongation was still not so high.For instance, the ultimate tensile strength and elongation of Mg–6.5Gd–2.5Dy–1.8Zn alloy [30] and Mg–8Gd–3Y–0.4Zr[31] is 392MPa and 362MPa, 6.1% and 7.6%, respectively.In 2018 and 2019, several cast Mg alloys with high strength were also reported, as summarized in Table 2. The important progress lies in the great improvement of the elongation of the cast alloys.
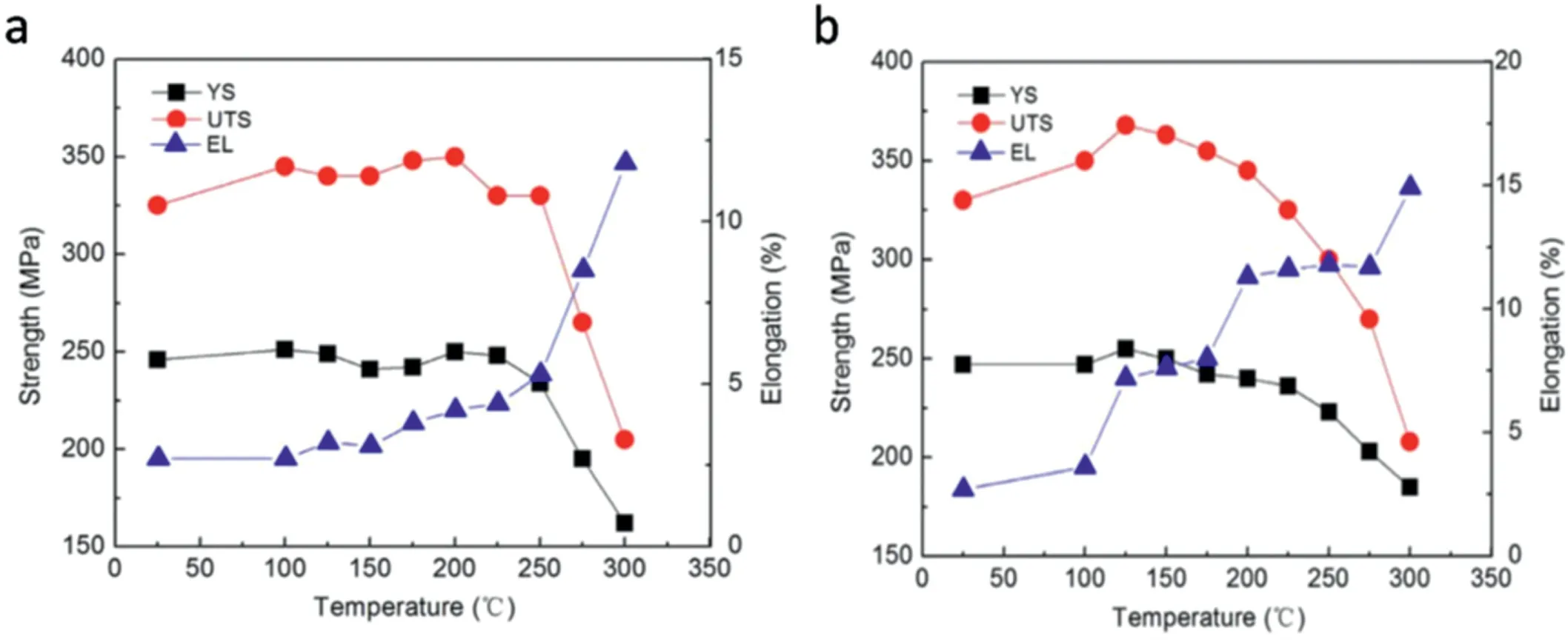
Fig. 1. Tensile properties of the T6 treated GW103K alloys under different heat treatment conditions at different tensile test temperatures: (a)525°C×12h+225°C×14h, (b) 525°C×12h+250°C×12h [26].
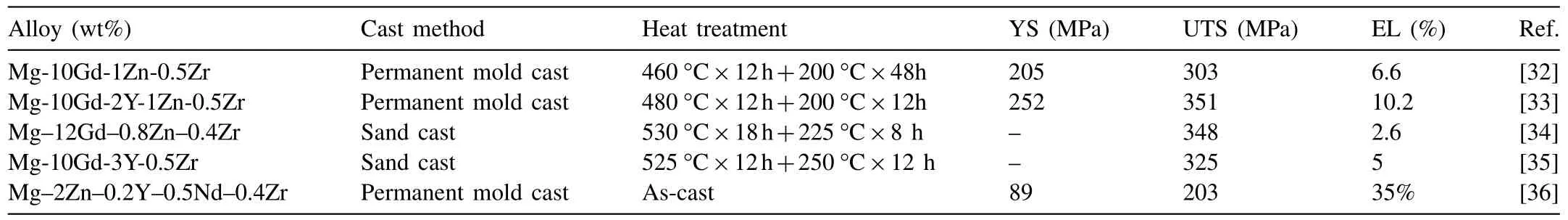
Table 2 Mechanical properties of high performance cast Mg alloys at room temperature.
Fusheng Pan’s group in Chongqing University successfully developed a cast magnesium alloy of Mg–10Gd–2Y–1Zn–0.5Zr with the elongation higher than 10%. The alloy with an ultimate tensile strength of 351MPa and a ductility of 10.2%in the peak aged condition [33]. Besides, a Y-free Mg–10Gd–1Zn–0.5Zr alloy with an ultimate tensile strength of 303MPa and a ductility of 6.6% in the peak aged condition was also developed [32]. Solution treated at 420°C for 12h exhibits the best mechanical properties as the alloy is mainly composed of matrix and block LPSO phase. The LPSO phase is identified as 10H and 14H types.
Wang et al. from Shanghai Jiaotong University developed a high strength sand cast Mg–12Gd–0.8Zn–0.4Zr alloy with the ultimate tensile strength of 348MPa and the elongation of 2.6% [34]. The highest strength of the alloys is obtained via solution treatment of 530°C×18h followed by peak aging of 225°C×8h.
Enhou Han’s group found an anomalous positive strainrate dependence of elongation in a Mg–10Gd–3Y–0.5Zr alloy (GW103) (Fig. 2) [35]. At a strain rate of 1×10−1s−1,the ultimate tensile strength of GW103 alloy reaches around 325MPa and the fracture elongation is 5%. Moreover, for the aging samples of high strength Mg–10Gd–3Y–0.5Zr alloy with dominantprecipitates, the precipitate spacing was deemed as the dominant influence factor for the ductility by influencing the dynamic recovery in stage III of work hardening [37]. Microstructural characterization by slip trace analysis revealed that exclusive deformation mechanism of pyramidal 〈c+a〉slip, non-Schmid behavior of basal slip, and slip transfer induced the high RT ductility.
Wang et al. [36] have developed a high plasticity as-cast Mg–2Zn–0.2Y–0.5Nd–0.4Zr (wt%) alloy with a high fracture elongation up to 35% ± 3% and a YS and UTS of 89±7MPa, 203±5MPa, respectively. The reported plasticity for as-cast Mg–2Zn–0.2Y–0.5Nd–0.4Zr alloy is higher than that reported for most as-cast Mg−Zn-based alloys. The high plasticity is due to twinning at the initial stage and activation of non-basal pyramidal slip systems in a later stage of deformation.
2.3. Low cost high performance Mg alloys
Cost generally plays a crucial role in the commercialization of cast magnesium alloys, thus the development of low cost magnesium alloy with high performance is of great importance for the industrialization of magnesium alloy. Some new types of cast magnesium alloys reported in 2018–2019 with low cost are listed in Table 3.
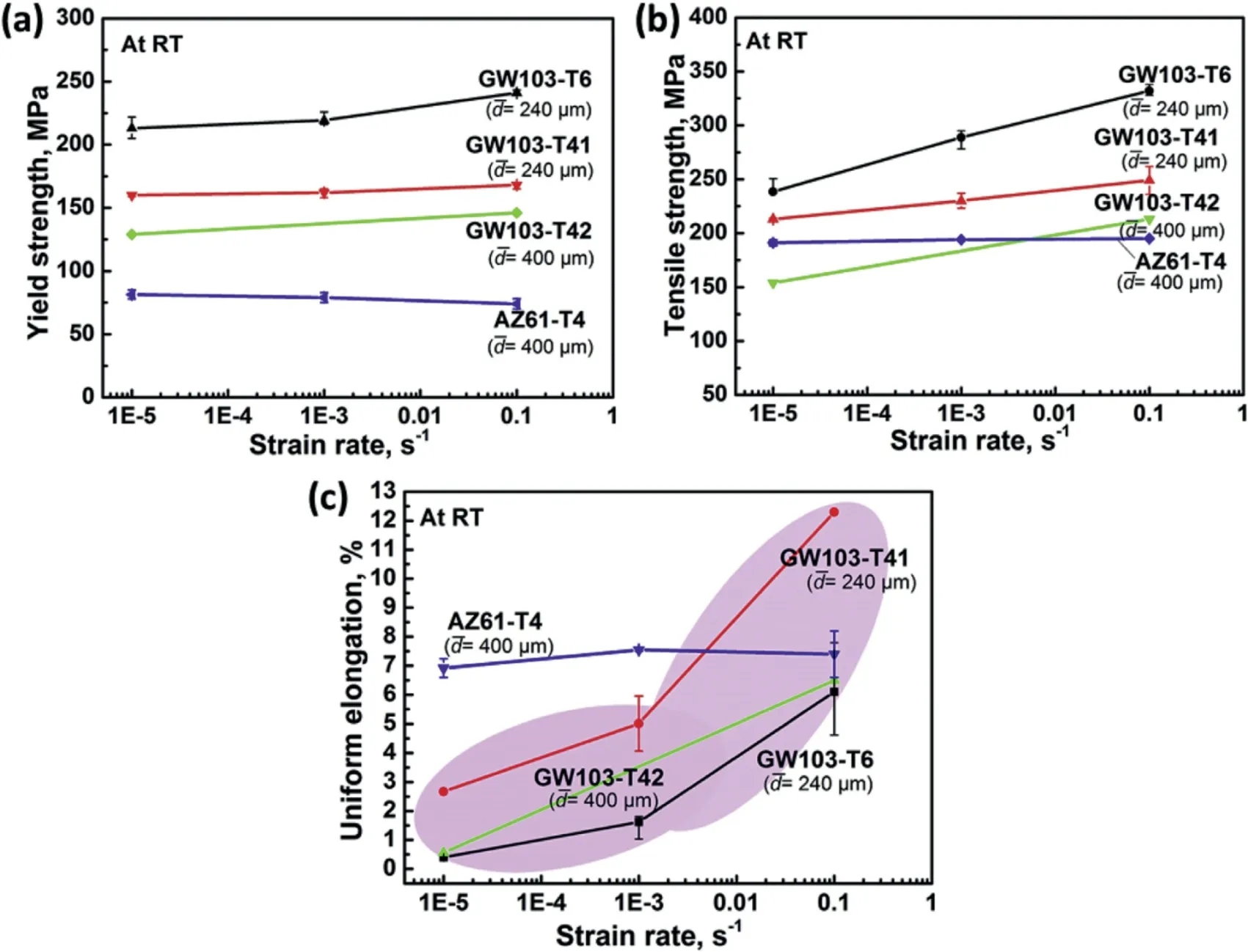
Fig. 2. The variations of (a) yield strength (at 0.2% plastic deformation), (b) ultimate tensile strength and (c) elongation-to-failure with strain rate of different alloys [35].
Table 3 Mechanical properties of low cost high performance cast Mg alloys at room temperature.
Cao and Song [38] have developed a corrosion resistant Mg5Y alloy with low cost compared to high purity Mg. The low corrosion rate was attributed to the protectiveness of a Ycontaining corrosion product film formed on the alloy surface.Interestingly, only a small quantity of Y-containing particles caused micro-galvanic corrosion of the Mg matrix in the distilled water as shown by the orange arrows in Fig. 3(a) and(b), while the particles indicated by the white arrows caused ignorable micro-galvanic corrosion.
Zeng’s group, in collaboration with D. Chen’s group,developed a Mg–10Zn–5Al (wt%) cast magnesium alloy with superior tensile properties and fatigue properties [39], as shown in Fig. 4. Unlike extruded magnesium alloys, the cast alloy exhibited almost symmetrical stress-strain hysteresis loops due to the presence of τ-Mg32(Al,Zn)49phase1It is of special interest to note that the τ-Mg32(Al,Zn)49 phase in cast magnesium alloys was first discovered by a double Nobel Prize Winner, Dr.Linus Pauling, and his colleagues in 1952 and published in a Nature paper(“Crystal structure of the intermetallic compound Mg32(Al,Zn)49 and related phases”, Nature, 1952, 169, 1057–1058).and nearly random textures. Although slight cyclic softening occurred at higher strain amplitudes, cyclic stabilization basically remained. This was also reflected by the nearly over-lapped cyclic and monotonic stress-strain curves. Fatigue crack initiated from the near-surface imperfections, and crack propagation was characterized by fatigue striation-like features along with tear ridges.
An outstanding performance and low cost Mg−3.5Al−4.2La−0.3Mn (wt%) alloy was successfully developed for high pressure die casting (HPDC) by Meng’s group [40]. The yield strength and elongation of the newly developed alloy exhibit better strength-ductility balance compared with the previously studied HPDC Mg and A380 alloys. The ultimate tensile strength of HPDC Mg−3.5Al−4.2La−0.3Mn alloy is slightly lower than A380 alloy but is the highest among the HPDC magnesium alloys.
With the aim to reduce the cost of Nd containing cast magnesium alloys, Feng et al. recently developed an Mg–1Nd–1Ce–0.5Zn–Zr alloy [41]. The yield strength, ultimate tensile strength and elongation in T6 condition of permanent mold cast Mg–1Nd–1Ce–0.5Zn–Zr alloy are 136MPa,237MPa and 9%, respectively. This alloy showed a potential application in car wheels since the requirement of which is 130 MPa–210MPa-7%.
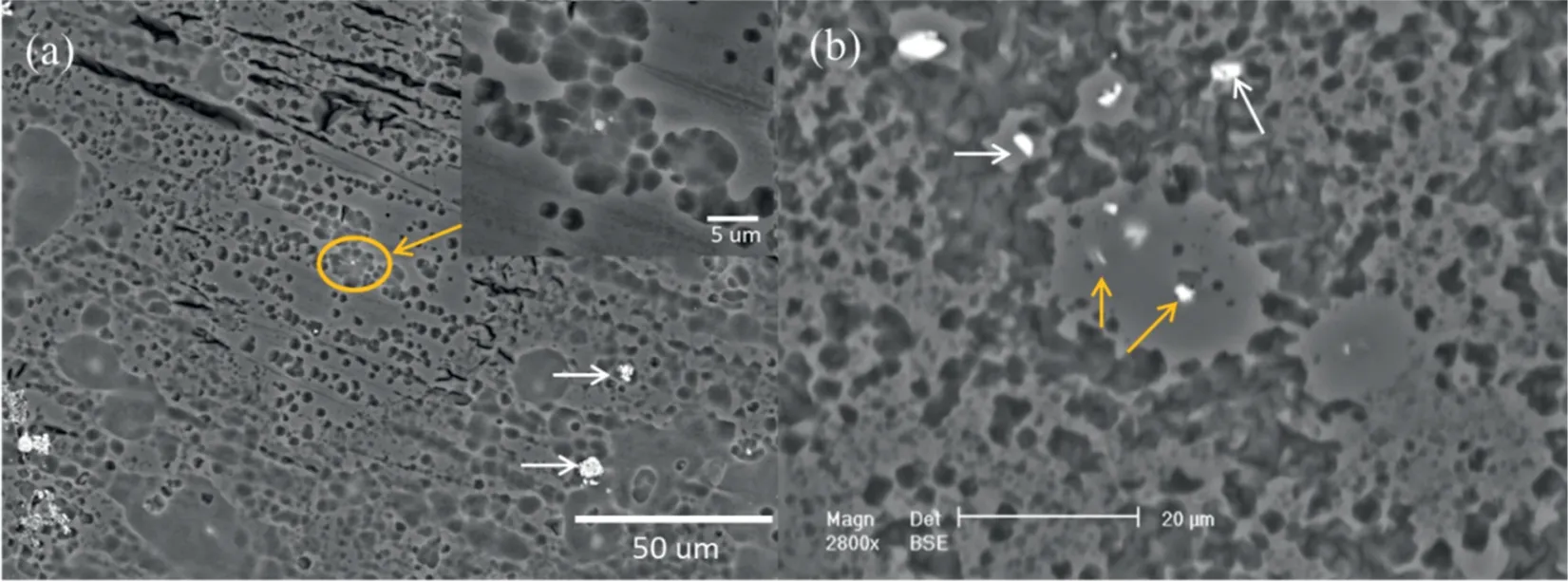
Fig. 3. Surface morphology of the Mg5Y after immersion in distilled water for 6h and removal of corrosion products: (a) as-cast Mg5Y, (b) solution treated Mg5Y [38].
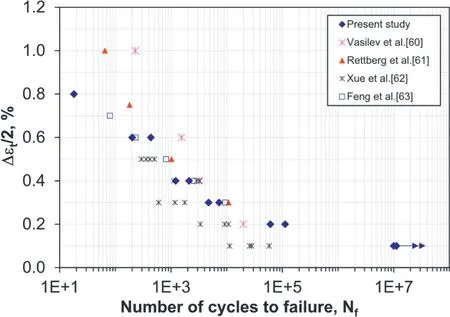
Fig.4. Fatigue life of the Mg–10Zn–5Al cast magnesium alloy in comparison with those reported in the literature [39].
In order to replace part of cast aluminum alloys, it is necessary that the tensile strength and elongation of new cast magnesium alloys with low cost should not be lower than 280MPa and 7%, respectively. Therefore, a lot of work needs to be done on the development of high performance cast magnesium alloys with low cost in the future.
2.4. Microstructure and performance of cast magnesium alloys
Microstructure plays a key role in the mechanical and corrosion performance of as-cast magnesium alloys. During 2018–2019, the work on the microstructure focused mainly on Mg–Sm–Zn–Zr, Mg–Zn–Gd, Mg–RE–TM (TM=Zn, Cu,Ni, Mn) alloys.
Li’s group [42] found that the net-like Mg41Sm5phase along the grain boundary in an as-cast Mg–Sm–Zn–Zr alloy (Fig. 5(a)) acted as a cathodic phase instead of corrosion barrier. This finding provides a novel solution to obtain reliable anti-corrosion performance through regulation of phases for Mg-RE alloys. It initiated and boosted galvanic corrosion of the adjacent α-Mg, accompanied by the accumulation of thick but loose corrosion products and severe localized corrosion.While in extruded alloy the localized corrosion was inhibited due to small size and homogeneous distribution of Mg41Sm5phase and a uniform corrosion film emerged,inhibiting the expand of corrosion in matrix. Consequently,the extruded alloy presented a three times better corrosion impedance compared with the as-cast alloy in NaCl solution(Fig. 5(b)).
Mg–Zn–Gd alloy is a research hot spot due to its high mechanical performance. Luo et al. [43] classified phase constitutions of as-cast magnesium (Mg)–Zn–Gd alloys(Zn/Gd=0.25–60, Zn 0–10 at%, Gd 0–2 at%, 48 samples)into five regions: (I) α-Mg+W-phase (+binary compounds),(II) α-Mg+W-phase+I-phase (+binary compounds), (III) α-Mg+I-phase (+binary compounds), (IV) α-Mg+binary compounds, and (V) α-Mg, as shown in Fig. 6.
Long-period stacking ordered structure (LPSO) phases attracted much attention due to its excellent contribution to yield strength and elongation in Mg–RE–TM (TM=Zn, Cu,Ni, Mn) systems. Besides the best-known 14H, 18R, 10H type, Liu et al. [44] discovered a new type LPSO, 12R type (Fig. 7), without Mg layer between L12cluster-rich layers, which shows the highest Vickers hardness in the LPSO phases.
3. Wrought magnesium alloys
3.1. Traditional commercial wrought Mg alloys
No matter composition or processing technology, the traditional commercial wrought Mg alloy has been very mature and stable. Therefore, the development in recent years is mainly focused on the evolution of its microstructure and crystallographic orientation [45–47]. For example, in recent two years, the research of AZ31 alloy mainly focuses on weakening the basal texture of the sheets[48,49],ascertaining the evolution of microstructure and texture in the process of dynamic recrystallization and static recrystallization [50–52],as well as the thermal stability of the typical texture [53].
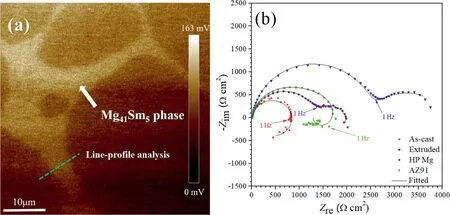
Fig. 5. (a) AFM surface voltage potential mapping of Mg–Sm–Zn–Zr alloys. The Volta potential of Mg41Sm5 phase was 30–40mV more positive than that of α-Mg matrix. (b) EIS of the as-cast and extruded Mg–Sm–Zn–Zr alloys, high purity Mg, and Mg alloy AZ91 in 0.6M NaCl solution [42].
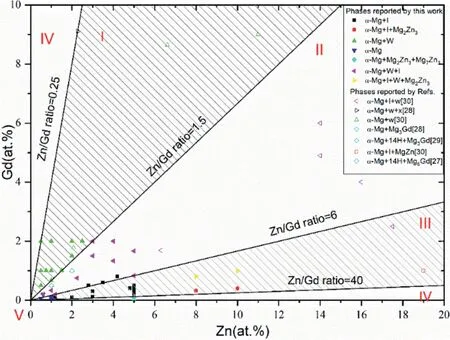
Fig. 6. Phase formation of Mg–Zn–Gd alloys on the Mg-rich corner(Zn 0–20 at%,Gd 0–10 at%): (I)α-Mg+W-phase(+binary compounds), (II)α-Mg+W-phase+I-phase(+binarycompounds), (III) α-Mg+I-phase(+binary compounds), (IV) α-Mg+binary compounds, and (V) α-Mg [43].
Zhao et al. [54] investigated the recrystallization behavior of cold-rolled Mg alloy strips during electropulsing treatment(EPT) process. AZ31 Mg alloy with homogeneous refined microstructure and excellent mechanical properties was obtained by the combined technology of equal-channel angular pressing (ECAP), rolling and electropulsing treatment (EPT).The yields strength (YS) and ultimate tensile strength (UTS)of AZ31 alloy were 320MPa and 430MPa, respectively [54].It is revealed that EPT works through its athermal effect as well as thermal effect, which increase the Gibbs free energy and the additional atomic diffusion flux and as a result accelerate the recrystallization of the deformed AZ31 alloy at lower temperature [55]. Abouhilou et al. [56] studied the microstructure and texture evolution of as-extruded AZ31 alloy after hot uniaxial compression and subsequent annealing. The alloy annealing at 450°C for 72h led to the increase of grains size to 28 and 25μm in the parallel and perpendicular samples, respectively. A random deformation texture was developed in the parallel sample while the deformation of perpendicular samples developed typical basal texture. Zhao et al.[53] investigated the thermal stability of two typical texture(<0002>//ED texture and <0002>⊥ED texture) components in an extruded magnesium AZ31 alloy. The results showed that normal grain growth took place in the AZ31 alloy during annealing at 300°C and 450°C. But the grain growth did not lead to the strengthening of either texture component.Both the < 0002 >⊥ED texture and < 0002 >//ED texture components show good thermal stability, without influencing the texture volume fraction.
Twinning and detwinning represent major deformation mechanisms in hexagonal close-packed (hcp) metals [57,58].Chen and co-authors [59] identified twin–twin interactions and contraction twin formation in an AZ31 magnesium alloy when the compressive direction was changed from the extrusion direction (ED) to the normal direction (ND). {10¯12}extension twins of multiple variants were observed after compressive deformation of 4.3% along ED. The detwinning of{10¯12} extension twins occurred along with the formation of{10¯11} and {10¯13} contraction twins, when the compressive direction was changed to ND. The extension twins in some grains almost fully vanished, making the grains back to a twin-free state. A new twin–twin interaction mechanism being different from double twinning (defined as twins within a twin) was identified, due to the impingement of {10¯11} contraction twins nucleated in the matrix grain on a pre-existing{10¯12} extension twin [59].
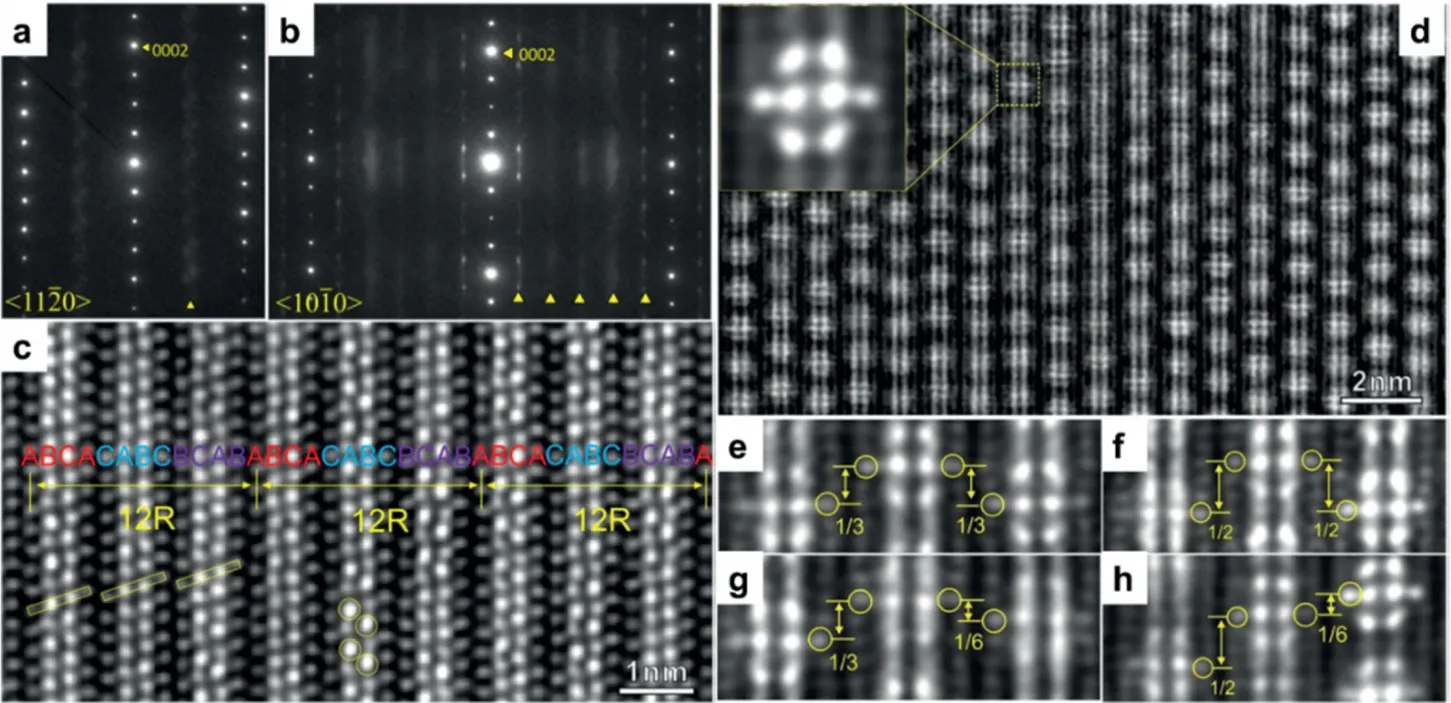
Fig. 7. The SAED patterns of 12R-LPSO with beam direction (a) [11–20] and (b) [10–10], atomic scale HAADF-STEM image from (c) [11–20] and (d)[10–10] directions, (e)–(h) the enlarged views of atomic image from (d) showing different shift distances between building clusters [44].
In addition, there is still great enthusiasm for the continuous improvement of traditional alloy composition. There are some reports about the effect of trace alkaline earth metals (Ca, Sr) on the recrystallization behavior, microstructure and properties of extruded AZ31 alloy in the past two years[50,60–62]. For example, traces of Sr (0.4 and 0.8wt%) were added to AZ31 alloy to weaken texture and improve the strength and plasticity [63]. The maximum intensity of the basal poles figure for AZ31 was 6.4 MRD(multiples of a random density) while that of AZ31+0.4Sr and AZ31+0.8Sr samples was 6.0 and 4.9 MRD,respectively(23%decrease by adding 0.8%Sr). The alloying elements Sr reduced the basal texture and homogenized the distribution of strain in uniaxial tension. Sr enhanced the combination of tensile strength and total elongation.
3.2. High strength wrought Mg alloys
In the past two years, high-strength Mg alloys have mainly concentrated on Mg-rare earth (RE) alloys and newly developed RE-free alloys, such as Mg–Al–Ca, Mg–Sn–Ca, etc.[29,64–66] A lot of research has been done on the optimization of alloy composition, aging treatment and plastic process parameters [67,68]. At present, the yield strength of Mg-RE alloy series has reached 480MPa, and that of RE-free alloy series has reached 450MPa [66,69]. In addition, the plasticity of high-strength magnesium alloys has been greatly improved. For example, the elongation of high strength (538MPa) Mg–Gd–Y–Zn–Mn alloys developed by Chongqing University could arrive at 13%. The mechanical properties of typical high strength wrought magnesium alloys developed recently are shown in Table 4. It is shown that the important progress occurred mainly in the improvement of strength-ductility balance.
3.2.1. Mg-RE-based alloys
Mg-RE alloy has always been an important research direction of high strength Mg alloy [29]. In the past two years,many scholars from different institutions have done a lot of work to improve the properties of Mg alloy via alloy composition, aging heat treatment, microalloying, and other processes[2,5,89].
Because LPSO is an intermetallic compound with high strength and superior deformation capacity, it is considered to be an important strengthening phase in the Mg-RE alloy [5,90,91]. The coefficient of thermal expansion of W(Mg3Y2Zn3) and LPSO phases were determined [92]. In-situ high-temperature XRD results showed that the coefficient of thermal expansion was derived to be 1.38×10−5K−1and 2.35×10−5K−1for W and LPSO phases, respectively. The density and type of dislocations were investigated in a plastically deformed high volume fraction (∼85%) of long-period stacking ordered (LPSO) phase of a Mg89Y7Zn4 (at%) alloy [93]. The order of magnitude of the dislocation density was ∼1014m−2after compression to the strain of ∼25%. It was observed that most of the dislocations formed in the LPSO phase during extrusion and compression were of -type. After extrusion, the yield strength of the alloy reached∼273 and 385MPa for the compression direction lying perpendicular and parallel to the extrusion axis, respectively. The influence of β′and β′precipitates on prismatic and basal planes and long-period stacking ordered (LPSO) fibers on the compressive behavior of high strength Mg–Gd–Y–Zn alloy was investigated by Garces et al. [67]. Results showed that the formation of β′prismatic plates and γ′basal lamellar precipitates increased the compressive yield stress from 310 to 409MPa. In the peak aged alloy, prismatic plates and basal lamellae interact with twins during the propagation and growth stages. β′precipitates are more efficient than γ′basal lamellae not only in hindering extension twinning but also in the hardening of the basal system.
Xu et al. [94] investigated the influence of solid solution solubility of Gd on the hardness and yield stress ofMg–Gd and Mg–Gd–Zr alloys with its contents varying from 2 to 15wt%. The results indicate that the hardness increased linearly with increasing Gd content. Grain size has little effect on the hardness of Mg–Gd alloy.Tensile and compressive yield strengths were both enhanced with increasing amount of Gd.The tensile/compressive(t/c)yield asymmetry of extruded alloys reduced.
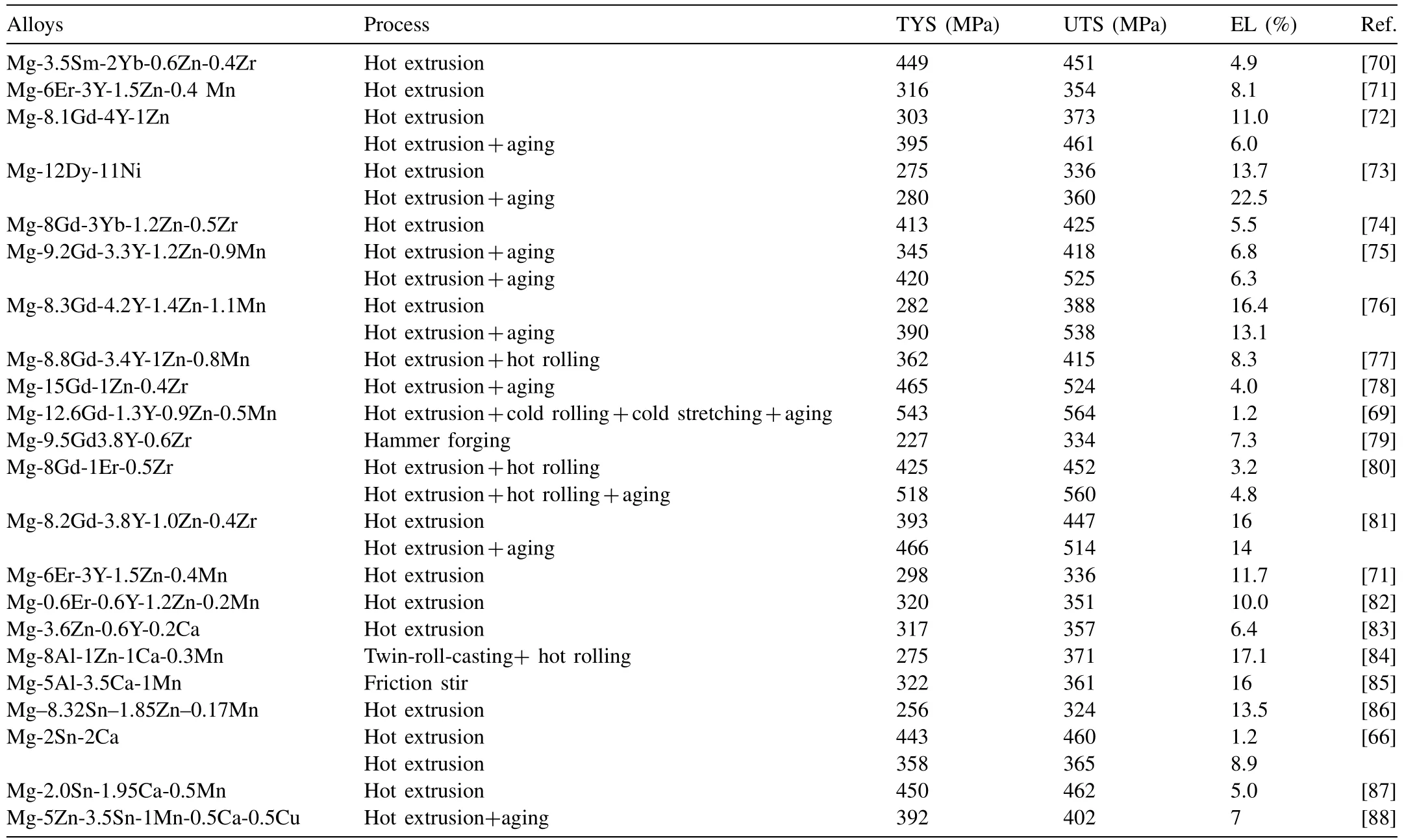
Table 4 The mechanical properties of high strength wrought Mg alloys at room temperature in 2018–2019.
Mg–Gd–Y-Zn alloy is considered to be one of the highest strength alloys. High-performance as-extruded and peak-aged Mg–9.2Gd-3.3Y–1.2Zn–0.9Mn (wt%) alloys have been fabricated by semi-continuous casting, heat-treating, extruding and aging processes [75]. After aging, the alloy with profuse LPSO and β′phases achieves the superior mechanical properties with ultimate tensile strength (UTS) of 525MPa, tensile yield strength (TYS) of 420MPa and elongation to failure(EL) of 6.3%. The alloy strengthening is mainly attributed to the bimodal grain microstructure, LPSO phase, Mg5Gd (β)phase and β′phase.Pan’s group[76]reported that the strength and ductility of as-extruded Mg–1.5Gd–1.3Y–0.6Zn–0.6Mn(at%) are simultaneously improved during aging at the peak hardness platform. The alloy aged for 100hours exhibits ultrahigh ultimate tensile strength (UTS) of 538MPa and good elongation to failure (EL) of 13.1% (as shown in Fig. 8).They [77] developed Mg–8.8Gd–3.4Y–1Zn–0.8Mn (wt%) alloy sheets, which prepared by large-strain high-efficiency rolling process. The as-rolled sample with ∼91% total reduction exhibits excellent mechanical properties with an ultimate tensile strength (UTS) of 434MPa, a tensile yield strength(TYS) of 318MPa and an elongation to failure (EL) of 10.7%parallel to the TD.
Remarkably enhanced mechanical performance of Mg–8Gd–1Er–0.5Zr alloy (UTS of 560MPa, YTS of 518MPa as well as elongation of 4.8%, depicted in Fig. 9(a)) with a lower rare-earth content has been achieved on the route of extrusion, rolling and aging process [80]. The β′phase (with a size of 100–200nm) was precipitated during rolling in accompany with refined grains and strong basal texture, and the layer-structured precipitates with nanoscale were formed during aging Fig. 9, which largely contributed to the enhanced mechanical properties of alloy.
After hammer forging,a large number of dislocation arrays with adjacent distances varying in a range of 0.3–1.4μm were obtained in Mg–9.5Gd–3.8Y–0.6Zr alloy [79]. The alloy with dislocation arrays presented unusual precipitation behavior at 265°C: Precipitation chains of β′phases and precipitationfree zones were obtained, which result from the preferable precipitation on dislocation arrays. Under the function of stress field around the dislocation arrays, β′phases precipitated at 265°C in the alloy have the same orientation. The UTS, TYS and elongation of the alloy after 8h aging at 265°C was 321MPa, 208MPa and 7.5%, respectively. An ultra-strong and ductile Mg–8.2Gd–3.8Y–1Zn–0.4Zr (wt%)alloy with a tensile yield strength of 466MPa, an ultimate tensile strength of 514MPa and an elongation to failure of 14.5% was developed via tailoring the microstructure through hot extrusion with forced-air cooling and T5 aging treatment [81]. The microstructure of the ultra-high-strength and ductility alloy is composed of fine DRXed grains pinned by the nanoscale β-Mg5RE particles, and coarse hot-worked grains with a strong basal texture. An exceptional grain refinement with an average grain size of ∼33nm is obtained by high pressure torsion (HPT) of a peak-aged Mg–8.2Gd–3.8Y–1.0Zn–0.4Zr (wt%) alloy [95]. A record hardness of 156±1 HV is achieved by subsequent post-HPT aging at lower temperature and shorter aging time [96], which is higher than any Mg-based alloy hitherto reported in the literature. This is attributed to the simultaneous hardening effects from grain refinement, high dislocation density and solute segregation.
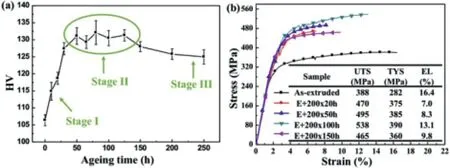
Fig. 8. (a) Age-hardening curve of the as-extruded alloy aged at 200 °C, and (b) tensile mechanical properties of the as-extruded alloy aged at 200 °C for 0h, 20h, 50h, 100h and 150h, tested at ambient temperature [76].
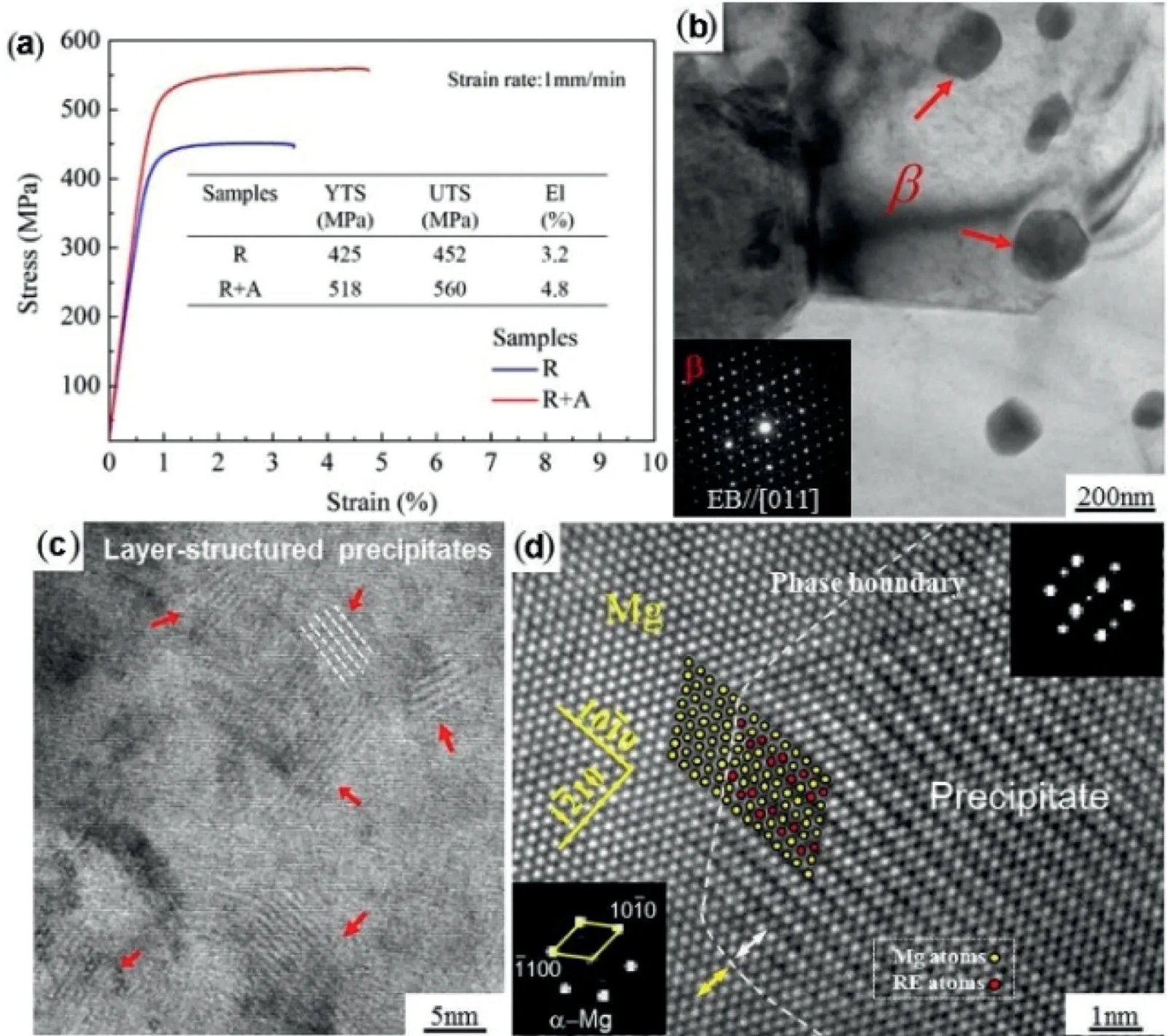
Fig. 9. (a) Strain-stress tensile curves of the R and R+A samples. TEM images of the (b) R sample and (c) R+A sample, and (d) HRTEM image of the layer-structure precipitate and Mg matrix with the FFT pattern viewed from [0001]Mg [80].
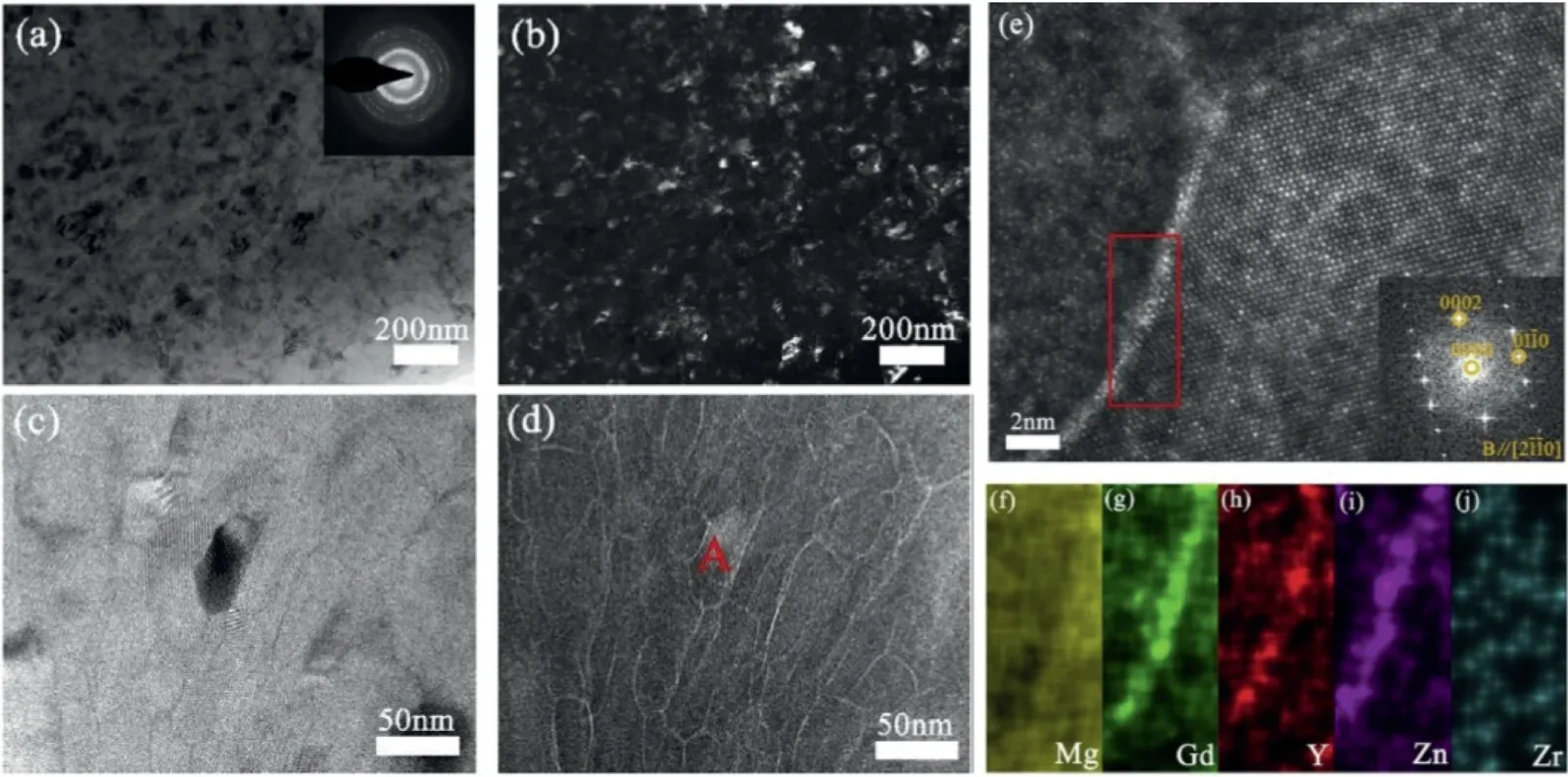
Fig. 10. Microstructure of the HPT-processed Mg-8.2Gd-3.8Y-1.0Zn-0.4Zr alloy after peak-aging at 120°C for 12 h: (a) TEM bright-field image and corresponding SAED pattern; (b) TEM dark-field image; (c) a high magnification TEM bright-field image; (d) HAADF-STEM image; (e) atomic resolution HAADF-STEM image of grain A marked in (d) and corresponding Fast Fourier transform (FFT) image, and elemental mappings of red rectangular region marked in (e): (f) Mg; (g) Gd; (h) Y; (i) Zn; (j) Zr [97].
Zheng and co-authors found that the aging response of the nanostructured Mg–8.2Gd–3.8Y–1.0Zn–0.4Zr alloy processed by HPT is significantly different from that of the conventional coarse-grained alloy [97]. As shown in Fig. 10, the main strengthening structure of the peak-aged Mg–Gd–Y–Zn–Zr processed by HPT is solute segregation at the grain boundaries, rather than the β´phase precipitates formed in peak-aged Mg-RE alloys processed by extrusion/rolling. The metastable β´phase precipitates do not form in the aged nanostructured alloy, and instead equilibrium β-Mg5(RE,Zn) phase forms during over-aging.This altered precipitation behavior is attributed to the high defect density (e.g., grain boundaries, dislocations and vacancies) introduced by HPT, leading to enhanced diffusion of solutes.
In addition to composition control, the precipitation induced by pre-deformation is also used to refine the precipitates, so as to improve the strength of the alloy. Rong et al.[78] studied the role of bimodal-grained structure in strengths and asymmetry behavior of GZ151K alloys in the as-extruded,compressive-yielded and fractured conditions. Eventually, the aged sample with a bimodal-grained structure exhibited ultrahigh tensile yield strength and ultimate tensile strength of 465MPa and 524MPa, respectively. Peng’s group [69] developed a super high-strength wrought GWZ1311M alloy fabricated by hot extrusion and strain aging. The strain before aging, including cold rolling or cold rolling plus stretching,results in strong work hardening and promotes the precipitation of γ′phase and chain-like structures during subsequent aging (Fig. 11). While the grain sizes and texture components in different aged samples do not show essential difference,the number densities of both γ′phase and chain-like structures are much higher in strain-aged samples than those in the aged sample without pre-deformation. The enhanced formation of γ′phase and chain-like structures are associated with the higher dislocation density imparted by the cold deformation before aging. As a result, the GWZ1311M alloy after strain aging at 200°C achieves super high tensile yield strength of 475±8MPa (only applying cold rolling before aging) and 543±1MPa (applying cold rolling plus stretching before aging), respectively (Fig. 11).
To meet the requirements of the high-temperature strength and heat-conductivity of Mg alloys, a type of particular microstructure, i.e., nano-spaced solute-segregated basal plane stacking faults (SFs) throughout both dynamically recrystallized (DRXed) and un-DRXed grains, is designed and obtained in the hot-extruded Mg-RE-Zn alloys[71,82].Based on the special microstructure, the wrought Mg alloys exhibit excellent performance at an elevated temperature of 300°C, and the ultimate tensile strength, yield strength and thermal conductivity are above 300MPa,270MPa and 70W/m·K,respectively(Fig.12).In addition,the novel SFs-reinforced structure in Mg-RE-Zn alloys, due to their relatively uniform electric potential distribution and special nano-structure, can reduce galvanic corrosion and promote the rapid formation of dense surface film, thus achieving the simultaneous improvement of high strength and corrosion resistance.
3.2.2. RE-free Mg alloys
Extraordinarily high yield strength has been obtained in Mg-RE alloys. However, high cost of rare earth elements hinders their widespread industrial applications. RE-free high strength wrought Mg alloys are promising candidates for the next generation high-strength low-cost wrought alloys[98,99].

Fig. 11. Flowchart, HAADF-STEM images showing the microstructures of EA, ERA and ERSA samples(The electron beam is parallel to (a), (c) and (e)[0001]α and (b, d and f) [1120]α, respectively.) and representative engineering stress-strain tensile curves of E, ER, ERS, EA, ERA and ERSA samples of a super high-strength wrought GWZ1311M alloy fabricated by hot extrusion and strain aging [69].
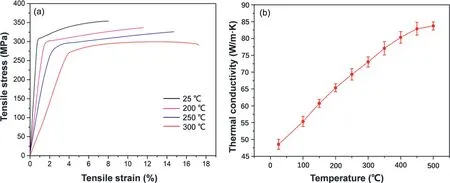
Fig. 12. Typical tensile stress-strain curves (a) and thermal conductivity (b) of a Mg–Er–Y–Zn hot-extruded alloy [71].
An ultrahigh strength AXM5303 (Mg–5Al–3Ca–0.3Mn)alloy with a yield strength of 420MPa, an ultimate tensile strength of 451MPa and an elongation to failure of 4.1% has been developed by extrusion [100]. The ultrahigh strength is mainly due to dense homogeneously dispersed Mg2Ca nanoprecipitates, grain refinement and strong basal texture. Further Al addition substantially decreases the number density of Mg2Ca precipitates, causing a decrease in strength. The limited ductility in as-extruded alloy is caused by the inhomogeneously compact distribution of the broken eutectic particles. The multi-pass equal channel angular pressing (ECAP)was employed on a high-calcium-content Mg–Al–Ca–Mn alloy to tailor its microstructure and mechanical properties. The almost complete DRX with an average grain size of ∼1.5μm was obtained after 12p-ECAP. The maximum strength and ductility were acquired for 12p-ECAP alloy with an UTS of 372MPa and fracture elongation of 8%.Mg–Al–Ca alloy with a higher Al content can also have higher properties. The ultimate tensile strength, 0.2% proof stress, and elongation to failure along the rolling direction of the sheet are 371MPa,275MPa, and 17.1%, respectively [84]. The high strength and ductility are attributed to the fine recrystallized grains and densely dispersed fine Mg17Al12precipitates. Moreover,the new rare earth-free Mg–5Al–3.5Ca–1Mn (AXM541) alloy was developed by friction stir processing (FSP) [85]. The synergistic effect of high heat input and the presence of fine(1–4μm) particles resulted in dynamic recrystallization via particle stimulated nucleation (PSN), with an average grain size of 4.5μm after FSP. This led to improved mechanical properties of the AXM541 alloy along the processing direction, with a tensile yield strength (TYS) of 322±14MPa and a total elongation of 16±3%.

Fig. 13. Engineering stress vs. strain curves of present as-extruded TXM220 alloy, and the recently reported Mg–1.0 Ca wt% alloy and TX22 alloy. (b) The typical low-magnification OM image, and (c, d) the corresponding inverse pole figure and the band contract images of as-extruded TXM220 alloy [101].
Pan et al. [66,87] reported the low-alloyed Mg–Sn–Ca–(Mn) alloy that exhibits tunable ultra-high tensile yield strength (360–450MPa) depending on the extrusion parameters applied. The ultra-high strength is mainly associated with the presence of surprisingly submicron matrix grains (down to ∼0.32μm).The ultrafine grain size,Ca enrichment in most large grain boundaries, and residual Mg2Ca nano-precipitates would in turn contribute significantly to the enhancement of the yield strength of the as-extruded alloys. Pan and Qin et al.[101] further optimized the alloy compositions and achieved exceptionally high yield strength, ∼377MPa in Mg–1.0wt%Ca binary alloy. By reducing the alloying element to only 0.1wt%, the yield strength of the as-extruded Mg–0.1Ca dilute alloy can also reach ∼290MPa (Fig. 13). Specially, an excellent combination of ductility, ∼12%, is also produced in the Mg–0.1Ca alloy.
Wang et al. [88] reported a new Mg–5Zn–3.5Sn–1Mn–0.5Ca–0.5Cu alloy with high strength, the yield strength,ultimate tensile strength and elongation of the alloy after double aging treatment reach 392MPa, 401MPa and 7%,respectively. The extrusion process refines the microstructure and leads to fully dynamic recrystallized grains with a tilted basal texture, which results in moderate mechanical properties of the extruded alloy. Fine and densely distributed Mg–Zn and Mg2Sn precipitates after double aging treatment,which contributes to the strengthening of the alloy.
Compared with high-strength aluminum alloys,the strength of high-strength magnesium alloys needs to be further improved, and the plasticity and anisotropy also need to be improved. In addition, the magnesium alloy with the highest strength is still Mg-RE series alloy, and its cost is very high. In order to expand the large-scale applications of highstrength magnesium alloys, reducing the cost is also the focus of future research.
3.3. Medium strength wrought Mg alloys
Low-cost medium strength wrought magnesium alloys have been paid more and more attention [102,103]. The strength of this kind of alloys is generally about 300–400MPa, and its main feature is composed of low-cost alloy elements, which can contain a small amount of rare earth elements or not [104,105]. The strength of magnesium alloys can be improved by precipitation strengthening and fine grain strengthening, mainly including Mg–Sn [106], Mg–Zn[107] and some low-alloyed alloys [108]. The mechanical properties of typical medium strength wrought Mg alloys over the past two years are summarized in Table 5.
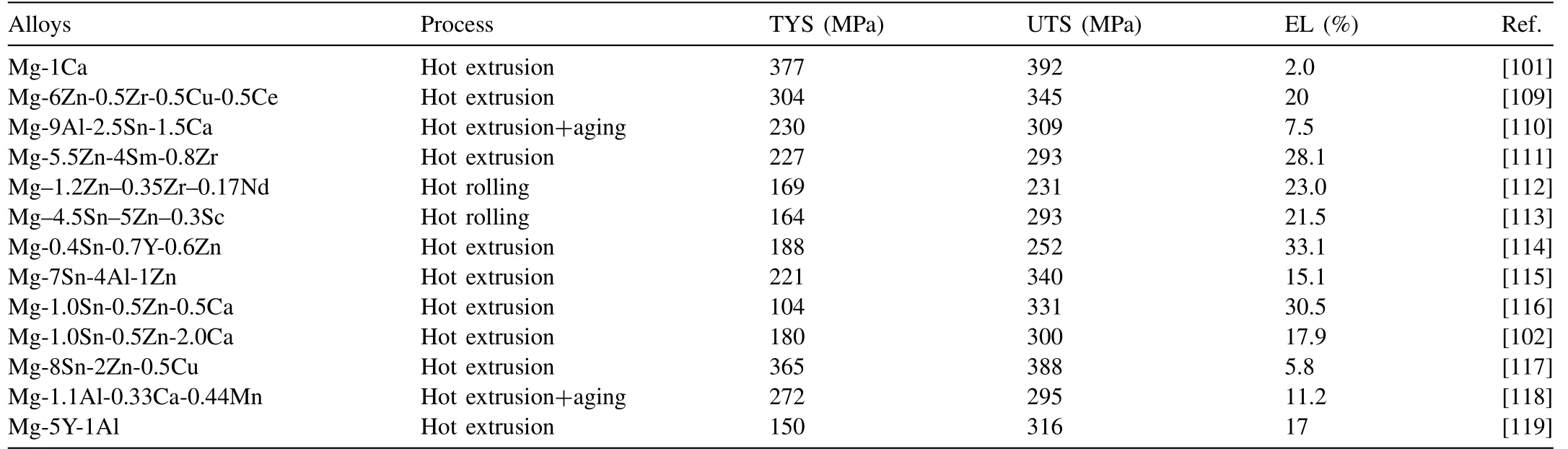
Table 5 The mechanical properties of typical medium strength wrought Mg alloys at room temperature in 2018–2019.
Pan’s group [120,121] reported that numerous fine precipitates in the ingot before extrusion can effectively refine DRXed structures during extrusion. Wrought Mg alloys containing a mass of single Mn phase can effectively restrict grain growth and result in fine microstructures [120]. Mg alloys with a high Mn content have recently elicited special attention given their high ductility. The Mg–3Mn alloys exhibit a high TYS of 212MPa and an elongation of 30% [122]. Thus far, Pan’s group have verified that Mg–Sn [123] and Mg–Zn[68] systems with a high Mn content can become ultrafine grained wrought Mg alloys. The average grain size of high Mn content Mg–Sn–Mn and Mg–Zn–Mn could be refined to∼2μm. The tensile and compressive strengths remarkably increased with an addition of high Mn content (2wt%). The UTS, TYS and elongation of Mg–2Zn–2Mn are 315MPa,290MPa, and 24%, respectively. The UTS, TYS and elongation of Mg–1Sn–2Mn are 321MPa, 233MPa, and 17.5%,respectively.
Yang’s group reported a microalloying approach for strengthening. Through adding two or more small amount of alloy elements on the basis of main alloying elements,and introduction of high-density nano precipitation phase,the strength of the magnesium alloy is improved. They[109] adopted the microalloying approach to improve the mechanical properties of Mg–6Zn–0.5Zr alloy by co-addition of trace Cu and Ce elements. The yield strength, ultimate tensile strength and elongation of the as-extruded Mg–6Zn-0.5Zr–0.5Cu–0.5Ce alloy were 304MPa, 345MPa and 20%, respectively.High-density nano-scale precipitates and a stronger basal texture led to an increase in the tensile yield strength of approximately 59MPa compared with that of the Cu and Ce free alloy. Huang et al. [110] reported a Mg–9Al–2.5Sn–1.5Ca alloy with a YS, UTS, and EL of 230MPa, 309MPa,7.5%, respectively. An increment in UTS of ∼46MPa and∼40MPa was yielded in peak-aged TXA322 and TXA329 alloys, while no obvious variations in UTS were present in peak-aged TXA324 alloy. It is found that age hardening behaviors of as-extruded Mg–2.5Sn–1.5Ca–xAl (2–9wt%) alloys are closely related with the Al concentration, and the aging nano-phases transform from the Guinier – Preston (GP)zones in the low-Al content TXA322 alloy to the Mg17Al12phases in the high-Al content TXA329 alloy. In particular,no apparent nano-precipitations are observed in the middle-Al content TXA324 alloy.
Although Mg–Zn system alloys have ZK series and ZM series alloys. Due to the rich resources and low cost of alloying element Zn, there is still a great interest in the development of high-performance Mg–Zn-based alloys. The asextruded Mg–5.5Zn–0.8Zr alloy with enhanced ductility was fabricated by 4%Sm addition[111].Compared with ZK60 alloy (14.1%), the elongation of Mg–5.5Zn–4Sm–0.8Zr alloys reached 28.1%, and increased by 100%. Hot rolling of the ZEK100 alloy at temperatures from 350 °C to 450 °C was investigated [112]. Results indicated that the increase in the rolling temperature caused reduction of tensile strength and improvement of ductility. The UTS, YS and elongation of the alloy rolled at 350 °C were 257MPa, 237MPa and 17%.
Mg–Sn–Zn alloy has been considered as a low-cost alloy with good strength and plasticity. In the past two years,a number of investigations were conducted to optimize the composition of this series of alloys [117,124]. The improvement of the microstructure and properties of Mg–Sn–Znbased Alloy by alloying elements addition has been widely studied. Alloying elements included: Sc, Y, Al, Ca, and Cu,etc. [114,116,119,125]. For instance, weakening and deflecting of the basal texture in an as-rolled Mg–4.5Sn–5Zn–Sc alloy was observed due to Sc addition [113]. The as-rolled Mg–4.5Sn–5Zn–0.3Sc alloy exhibits the optimum mechanical properties. The UTS, YS and elongation of the alloys were 293.0MPa, 164.2MPa and 21.5%, respectively. Park and co-authors [115,126] investigated the effect of Sn on the microstructure and properties of extruded Mg–Sn–Zn alloys. They found that an addition of 4wt% Al to Mg–7Sn–1Zn alloy effectively promotes the dynamic recrystallization and dynamic precipitation behavior. The strengths of the extruded alloy were considerably improved with Al addition.The YS, UTS and elongation of extruded Mg–7Sn–4Al–1Zn alloys were 221MPa, 340MPa and 17.7%. Zhong and coworkers[86]investigated the influence of aging prior to extrusion(APE)on the tensile strength and ductility of as-extruded Mg–8.32Sn–1.85Zn–0.17Mn alloy. Results demonstrated that APE treatment improved dynamic recrystallization, thereby refining the grain size of the as-extruded alloy. YS and UTS of the alloy increased from 242MPa and 304MPa to 257MPa and 325MPa, respectively, after APE treatment. The ductility of the APE alloy markedly increases from 7.1% to 13.5%.
Moreover, low alloying is a new development direction of wrought Mg alloys in recent years[68].As is well known,the reduction of alloying elements tends to weaken the solid solution strengthening and precipitation strengthening. Therefore,the properties of low alloying Mg alloys are usually improved by fine grain strengthening [70,127,128]. The reduction of alloying elements leads to the low deformation resistance in the process of plastic processing, which can be relatively easily processed to obtain fine grain structure at low processing temperatures [129]. Low-alloyed Mg alloys have been developed into a series of medium-strength alloys.
A low-alloying Mg–1.38Zn–0.17Y–0.12Ca (at%) alloy with 1.38 at% Zn, 0.17 at% Y and 0.12 at% Ca has been developed and then subjected to extrusion [83]. Due to the significantly refined grains, the precipitation of nano-sized Iphases and the texture variation affected by extrusion temperature and speed, superior mechanical properties with a YS of 317MPa, an UTS of 357MPa and an EL of 6.4% were achieved.
3.4. High plasticity wrought Mg alloys
Because the Mg alloy has a hexagonal close-packed(HCP) structure, it is easy to form a basal texture after plastic processing, and the critical resolved shear stress (CRSS)difference between the basal and the non-basal slip systems is too large, which inevitably leads to the reduction of the plasticity of the alloy [130,131]. Pan’s group [12] and other researchers [132–134] have shown that some elements can reduce the difference of CRSS between the basal and nonbasal slip systems, which is conducive to the activation of non-basal slip systems, and can simultaneously improve the strength and plasticity of Mg alloys. Based on the theoretical calculation and experimental research,Pan et al. [130] developed a model of“solid solution strengthening and ductilizing”(SSSD model), which can be used to develop high plasticity magnesium alloys.On the other hand,the grain size is smaller than ∼2μm, the CRSS difference between the basal and non-basal slip systems decreases, the generation of extension twins also can be effectively inhibited [68]. The plasticity and anisotropy of the fine-grained Mg alloys can be improved.Therefore, the main direction is to improve the plasticity of the alloys by weakening the texture of the Mg, reducing the CRSS difference between the basal and non-basal slip systems (alloying element) or refining the grains to <2μm.The mechanical properties of typical high plasticity wrought Mg alloys over the past two years are summarized in Table 6.
Wang et al. [139] found that the extruded Mg–8Al–0.5Zn(AZ80) alloy with an addition of 0.8wt% Ce owns a superior combination of tensile properties, i.e., a high elongation of∼29–34% and a relatively high UTS of ∼320–327MPa.The AZ series Mg alloys, such as Mg–7Al–5Zn (AZ75)[140] and AZ91 [141] alloys processed by hard-pate-rolling(HPR) exhibited the optimum superplasticity at 300 °C and 1×10−3s−1, i.e., ∼615% and ∼580%, respectively. The superplasticity of uniform fine-grained HPRed Mg–7Al–5Zn(with an average grain size of ∼6μm) has been attributed to the enhanced grain boundary sliding (GBS) promoted by numerous Mg17(Al, Zn)12particles along grain boundaries during tension.More importantly,the work reported by Zhang et al. [141] challenged the traditional view that superplasticity was usually achieved in uniform fine-structured materials.
Gd atoms can significantly reduce of the generalized planar fault energy in the prismatic slip system and accelerate the dislocation nucleation in non-basal planes. Mg–Gd alloy with a low Gd content is considered to be a super-high plasticity magnesium alloy. Based on the high plastic Mg–2Gd alloy,Hu et al. developed the high plasticity with high strength Mg–Gd–Zn and Mg–Gd–Mn alloys. Mg–2Gd–0.5Zr–3Zn alloy shows well-balanced strength and ductility with a tensile yield strength (YS) and ultimate tensile strength (UTS) of 285 and 314MPa, accompanied by a high tensile elongation of 24% [137]. The enhanced strength is attributed to grain refinement, precipitation strengthening, and texture sharpening induced by alloying with Zn. Moreover, the strength of the as-extruded Mg–2Gd alloys gradually increases as Mn content increases from 0.5 to 2.0wt%. The as-extruded Mg–2Gd–0.5Mn alloy has the best ductility with an elongation of more than 50%. Mg–2Gd–2.0Mn alloy possesses the highest strength with YS and UTS of 189 and 243MPa, respectively[12].
In the aspect of grain refinement to improve the plasticity of alloy, Peng et al. [128] reported that low content Al alloying in an Mg–1Mn alloy exhibited high tensile yield strength(248MPa) and ductility (33%), depending on the phase composition. The phase composition of the targeted Mg–1Mn–xAl (x=0.3wt%, 0.5wt%, 1.0wt%) alloys were composed of α-Mn+Al8Mn5, Al8Mn5and Al8Mn5+ Al11Mn4, respectively. Among them, Al8Mn5particles were the most effective grain refiner.Mg–1Mn–0.5Al alloy(∼1.9μm)containing specific Al8Mn5particles possessed the finest microstructure and exhibited the best mechanical properties.
Although the plasticity of magnesium alloys has reached a very high level (more than 30%) and the theory of deformation mechanisms has been established,the high plasticity alloy systems are inadequate. Thus, further systematic research is still needed.
3.5. Superlight wrought Mg alloys
Because of its low-density Mg–Li-based alloy is widely used in the field of aerospace and electronic 3C, etc. [142].Mg–Li alloy has good plasticity but low strength and poor corrosion resistance [143]. Researchers have always been committed to improving the strength of the alloy, with the main means including: alloy composition control and grain refinement [144,145]. The mechanical properties of typical Mg–Li-based wrought Mg alloys over the past two years are summarized in Table 7.
Table 6 The mechanical properties of typical high plasticity wrought Mg alloys at room temperature in 2018–2019.
Table 7 The mechanical properties of Mg-Li-based wrought Mg alloys at room temperature.
When Zn and Y (with a Zn/Y atomic ratio of 6) were added, I-phase (Mg3Zn6Y) with an icosahedral quasicrystal structure could be in-situ formed in Mg–Li–Zn–Y alloys[146], which resulted in the improvement of their mechanical strength, corrosion resistance and thermal stability and anisotropy. Specifically, the YS, UTS and EL are 137MPa,156MPa and 13.5%, respectively, for the YS, UTS and EL of “TD” samples, and 149MPa, 168MPa and 28.2%, respectively, for the “ED” samples. This anomalous phenomenon was not observed in HCP-structured and (HCP+BCC) duplex structured Mg–Li based alloy. The I-phase strengthened Mg–Li alloys, being as the promising candidate materials,could have a great potential in the engineering fields.
To obtain a super-lightweight alloy with high strength and elongation,LA141 alloy was cryogenic rolling processed with a large total reduction of 90% [147]. Nano-twins were successfully introduced by cryogenic rolling under large strain.In addition, there is obvious recrystallization grain formation at the end of the twin bands, as shown in Fig. 14.
Yang et al. [148] investigated microstructure and mechanical properties of Mg–9Li–3Al–2.5Sr alloy rods extruded at different temperatures. With increasing extrusion temperature,the grain size of the extruded alloy increases, and the strength decreases whereas the elongation increases. The alloy extruded at 250 °C possesses the highest strength of 238MPa.
Because the β phase (rich in Li) is very soft, the strength of Mg–Li alloy is still low. In the future, it is very important to improve the strength of the alloy while keeping good plasticity.
3.6. Magnesium matrix composites

Fig. 14. TEM micrographs and SAED image of the cryogenic rolling processed LA141 alloy [147].
One of research directions of developing high strength Mg alloys is to develop Mg-based composite by adding reinforcement particles such as ceramics [151,152]. However,the chemical activity of Mg is very high, which makes it difficult to form a good combination with reinforcement, thus restricts the development of Mg-based composites. In the past two years, the combination of the reinforcement and Mg has been improved by the pretreatment of the reinforcement. The in-situ modified graphene oxide was observed to be more uniformly distributed in Mg.The yield strength and elongation of the new composite increased by a record amount,up to 85.7%and 61.4%, respectively, as compared with AZ91 alloy [153].
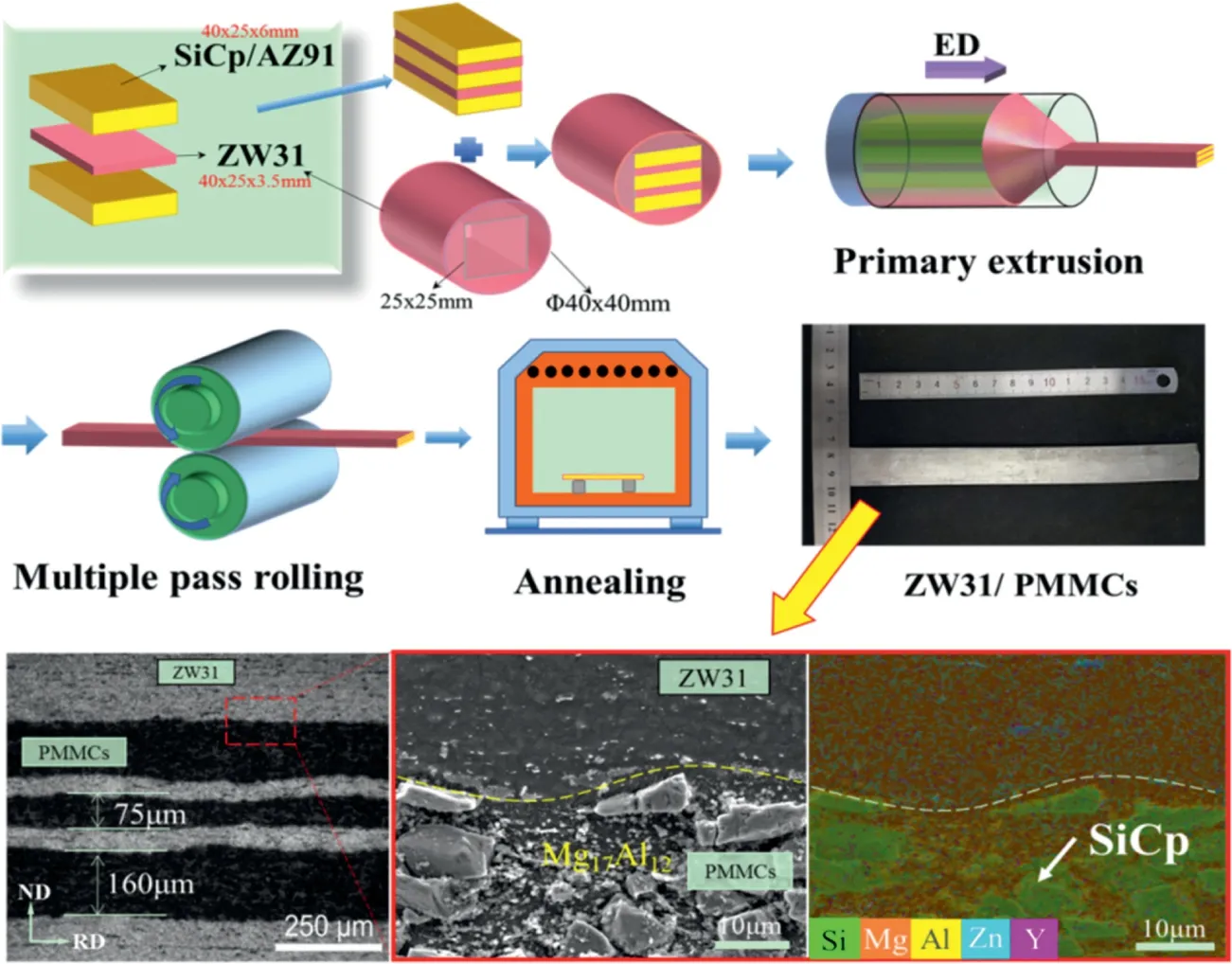
Fig. 15. The fabrication process and microstructures of the SiCp reinforced Mg matrix laminate. [156].
The in-situ synthesized MgO nanoparticles can significantly improve the interfacial bonding between GNS and α-Mg owing to the formation of semi-coherent interface of MgO/α-Mg and the distortion area bonding interface of GNS/MgO. By filling 0.5wt% of GNS, the yield strength and elongation of the composite increased by 76.2% and 24.3%, respectively,as compared with the matrix alloy [154].
3.6.1. Ceramic reinforced Mg composites
The AZ91 alloy reinforced by SiCpwas fabricated by stir casting technology, and then it was subjected to extrusion at 250°C with the speed of 0.01, 0.1 and 1mm/s. Owing to the low temperature and extrusion speed, a large amount of fine Mg17Al12phase precipitated in the Mg matrix [155].With the help of the fine precipitates and extra SiCp, the Mg matrix composite with a high strength (∼441MPa) and a high modulus (∼60GPa) was obtained by Deng et al.’s group[156]. To realize the formation of high modulus Mg matrix sheet,the ductile Mg–Zn–Y alloy(ZW31)was introduced into the 10μm 15vol.% SiCp/AZ91 composites (PMMCs) to coordinate the deformation of PMMCs during rolling process,then the SiCpreinforced Mg matrix laminate with a thickness of ∼1mm was prepared successfully [156], as shown in Fig. 15.
Besides, a novel Mg–4Zn–0.5Ca matrix composite reinforced by trace concentration of 0.5vol.% TiC nanoparticles has been successfully developed and then extruded [157]. The formability of this nanocomposite at lower temperatures was improved compared with the unreinforced matrix alloy. A YS of ∼355MPa, a UTS of ∼386MPa and an EL of ∼10.2%were obtained, which are associated with a combined effect of the addition of TiC nanoparticles, grain refinement and a large amount of precipitated MgZn2phases.
3.6.2. Nano-carbon reinforced Mg composites
The toughness mechanism for CNTs/Mg composites has been investigated via compression test [158]. As shown in Fig. 16, the formed twins, caused by CNTs interacting with dislocations, result in crystal rotations and promote the nonbasal slip at the hardening stage under the effect of twin shear,thus improving the toughness of CNTs/Mg composites. To improve the interfacial bonding between CNTs and Mg matrix, nano-Mg2Ni phase at the interface was in-situ fabricated using two-step process (Fig. 16(j) and (k)), showing an increase of 77%and 48%,respectively,in the yield and ultimate tensile strengths compared with the Ni-free CNTs/Mg composite.In a Mg–9Al alloy,it was observed that the addition of MWCNTs significantly affected the size of Mg17Al12phase,which decreased from micron to nano length scale [159].
Homogeneous magnesium alloy (ZK60) reinforced by a low content of GNPs was fabricated by melt stirring and hot extrusion processes [160]. GNPs were pre-dispersed with Mg powders and extruded into rods used as precursor for melting,which effectively guaranteed the integrity and dispersion of GNPs (Fig. 17(a)). Compared with ZK60 alloy, the composite with only 0.05wt%GNPs can have 62%enhancement in yield strength up to 256MPa, exhibiting an ultra-high strengthening efficiency of 1550 (Fig. 17(b)). In addition, the structural feature of GNPs was investigated to largely affect the strengthening in magnesium matrix composites [161]. During the composite fabrication,Mg atoms restored the sp2structure of graphene and reacted with the oxygen-containing groups on graphene (Fig. 17(c)), leading to a strong interfacial bonding.Meanwhile,graphene defects scattering in nanoscale triggered nucleation of Mg nanograins,which connect the“hard”GNPs and “soft” Mg matrix for an effective transfer of stress and strain, as shown in Fig. 17(d).
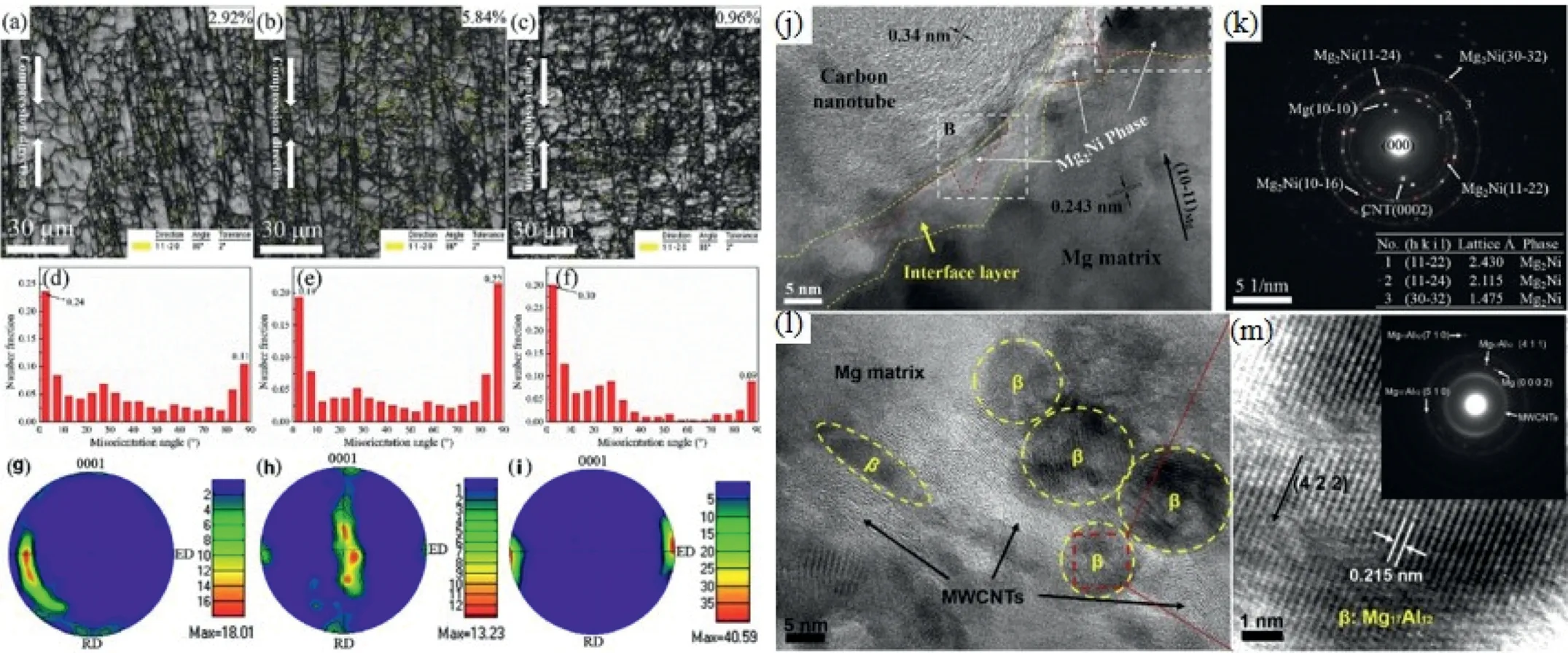
Fig. 16. IQ mapping, misorientation angle distribution and pole figure of the 1.0wt% CNTs/Mg composite at different stages: (a), (d), (g) elastic stage, (b),(e), (h) yield stage, (c), (f), (i) hardening stage (j) HRTEM observation of Ni-CNTs/Mg composite, (k) SAED pattern of (a). (l) HRTEM observation of Mg-9Al/0.4MWCNTs composite, (m) partial enlargement at selected region in Fig. 4(c) [159].
3.6.3. Metal reinforced Mg composites
The bimetal composite rods composed of a softer AZ31 sleeve and a harder WE43 core were fabricated via a special process by combining hot-pressing diffusion with co-extrusion(Fig. 18), with particular attention to the characterization of microstructure and mechanical properties [162]. The results showed that a well-bonded interface with a diffusion layer of ∼20μm in thickness was achieved. The texture in the interfacial region adjacent to the WE43 core changed with the basal poles largely perpendicular to the extrusion direction.Compared with the monolithic Mg billet,the co-extruded AZ31/WE43 bimetal composite rods could achieve a gradient of both composition and microstructure. Such gradients along with the superior interfacial bonding led to a higher compressive and tensile yield strength of AZ31/WE43 bimetal composite rods compared with the AZ31 sleeve. This study indicated that combining hot-pressing diffusion with co-extrusion is an effective method to fabricate the bimetal composites with superior mechanical properties.
4. Functional Mg materials
4.1. Bio-magnesium alloys
As is known to us all, magnesium alloys are biocompatible and biodegradable which are promising to be used as implantable medical devices, such as bone implants,vascular stent, etc. However, the over-rapid corrosion rate of magnesium in physiological environment and the insufficient mechanical property have hindered the application of biodegradable magnesium alloys. Therefore, several researchers designed magnesium alloys and carried out the in-vitro and in-vivo studies, aiming to find biodegradable magnesium alloys with better integrated performance.
Several alloys were reported for orthopedic implant application during 2018–2019. Yufeng Zheng’s group developed a ternary Mg–1.8Zn–0.2Gd alloy which is suitable for the orthopedic implant application [163]. Mg–1.8Zn–0.2Gd alloy showed excellent strength and toughness and its degradation rate was comparable with that of high pure Mg, and was not cytotoxic to L929, MG63 or VSMC cells. With regard to the in-vivo implantation, Mg–1.8Zn–0.2Gd alloy showed fast osseointegration without any disturbance of bone remodeling in the first 2 months, implying its good biocompatibility and osteoconductivity. Yufeng Zheng’s group also studied in-vitro and in-vivo performance of Mg–30Sc alloys for orthopedics applications [164]. The single β phased Mg–30Sc alloy showed the best mechanical performance with an ultimate compressive strength of 603±39MPa and a compressive strain of 31±3%. The incorporation of Sc into corrosion layer led to the formation of double-layered corrosion product and thus improved the corrosion resistance of alloy. The β phased alloy showed no cytotoxicity on MC3T3-E1 cells.After implantation into the femoral condyle of SD rats for 24 weeks, the β phase alloy showed good osseointegration and superior in-vivo degradation performance (0.06 mm y−1),indicating its potential usage within bone. Shaokang Guan’s group developed a novel Mg–Zn–Y–Nd–Zr alloy for the potential application of bone implants [165]. No toxic effect on L929 and MC3T3-E1 cells, as well as a function of promoting the osteogenic differentiation, indicating excellent cytocompatibility of the novel Mg–Zn–Y–Nd–Zr alloy. Hou et al.[166] developed the ZX11 alloys that exhibited a fairly low degradation rate of <0.2mm/year in α-MEM with 10% FBS,limited cytotoxicity to primary human osteoblasts, adequate mechanical strength and integrity (with a YS of 213MPa and a UTS of 256MPa), which offers a possible application as bone plates of the alloys.Tanja Kraus et al.[167]reported that biodegradable WZ21 alloy with moderate, homogeneous degradation characteristics is potential for growth plates application. The degradation characteristics of moderately corroding Mg-alloys (e.g. WZ21) thus seem to satisfy the prerequisites for use in paediatrics when fixation across or close to the growth plate is required.Hong et al.[168]reported that matrix GLA protein (MGP) can locally inhibit the mineralization of WE43 alloy which helps to solve pathogenic calcification. In the in vivo mouse intramuscular model conducted for 4 and 6weeks, WE43 rods were embedded in collagen scaffolds,seeded with cells overexpressing MGP. Results demonstrate that minerals formed on the Mg implant surface can be significantly reduced when MGP was delivered in the vicinity.
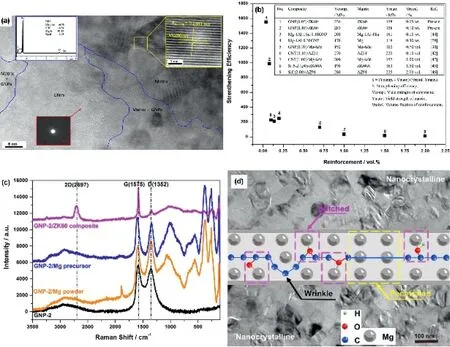
Fig. 17. (a) GNPs stretching in magnesium matrix like an integral whole, (b) strengthening efficiencies of this work compared with other results [160]. (c)Raman spectra of defective GNPs related products, (d) interaction between GNPs and Mg matrix on cross section [161].
Magnesium alloys were also designed for implantable stent. Shaokang Guan’s group used a high plasticity cast Mg–Zn–Y–Nd alloy with an elongation up to 35% was to fabricate micro-tubes for biodegradable vascular stents [169].The micro-tubes having an outer diameter of ∼2.0mm and wall thickness of ∼0.15mm, exhibit a high tensile strength of 298MPa and a large breaking elongation of ∼20% as well as a good corrosion resistance in simulated body fluid solution,providing the precursor for laser engraving stents. The performance of biodegradable magnesium alloy stents (BMgS) requires special attention to non-uniform residual stress distribution and stress concentration, which can accelerate the localized degradation after implantation. Chen et al.[170] reported a novel concept in stent shape optimization using a finite element method (FEM) toolkit which enables in-depth understanding of how deformation history affects the biomechanical performance of BMgS. They developed a Rapamycineluting polymer coated Mg–Nd–Zn–Zr alloy for BMgS applications with uniform degradation behavior, excellent in-vitro and invivo performance. Neither thrombus nor early restenosis was observed in the coated BMgS after planted in the iliac artery of New Zealand white rabbit for 5 months.
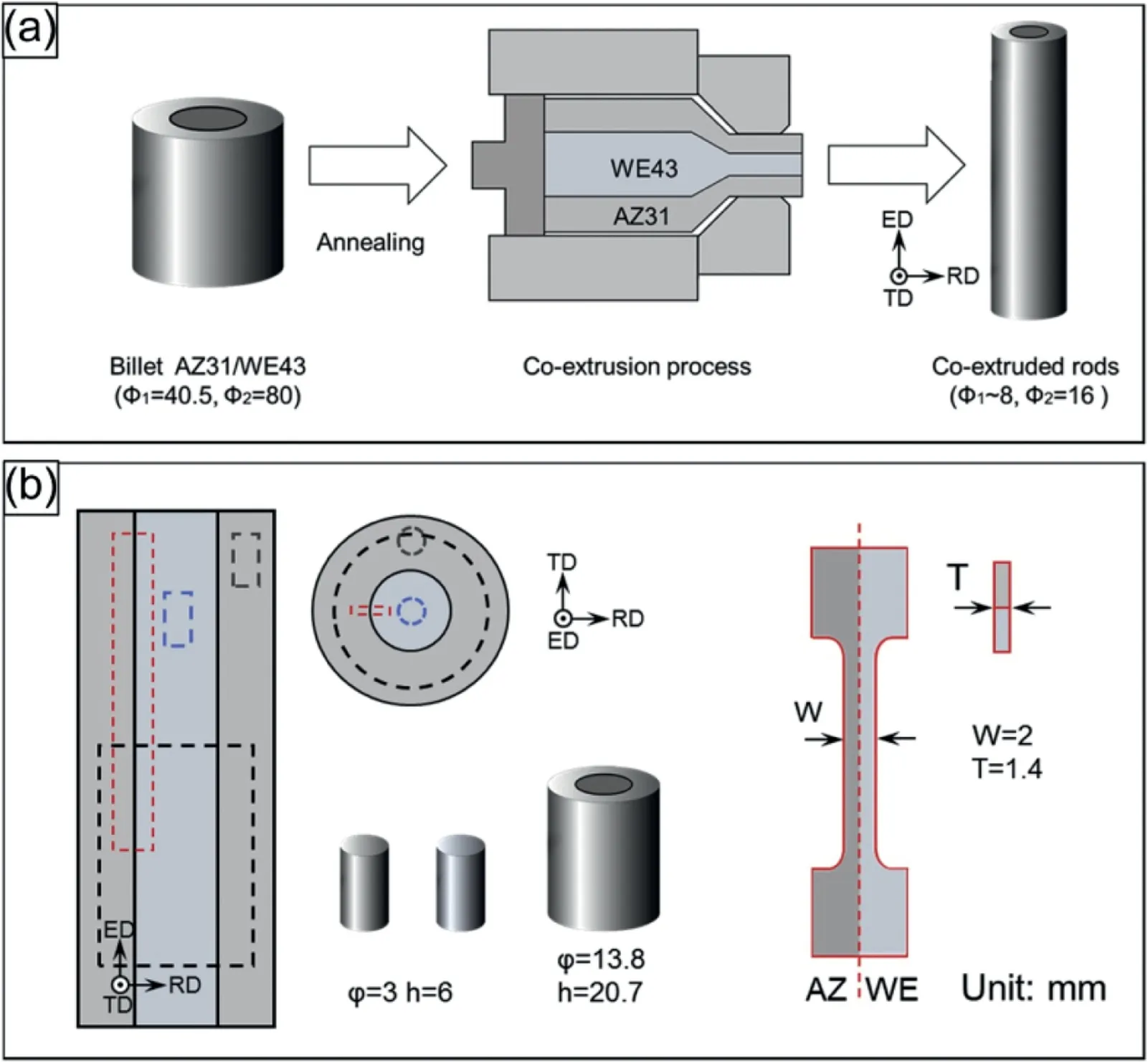
Fig. 18. A schematic diagram showing (a) the fabrication of Mg/Mg bimetal composite rods by hot-pressing diffusion and co-extrusion; (b) the specimens for the compression and tensile tests [162].
Other researchers attempt to develop alloys with better biocorrosion resistance or to bring in other functions, such as antibacterial properties. It is found that an addition of Li in an Mg–xLi–1Ca alloy leads to a marked reduction in mechanical strength and an increase in ductility, giving rise to a decreased corrosion resistance of Mg–Li–Ca alloys in Hank’s solution [171]. Besides, Mg–Li–Ca alloy with a high content of Li cannot possess good biocompatibility. In a further study[172], Mg–3.5Li–0.5Ca alloys show an enhanced mechanical strength than pure Mg and suitable corrosion resistance,good in-vitro and in-vivo performance. Wang’s group have developed a Mg–4Zn–1.5Sn alloy with a superior alliance of cytocompatibility, suitable mechanical properties and low biocorrosion rate [173]. The developed Mg–4Zn–1.5Sn alloy exhibits a UTS of 238MPa, a YS of 142MPa and an elongation of 20.9%, as well as a low mass loss rate of 0.45mm/y without any cytotoxicity to osteoblasts. The in-vivo investigations should be carried out in the future for the Mg–4Zn–1.5Sn alloy as a new biodegradable magnesium alloy. Du et al.[174] developed a Mg–4Zn–0.2Mn–0.2Ca alloy with a corrosion rate of ∼0.31mm/year in Hank’s physiological solution for 40 days. The decreased corrosion rate results from the formation of uniformly distributed Mg–Zn–Ca ternary phase,which acts as a temporary local corrosion barrier against the corrosion attack of the matrix at the initial stage of corrosion.Guan’s group[175]also found that the Mg−Zn−Y−Nd−xAg(x=0.2,0.4,0.6,0.8)alloys exhibit certain antibacterial property. Especially, the as-extruded Mg–Zn–Y–Nd–0.4Ag alloy exhibits a good combination of corrosion resistance, antimicrobial and mechanical properties. This alloy has a potential application in treating orthopedic infections.
Moreover, additive manufacturing was recently applied to fabricate porous bio-degradable WE43 alloy [176,177]. Results reveal that the mechanical properties, biodegradation rate,cytotoxicity in vitro of WE43 scaffolds are satisfying.Although pure WE43 itself may not be an ideal surface for cell adhesion, with the right design and coating, Mg-based biomaterials could be part of a new generation of functional degradable biomaterials, particularly in orthopedic applications. Spark plasma sintering (SPS) was also used to fabricate porous magnesium scaffolds. Kang et al. [178] reported that SPS prepared Mg scaffolds with hybrid PEI–SiO2 coating exhibited superior bioactivity due to the improved corrosion resistance and hydrophilicity in both in-vitro and invivo animal tests. Bagherifard et al. [179]reported a novel severe shot peening treatment on AZ31 alloy which highly improves its mechanical properties. Shot peening was found to decrease corrosion resistance and lead to a small decrease in osteoblast viability during the first three days of culture.
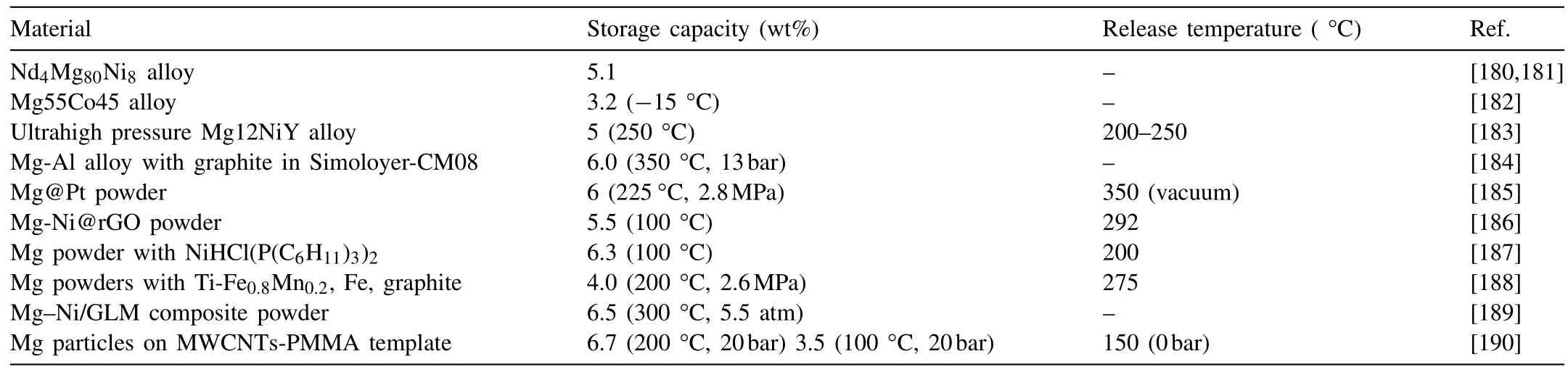
Table 8 Recently developed Mg based hydrogen storage materials.
However, the cells fully recovered by day seven, indicating that there was no lasting cytotoxicity induced by the degradation of the SSP treated samples.
4.2. Hydrogen storage Mg materials
Mg-based materials are thought to be promising candidates for future hydrogen storage applications due to their low cost,abundant resources and large hydrogen storage capacity.However, they suffer from the challenges of sluggish kinetics and large volume change after hydriding/dehydriding (H/D) process. Thus, researchers mainly focus on hydrogen storage capacity,hydriding/dehydriding rate and cyclic stability,as listed in Table 8.
The composition of alloys directly determines the hydrogen storage capacity that the alloy can reach. Based on the review on the enhancement and mechanism of kinetics in Mgbased hydrogen storage materials [180], Li’s group proposed a design idea for Mg alloys that in-situ hydrogenation Mg-RE alloy to form nano-REHxuniformly distributed hydrogen storage alloys. After the thermodynamic calculations and kinetic investigations (Fig. 19(a)), a Nd4Mg80Ni8alloy was designed which shows good comprehensive hydrogen storage properties (high capacity of 5.1wt% H2and fast hydriding/dehydriding rate), especially for remarkable cycling stability even after 819 hydriding/dehydriding cycles (Fig. 19(b)and (c)) [181]. In-situ synchrotron powder X-ray diffraction and three-dimensional atom probe tomography analysis reveal that the catalytic NdH2nanocrystallites are in-situ formed during the first hydrogenation of Nd4Mg80Ni8and densely distribute in the matrix of α-Mg and Mg2Ni (Fig. 19(d)),which plays a key part in achieving excellent hydrogen storage properties. Li et al. [182] have successfully synthesized a Mg55Co45 metastable alloy with a BCC structure, which exhibits a hydrogen storage capacity of 3.24wt% (hydrogen per metal or H/M=1.28, H/Mg=2.33) at −15 °C. Such an absorption temperature in Mg-based BCC structure is the lowest temperature reported for Mg-based materials to absorb hydrogen.The enhanced kinetics are due to large surface area,short diffusion distance of nanostructure, numerous defects and BCC crystal structure of the nano Mg55Co45 metastable alloy.Peng’group firstly observed two-step reaction process and catalyst formation of 18R-type long period stacking ordered(LPSO)Mg12NiY alloy on an atomic-scale[183].In addition,the MgH2compound is readily identified in the Mg–Mg6Pd interface, which is also not stable. It can spontaneously change into MgO increasing the hydrogenation process,which is also the reason for the failure of hydrogen-storage Mg materials[191].Hardian et al.explored the possibility to produce high quality MgH2 using waste Mg–Al alloys [184]. Their aim is to reduce the production cost. The results showed that the highest hydrogen absorption capacity with about 6wt%and the fastest hydrogenation kinetic with 26.9% conversion per minute were achieved by milling Mg–Al alloy with 5wt%graphite in Simoloyer-CM08 ball-mill (R2-120) for 120min.This material has a full reversibility even after more than 40 hydrogenation/de-hydrogenation cycles without any obvious drop of hydrogenation/de-hydrogenation rate.
Magnesium powders are also potential hydrogen storage materials, several catalysts were added to improve the hydrogen storage properties.Lu et al.[185]synthesized a core-shell nanostructured Mg@Pt composite,consisting mainly of icosahedral Mg particles as the core with nano-sized Pt particles distributed homogeneously on different surfaces. Both hydrogen absorption kinetics and dehydriding kinetics were improved, compared with those of the pure MgH2powders. Lan et al. [186] incorporated reduced graphene-oxide-supported nickel (Ni@rGO) as catalysts into magnesium powders with the aim to improve the hydrogen storage properties. Mg–Ni@rGO composites absorbed 5.5wt%of H2at a temperature of 100 °C, whereas the pure Mg and Mg@rGO composite absorbed almost no hydrogen. The heating temperatures required for releasing 5.0wt% of H2was reduced from 334 °C to 292 °C for (i) pure Mg and Mg–rGO and (ii) Mg–Ni and Mg–Ni@rGO. A nickel hydride complex NiHCl(P(C6H11)3)2precursor was mixed with Mg powders by Galey et al. to improve the hydrogen storage properties [187]. The addition of 20wt% of precursor into Mg can release hydrogen at 200°C and absorb 6.3wt% of H2at 100 °C in less than 1h. The significantly enhanced H2storage properties result from the influence of the highly dispersed nickel on both kinetics and thermodynamics of the Mg/MgH2 system. Majid [188] coadded Ti-Fe0.8Mn0.2, Fe, graphite to Mg powders and found that the composite can absorb 4.0wt% H2at 200 °C under a hydrogen pressure of 2.6MPa. Such a composite also reduces the desorb temperature to 275 °C from 440 °C of pure magnesium. Tarasov et al. [189] introduced Ni/GLM(Graphene Like Material) to Mg powders, and the composite has a high reversible hydrogen capacity exceeding 6.5wt%H2. Besides, the Mg–Ni/GLM composite also displays high rates of hydrogen absorption and desorption.
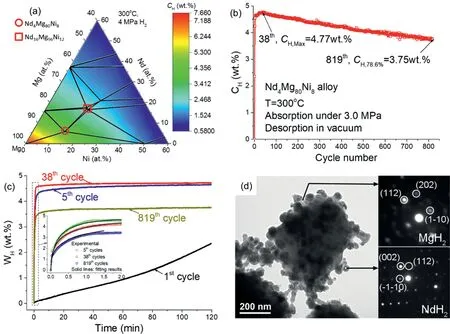
Fig. 19. (a) The distribution of hydrogen storage capacity in Mg-rich corner of Mg–Ni–Nd system, (b) the hydrogen storage capacity of Nd4Mg80Ni8 alloy versus cycle number, (c) the hydriding behaviors of this alloy at different cycles, (d) bright field TEM image of fully hydrogenated Nd4Mg80Ni8 powder after 38 H/D cycles with the SAED patterns of NdH2 and MgH2 [181].
In addition, Liang et al. [190] designed a nano-sized Mg supported on a porous structured MWCNTs-PMMA template to satisfy H2storage demand.A hydrogen absorption capacity of 6.7wt%at 20bar and 200°C was achieved,which was very close to the theoretical capacity of 7.6wt%. The composite could release 3.7wt% of H2at 150 °C and 0bar H2, showing a faster desorption kinetic than the materials reported in the literature. In addition, the composite sample could perform a reversible stabilized uptake/release cycles.
4.3. Mg batteries
4.3.1. Mg–air battery
Metal-air battery is a promising candidate for the power source of electric-driven vehicles (EVs), due to its high practical energy densities. Magnesium is a promising anode material for metal–air batteries, due to its high theoretical specific capacity (2200mA g−1), discharge voltage (3.03V),relative low cost and abundant reserves on earth. In 2018–2019, several magnesium alloys were used as anode materials for Mg–air batteries, such as Mg–Ca, AZ31–Gd, AZ91–Ca–Sm–La, Mg–Li–Al–Zn, Mg–Al–Pb, Mg-Al-Pb-RE, Mg–Al–Pb–La, etc. [192–201]. The energy density, specific capacity,anode efficiency of these alloys are listed in Table 9. Among these alloys, Mg–Al–Pb based alloys show the highest anode efficiency of 62.9%−64.8% at a current of 10 mA·cm−2.
Mikhail L. Zheludkevich’ group thoroughly investigated the self-corrosion and discharge performance of Mg–Ca anode [202]. Mg–0.1Ca exhibited the best discharge potential,high utilization efficiency and capacity among all alloys. Further addition of Ca tends to decrease the utilization efficiency due to the increasing amount of Mg2Ca phase. Deng et al.[192] also revealed that cooling rate impacts the microstructure,self-corrosion and discharge performance of Mg–0.5wt%Ca alloy. The water-cooled Mg–0.5Ca alloy shows a lower self-corrosion rate than the air-cooled one. Moreover, Deng et al. [203] selected high pure Mg and five Mg alloys (AZ31,AM50, Elektron 21, WE43 and ZE41) as anodes for primary aqueous batteries under relatively small load to evaluate theirutilization efficiency. They revealed that “chunk effect” can cause large efficiency loss of Mg anodes with up to 50%,which decreases with the increase of current density. In some cases, the anodic efficiency loss caused by “chunk effect” exceeds that caused by self-corrosion of anode, especially at low current density.
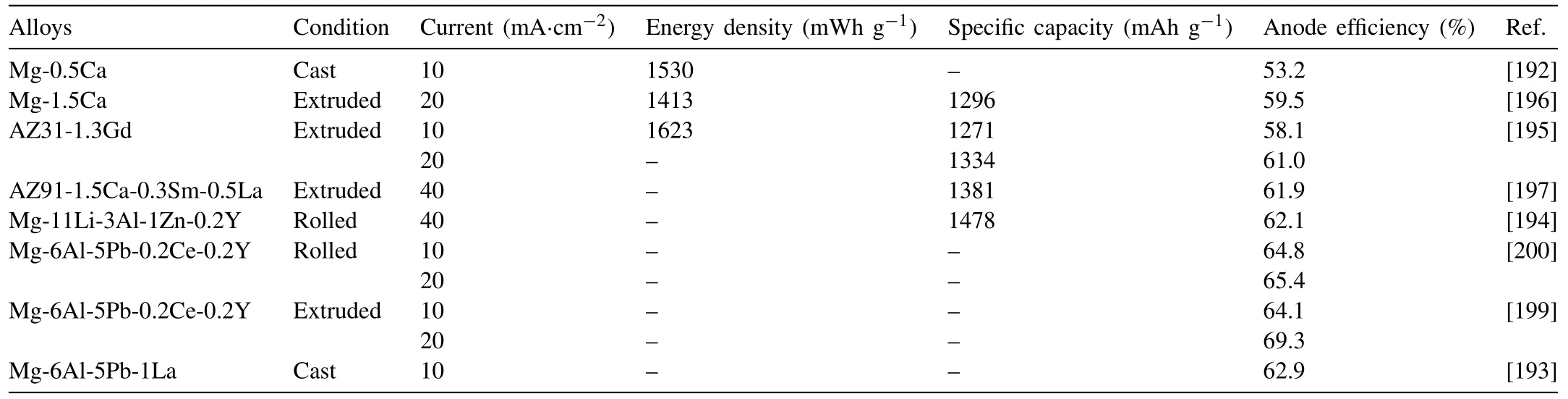
Table 9 Energy density, specific capacity, anode efficiency of Mg alloys as an anode for Mg-air battery.
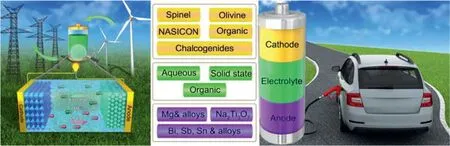
Fig. 20. The schematic illustration of rechargeable magnesium battery [204].
To further improve the anode efficiency of Mg alloy, the solution was modified. Guangsheng Huang’s group[201] added phosphate and vanadate as an electrolyte which improves the anode efficiency of AZ31 from 59.7% to 72.5%and 68.8% at a current of 10 mA cm−2, respectively. Reduced graphene oxide/Mn3O4 nanocomposite was also introduced as additives by Ma et al. [198] for Mg–6Al–1In (wt%)alloy anode. The assembled magnesium-air battery possesses an energy density of 1620Wh kg−1and an anode utilization of 82%, being much higher than those achieved with a NaCl solution and a commercial air cathode (1115Wh kg−1and 52%).
4.3.2. Rechargeable Mg battery
Rechargeable Mg batteries (RMBs, Fig. 20) have been regarded as one of the most potential energy storage devices owing to its abundant ores, atmospheric stability, low cost and eco-friendly, high theoretical specific capacity of 2205mAhg−1and volumetric capacity of 3833mAhcm−3.However, due to the strong polarization of Mg ions, the existing electrodes and electrolyte cannot fully facilitate the Mg2+ion insertion/extraction. In this regard, finding suitable electrode materials and electrolytes with high Mg insertion kinetics, excellent reversibility and low cost are still the challenges that hinder the practical application of RMB [204].The properties of rechargeable Mg batteries are summarized in Table 10.
Zhao et al. [205] reported a new cathode material for rechargeable magnesium battery: chloride ion-doped polypyrrole/carbon nanotube (PPyCl/CNTs) nanocomposite. The PPyCl/CNTs nanocomposite can deliver 95mAhg−1capacity and a high Coulombic efficiency of about 96%. Cui et al. [206] used the 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) to serve as a cathode material for RMBs in non-aqueous system, fast diffusion kinetics and remarkable Mg-storage performance are obtained. The PTCDA exhibits a reversible capacity of 126mAh g−1(at 200mAg−1),an excellent rate performance, and a good cycling stability(100mAh g−1even after 150 cycles). Xu et al. [207] have newly designed [Mg(DG)2][HMDSAlCl3]2/DG electrolyte with superior properties. The anodic stability (3.5V vs. Mg)is excellent, the Mg plating/striping cycling efficiency approaches ∼100%. In addition, at a high current density up to 500μAcm−2, a long Mg plating/striping cycle life of over 260 cycles is achieved.
Yang et al. [208] have reported CuSe nano-particles as the cathode material for RMBs which was synthesized by a costeffective and facile microwave-assisted method. CuSe nanoparticles show an excellent electrochemical performance,such as a high specific capacity of 241.2mAhg−1at 20mAg−1and 200mAhg−1at 50mAg−1, a good rate capability, and a cycling stability. The CuSe nano-particles can maintain a discharge capacity of 107.7mAhg−1at 100mAg−1current density until 150 cycles. A graphene wrapped VS2 (VS2-GO)cathode for hybrid magnesium-based batteries is designed and presented by Sun et al. for the first time [209]. The cathode exhibited a high discharge capacity of 235mA h g−1,an ultra-high rate capability (129mA h g−1at 80 C) and a long life (capacity of 146mA h g−1even after 10,000 cycles at 5 C). Zhou et al. [210] are the first to report that Ni–Fe bimetallic diselenides microflowers (Ni0.75Fe0.25Se2,NFS) as cathode materials exhibits a considerable reversible capacity of 190mAhg−1and an excellent Mg-storage cycling stability (148mAhg−1even after 500 cycles).
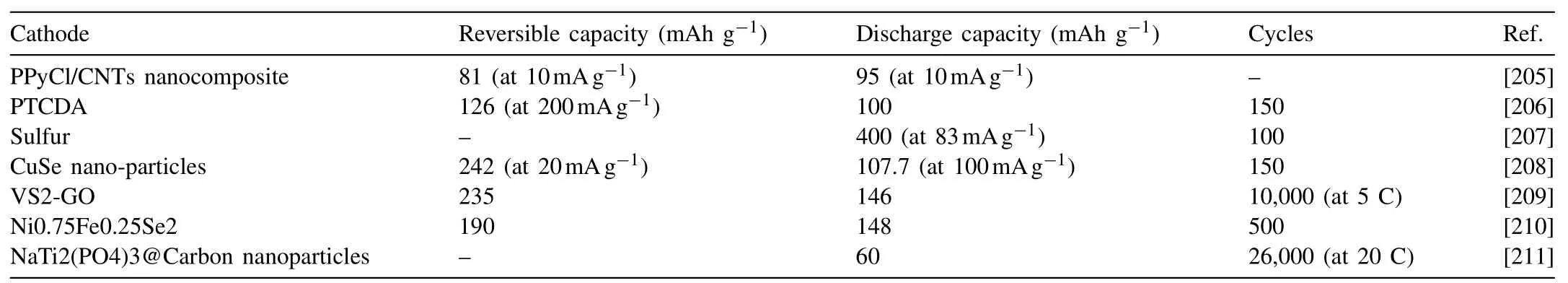
Table 10 Summary of rechargeable Mg battery.
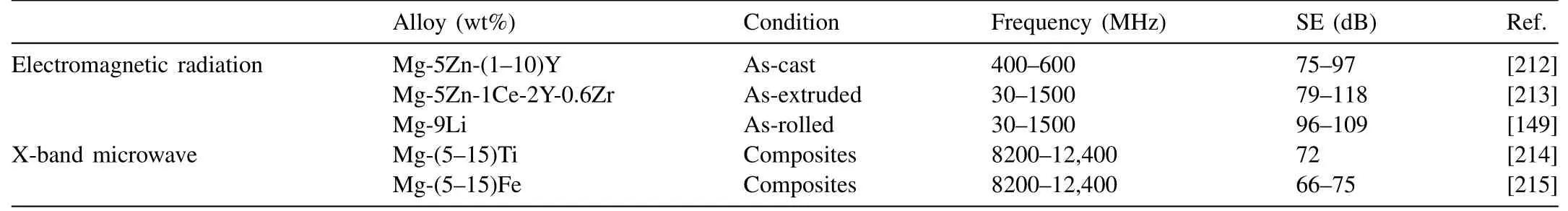
Table 11 Shielding properties of magnesium alloys.
Zeng et al. [211] designed a new Na–Mg hybrid battery with the NaTi2(PO4)3@Carbon nanoparticles as a cathode material, the Mg metal as an anode and the Mg(TFSI)2+NaBH4–triglyme/1,2-Dimethoxyethane (DME)as a hybrid electrolyte. The corresponding average capacity decays only 0.004% and 0.008% per cycle after 1000 cycles at 2 C and 10C, respectively. About 60mAh g−1can be obtained after 26,000 cycles at 20C. The capacity retention of 93.7% and 90.2% can be obtained at 5 and 10C, respectively,which is highly impressive.
4.4. Electromagnetic resistant Mg alloys
Electromagnetic radiation pollution has become a problem to be solved. Shielding effectiveness (SE) is generally measured in decibels(DB).Several researchers attempt to develop Mg alloys with high electromagnetic shielding properties.Table 11 presents the shielding properties of magnesium alloys.
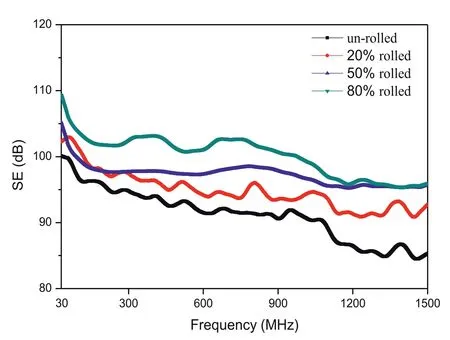
Fig. 21. Shielding effectiveness of the Mg-9Li alloy with different rolling strains [149].
Xianhua Chen’s group [212] have developed Mg–5Zn–xY alloys for electromagnetic radiation shielding applications.With the addition of Y content from 1wt% to 10wt%, the SE of the alloys decreases from 97dB to 75dB at 400–600MHz.SE values of Mg–Zn–Y alloys are higher than that of pure Mg (about 60–65dB at 400–600MHz) and 2024 Al alloy(about 35dB at 30–1500MHz). Besides, high strength and high electromagnetic shielding capacity of extruded Mg–5Zn–1Ce–2Y–0.6Zr alloys have also been developed with a SE of 79–118dB at 30MHz to 1.5GHz [213]. The SE was significantly higher than that of some aluminum alloys which are desirable materials to counter the problem caused due to electromagnetic pollution. Wu’ group [149] found that a rolled Mg–9Li alloy exhibited a good shielding effectiveness(SE) due to a high amount of phase boundary (alpha/beta),fined-grained boundary and obvious texture. Fig. 21 shows the SE curves under the frequency of 30–1500MHz. With increasing rolling strain, the overall SE improves. The as-rolled Mg–9Li alloy with a strain of 80% possesses the SE values of 96–109dB, which are obviously higher than the normal magnesium alloys with a SE of ∼60dB.
Gupta’s group focused on the SE of Mg–Fe and Mg–Ti composites to shield the X-band microwave range (8.2–12.4GHz) [214,215]. Mg–(5–15wt%) Ti micro-composites displayed better EM shielding (72dB) than pure magnesium, although the Ti content has little effect on the EM shielding properties [214]. In the case of Mg–Fe composites [215], the SE values in the X-band are 66dB, 73dB and 75dB for Mg, Mg–5Fe and Mg–15Fe, respectively, indicating that Fe can be used as reinforcement to enhance EMI SE of magnesium. The enhancement in EMI shielding is ascribed to an increase in the amount of ferromagnetic iron reinforcement that promoted the absorption capability of the material.
4.5. High damping magnesium alloys
Although some of Mg alloys have high-strength properties,the damping capacity of those Mg alloys is always poor. It is challenging for such high-strength Mg alloys to meet industrial requirements by improving damping capacity. Therefore,an effective way to enhance damping capacity of Mg alloys with high mechanical properties is crucial for engineering applications of such alloys.
Tang et al. [216] reported that the extruded Mg–Y based alloys exhibited a promising potential for developing highperformance damping alloys, especially for the elevatedtemperature applications. The extruded Mg–1Y sheet exhibited slightly higher yield strength and Q−1, comparing with high-damping Mg–0.6Zr at room temperature. At an elevated temperature of 325 °C, the Mg–1Y sheet had a similar Q−1but over 3 times higher YS than that of the pure Mg.
Chen’ group [217] developed an extruded Mg–7Gd–4Y–2Zn–0.5Mn–0.8Si alloy with excellent damping properties and elastic modulus of ∼49.3GPa. This alloy exhibited superior damping properties at room temperature to that of the extruded pure Mg at high stain amplitudes. The mechanism of the increased damping capacity was associated with the presence of abundant thermal mismatch dislocations around the phase interfaces. However, at higher temperatures the extruded Mg–7Gd–4Y–2Zn–0.5Mn–0.8Si alloy showed a relatively higher damping capacity compared with that of the Si-free alloy but lower than that of pure magnesium.
Niu et al. [218] revealed that Mg–0.6Zr–4Y alloys presented a good damping properties with good comprehensive strength and elongation among Mg–0.6Zr–(1–5)Y alloys. Increasing Y from 1.0 to 5.0wt% can significantly affect damping properties under the condition of ε > εcr=2.0×10−2,while no visible effect of increasing Y content on damping properties at ε < εcr=2.0×10−2was found.
Yu et al. [219] discovered that the addition of Ti2AlC particles into AZ91D alloy improves its damping properties, especially at elevated temperatures. With increasing temperature, especially above 200°C, the interfacial damping capacity becomes dominant and dynamic modulus of composites decreases.
4.6. Other functional Mg materials
4.6.1. Intelligent sacrificial anode
Song’s group used Mg as an intelligent sacrificial anode which can automatically detect the corrosivity of concrete and intelligently provide a cathodic protection current to inhibit the corrosion of steel [220]. Magnesium has a more negative equilibrium potential (−2.37V vs. SHE), which makes it an effective sacrificial anode material in high impedance circumstances(for instance,in concrete).In addition,Al alloying can enhance the sensitivity of the Mg anode to chloride ions and improve its cathodic protection effect, and thus enhance the intelligence level of the Mg as a corrosivity detector and corrosion protector.
4.6.2. High thermal conductivity Mg alloys
It is very difficult for Mg alloys to be strong and also highly thermal conductive since high strength requires defects to hinder the movement of dislocations whilst high thermal conductivity requires a perfect crystal for the movement of phonons/electrons. Pan’s group [221–225] did a lot of work on the effect of alloying elements on thermal conductivity of magnesium alloys before 2018, which provides an important basis for the development of high thermal conductivity magnesium alloys.
In 2019, a new magnesium alloy with both very high strength and high thermal conductivity, Mg–4Al–xLa alloy,was reported in [226]. Both the thermal conductivity and strength of Mg–4Al–xLa (x=2, 4, 6wt%) alloys increase with increasing La content, as shown in Table 12. The extruded Mg–4Al–6La alloy exhibits a thermal conductivity of 130W/m K, an ultimate tensile strength (UTS) of 337MPa and an elongation to failure of 8.4%. The mechanism of the simultaneous increase of strength and thermal conductivity of the Mg–Al–La alloys is that the precipitation of Al11La3during extrusion strengthens the alloy as well as maintains high thermal conductivity through consuming solute atoms. Zhu et al. [227] designed two Mg–La–Zr alloys with high thermal conductivity of 137 and 114W/m K through assessing the Mg–La–Zr system using CALPHAD method.
5. Cast and processing technologies
5.1. Melting and casting technologies
Current magnesium alloy castings are generally categorized into permanent mold casting,sand casting,squeeze casting, investment casting, low pressure casting, and high pressure die casting. Many progresses were made on these casting technologies in the past two years.Prior to casting,the quality control of the melt plays an essential part in the mechanical and corrosion behavior of the magnesium alloys [170].
By using the melt self-purification method based on low temperature melt treatment without adding any fluxes, Pan and Chen et al. in Chongqing University could significantly reduce the impurity Fe concentration to as low as 10ppm inMg–Gd–Zn–Ag–Zr alloy with a high RE content. The lowcost purification technology has already achieved large-scale industrial applications, providing an important basis for the development of high-purity magnesium alloys.
Table 12 Mechanical properties and thermal conductivity of magnesium alloys.
Fig. 22. (a)–(d) The solidification microstructures of Mg–24wt%Nd alloys in different conditions, (e) the typical stress strain compression curves, (f) Vickers hardness evolution alloys [228].
Zhai et al. [228] reported that 1T static magnetic field altered the growth direction of primary α-Mg (Fig. 22(a)–(d))and influenced the type of second phase in Mg–24wt%Nd alloys. It is revealed that the magnetic field makes the critical undercooling of Mg12Nd more than that of Mg41Nd5, which led to Mg41Nd5more likely to form in cast samples with higher strength and hardness of alloys (Fig. 22(e)–(f)). Furthermore, the application of 1T static magnetic field during the heat treatment of Mg–Al–Gd alloys reduced the laths of 15R-type LPSO from 236nm to 47nm and enhanced the ultimate compressive strength from 447MPa to 473MPa [229].
Squeeze pressure was observed to be an significant factor for the microstructure and mechanical performance of as-cast Mg–15Gd–1Zn–0.4Zr (GZ151K, wt%) alloy [230]. As-cast GZ151K alloy is a typical high strength alloy with an ultimate tensile strength of 405MPa and an elongation of 2.9%.The applied pressure in the squeeze casting further refines the grain size from 41μm to 28μm.
Yang et al.introduced a high shearing dispersion technique(HSDT) to incorporate 0.5% AlN/Al nanoparticles (NPs) in Mg–2.85Nd–0.92Gd–0.41Zr–0.29Zn (El21) alloy [231]. They observed that after adding AlN nano-particles the creep resistance of El21 alloy was significantly improved by one order of magnitude. The eutectic particles were modified to be less continuous, much thinner and more homogeneously distributed in the matrix. They are beneficial for resisting the grain boundary sliding and hence enhance the creep resistance. Yang et al. [232] also investigated the effects of 0.25%Al and HSDT on the microstructure and creep resistance of El21 alloy. The creep resistance of El21 alloy was obviously improved by about one order of magnitude with the combination of Al addition and HSDT. This is attributed to the dendritic and denser Mg3RE phase. Al2Nd and Al2Zr particles also act as efficient obstacles to grain boundary sliding and inhibit the movement of dislocations, and consequently enhance the creep resistance of El21+0.25% Al.
5.2. Processing technologies of wrought Mg alloys
5.2.1. Low temperature extrusion
In the past two years, a large number of reports about the extrusion process have been published, aiming to obtain high performance extruded Mg alloys [45]. As is well known,the formation of microstructure and the mechanical properties of extruded alloys depend on the extrusion temperature to a great extent, which is an important parameter of extrusion processing. At present, the main mechanism of plastic deformation of extruded Mg alloys is cross-slip and climb.Dynamic recrystallization (DRX) is usually easy to occur at high temperatures. Mg alloys can commonly have a complete recrystallization microstructure at an extrusion temperature higher than 350 °C. The increase in the extrusion temperature promotes the DRX. In Ref. [233], with an increase in the extrusion temperature, the predominant DRX mechanism of the alloy changed from twinning-induced DRX and continuous DRX to discontinuous DRX, which led to an increase in the DRX fraction of the extruded alloy. Kang et al. [83] reported that the average size and volume fraction of DRXed grains increased with increasing extrusion temperature in a Mg–1.38Zn–0.17Y–1.2Ca(at%)alloy.However,this is not always the case, and can exhibit different phenomena. Yu et al.[234] reported that the long period stacking ordered (LPSO)phase promoted the deformation of parent grains and caused the strain concentration. Then the strain concentration could be rapidly released as the movement of dislocations became easier at the elevated extrusion. Thus, the increased extrusion temperature suppressed the DRX due to the presence of LPSO phase in Mg–11.5Gd–4.5Y–(1Nd/1.5Zn)−0.3Zr alloys.
At lower extrusion temperatures, the critical resolved shear stress of the basal slip and extension twining is much lower than that of the non-basal slip [235]. Therefore, the basal slip and tension twining are easily generated to accommodate the plastic strain.The dislocations accumulate near the twin bands and boundaries, leading to DRXed region. As the extrusion proceeded, the DRXed region expands further into the grains,leading to a gradual decrease in the size and fraction of the unDRX grains. However, the driving force of DRX is relatively low. Therefore, unDRX regions with sufficient dislocation and hard orientation will be preserved. The microstructure of the low temperature extruded Mg alloy consists mainly of the fine equiaxed DRX grains (∼1μm), elongated coarsen unDRX grains regions. For example, a Mg–2Zn–1Al alloy with a bimodal structure having a high strength of 332MPa and a considerable ductility of 23% of was successfully fabricated via the low temperature extrusion at 110 °C (Fig. 23)[129].
5.2.2. Continuous extrusion
Accumulating strain in the conventional hot extrusion has a large potential for grain refinement,such as pre-deformation process before extrusion and multiple deformation [236,237].Excellent grain refinement can be achieved from increasing strain before or during extrusion. It is of great significance to explore the cumulative deformation in conventional extrusion for refining Mg alloys. Pan’s group [238] presented a simple cumulative deformation method called continuous forging extrusion (CFE) to fabricating ultrafine-grained (UFG) Mg alloys. In the conventional extrusion, the billet is upset and fulfilled with the container before extrusion other than squeezed into the mold immediately because the mold is sealed by the left tailings from last extrusion.This upsetting processing will induce strain to the billet, while this strain is ignored because the size of ingot is closed in the container and this strain is relatively small. In the currently proposed CFE, the induced strain from the upsetting processing is magnified by a reduced diameter of AZ31 billets before extrusion and a UFG structure(∼0.6μm) is obtained, thereby enhancing the strength and ductility simultaneously. Torsion shear stress plays an important role in grain refinement whether it is in twist extrusion or high-pressure torsion process. Pan and co-authors [239] presented a simple on-line twist extrusion process to fabricate UFG structures of an extruded rod. The schematic illustration of OLTE is shown in Fig. 24(a). The proposed method can be used to refine the grains of the extruded bar from the surface to the center,thereby improving its strength,yield asymmetry,and ductility.The recrystallized UFG structures with ∼1.2μm in a ZK60 alloy exhibits relatively high TYS, UTS, CYS and yield asymmetry σCYS/σTYSof 245MPa, 347MPa, 227MPa and 0.93, respectively, as well as 28% fracture elongation(Fig. 24(b)). The advantage of the continuous extrusion process versus other intense plastic straining processes lies in its high productivity and the feasibility of bulk materials.
5.2.3. Hard-pate-rolling
Fig. 23. (a) Schematic diagram of experiment flow, (b) Mechanical properties of extruded Mg–2Zn–1Al alloys [129].

Fig. 24. (a) Schematic diagram of OLTE process and (b) stress–strain curves of ZK60 alloys [239].
Zha et al. [240] and Zhang et al. [241] have applied hardpate-rolling (HPR) to Mg–9Al–1Zn (AZ91) [240] and Mg–8Al–2Sn–1Zn (ATZ821) alloys [241]. Both HPRed AZ91 and ATZ821 alloys exhibit a high strength(UTS of ∼374MPa and∼362MPa, respectively) and ductility (uniform elongation of∼19.6% and 17%, respectively). The increased tensile properties have been attributed to the formation of bimodal structures (Fig. 25(b) and (d)). At the initial stage of tensile tests,deformation is mainly accommodated by fine grains with a weak basal texture (Fig. 25(f)). When the applied resolved shear stress exceeds the CRSS value of pyramidal slip in Mg alloys,the coarse grains begin to play a prominent role in storing dislocations and hence promote work hardening, leading to a simultaneous higher strength and ductility in comparison with fine-grained Mg alloys.
5.2.4. Composite extrusion
Chai et al. [48] reported that grain refinement and tilted texture were detected in composite extruded AZ31/AZ31 and AZ31/4047 Al sheets. The schematic diagrams of the fabrication for three sheets are shown in Fig. 26. The compression-tension yield ratio increased gradually from AZ31 to AZ31/AZ31 and AZ31/4047 Al sheets, which was attributed to the intense shear deformation at the interface during the composite extrusion. This provides a new and effective method to weaken the texture of extruded Mg alloys and improve their mechanical properties. Lee et al. [45] presented a novel intense plastic straining process called multipass caliber rolling. The strength and ductility of AZ31 Mg alloy were simultaneously enhanced through the application of caliber rolling. The AZ31 rod was inserted into the first caliber with its basal plane parallel to the normal direction(ND). The rods were rotated 90° counterclockwise around the RD for each caliber-rolling pass.
5.3. Other technologies
5.3.1. Thermal explosion synthesized Mg2Si0.5Sn0.5 compounds
Sb-doped Mg2Si0.5Sn0.5materials with decent thermoelectric performance were successfully prepared by a fast thermal explosion reaction for the first time by Zhang et al. [242].X-ray diffraction (XRD) patterns indicate that the immiscible composition of 0.5Mg2Si-0.5Mg2Sn is easily converted into a single-phase Mg2Si0.5Sn0.5solid solution via this new method, as shown in Fig. 27. With increasing Sb content,the dimensionless thermoelectric figure of merit ZTpeak(peak value) and ZTave(average value) are greatly improved, which are quite comparable to or even better than the values obtained by other methods. Therefore, a significant foundation has been laid for the future synthesis of other Mg-based and functional materials.
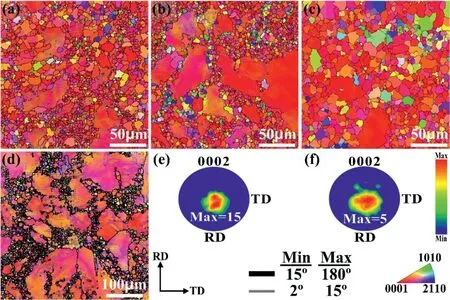
Fig. 25. (a)–(c) EBSD OIM maps of AZ91 samples processed by HPR at (a) 300°C, (b) 350°C, (c) 400°C, followed by annealing at 350°C for 5min [240].(d)-(f) EBSD results of ATZ821 samples, HPRed at 350°C followed by annealing at 350°C for 5min [241]: (d) EBSD OIM map, (e) microtexture of all grains and (f) microtexture of fine grains in (d).

Fig. 26. Schematic diagrams of the fabrication for three sheets and the details of composite extrusion process [48].
5.3.2. Directional solidification
Yang’s group reported a directional solidification technique to prepare Mg alloys with different grain morphology and orientation, which results in different mechanical and corrosion properties. It is showed that {0002} oriented planes of directionally solidified Mg–4 wt% Zn alloy had better corrosion resistance than {11–20} and {10–10} ones in 0.9wt%NaCl solution, which is attributed to a synergistic effect of surface energy, atomic packing density and the stability of oxidation film [243]. Furthermore, the degradation behavior of directionally solidified Mg–4Zn alloy with cellular structure in Hank’s solution at 37°C had a more positive corrosion potential, lower corrosion current density, larger impedance and more protective film than the samples with columnar dendritic and equiaxed dendritic structure [244]. Besides, the directionally solidified Mg–4Zn alloy with cellular grains exhibited mechanical anisotropy when the compression direction was parallel and vertical to the growth direction.The samples cut longitudinally had higher compression strength, lower compression rate and different variation tendency of work hardening rate compared with the samples cut transversely on account of the activate of{10–12}twin[245].
6. Corrosion and surface treatment of Mg alloys
6.1. Corrosion behavior of magnesium alloys
6.1.1. Environmental corrosion of magnesium alloys
There are relatively fewer investigations into the influence of environmental factors on the corrosion performance of Mg alloys, especially in the polluted marine atmospheric environment.
Fig. 27. Mg2(Si0.5Sn0.5)1-xSbx compounds prepared by thermal explosion (TE) combined with plasma activated sintering (PAS) exhibit superior peak ZTpeak and average ZTave [242].
Song’s group found that in the standard artificial seawater, the corrosion rate of the pure Mg was measured to be only a half of that in a 3.5wt% NaCl solution, while the serious localized corrosion of Mg in the NaCl solution was even inhibited [246], as shown in Fig. 28. The main constituents MgCl2and Na2SO4in the artificial seawater appeared to be inhibitive for the corrosion of Mg. These findings would further clarify the corrosive and inhibitive effects of other anions and cations on Mg dissolution in the artificial seawater. The new knowledge will be useful for the application of Mg in the marine environment.
Song’s group also investigated the corrosion of magnesium alloys in Haze, which has recently become severe air pollution issue [247]. The most deleterious ion in haze is NH4+.The presence of NH4+in haze was found to accelerate the corrosion of Mg significantly, overwhelming the acceleration effect of Cl−. When NH4+is present in the solution, it can penetrate into the surface film and combine with the MgO in the inner layer, accelerating the dissolution of the MgO. After perforation of the thin protective MgO film by the NH4+, the Mg substrate will actively react with the solution, resulting in a high corrosion rate.
6.1.2. Oxidation of single crystal Mg
Understanding the oxidation process of active metals plays a crucial part in improving their mechanical/oxidation properties. Using in-situ environmental transmission electron microscopy and density-functional theory, Qiuming Peng’s group firstly clarify the oxidation process of a single crystal Mg at the atomic scale by using a new double-hole technique. A unique incipient interval-layered oxidation mechanism of the single crystal Mg has been confirmed, in which O atoms intercalate through the clean (2–1–10) surface into the alternate-layered tetrahedral sites, forming a metastable HCP-type MgO0.5 structure [248].
In addition, the oxidation process of Mg also can be detected in oxidative gas condition. Peng ’s group reported observations of rapid oscillation sublimation of Mg at room temperature in the presence of both CO2gas and electron irradiation. The sublimation is mainly related to phase transformation of amorphous MgCO3. Differing from the direct formation of gas-state MgCO3, which attributes to the sublimation of pure Mg under a mild electron beam dose,a unique oscillation process is detected in the process of Mg sublimation under a harsh electron beam dose.The main reason stems from the first-order reaction of a reversible decompositionformation of amorphous MgCO3[249].
6.1.3. Corrosion mechanism of magnesium alloys

Fig.28. Comparison of corrosion rate for each pure Mg specimen in different solutions evaluated from average hydrogen volume: (a) addition of various concentrations of X; (b) addition of 0.02 normal concentration X [246].
To date, one of the most important findings on the corrosion mechanism of Mg alloys is the discovery of exfoliation corrosion (EFC) of as-extruded Mg–Li–Ca alloy [250]. EFC of extruded Mg–1Li–1Ca alloy occurred after experiencing three to five-months’ soaking in 3.5wt% NaCl solution. This scenario is attributed to typically elongated microstructure,and the formation of carbonates, e.g., Li2CO3, CaCO3and Mg5(CO3)4(OH)2along grain boundaries or inside the grain interiors. As a result, “wedge effect” and “hydrogen blister”,resulting from the enhanced volume of corrosion products and hydrogen evolution, respectively, lead to EFC. Moreover, it is demonstrated that the galvanic corrosion occurs between the outer skin and the interiors. EFC may be a synergic process of pitting corrosion, filiform corrosion, and IGC as well as stress corrosion.
The purity of water was observed to be important for the corrosion of magnesium alloys. Jiang et al. found that in the deionized water, only partially protective Mg(OH)2corrosion product layer was formed on the surface of Mg0.5ZnX(X=Ca and Ge) [251]. When non-deionized water with rich Ca2+was utilized, a new corrosion product layer with rich CaCO3was formed covering the top of Mg(OH)2layer, leading to be higher corrosion resistance by one order of magnitude than that tested in the deionized water. Jin et al. investigated the microstructure and corrosion performance of as-cast and solution-annealed Mg–0.5Zn–0.2Ca alloys [252].Corrosion sequence of the intermetallic particles and the related mechanisms were unveiled via quasi-in situ corrosion observations. Mg2Ca acted as an anodic phase respective to other intermetallic particles, which corrode preferentially in 0.9% NaCl solution. Ca2Mg6Zn3and MgCaSi are effective cathodes. They suggested that Si content needs to be thoroughly measured and carefully considered, which should be far below 150ppm. The amount of MgCaSi is of importance to influence the corrosion resistance.
Wolff et al. found that iron pick up took place from the steel bottom plate into the specimen during the sintering of Mg–2.6Nd–1.3Gd–0.5Zr–0.3Zn (EZK400) alloy [253]. The biomedical EZK400 Mg alloys was prepared by metal injection moulding (MIM) and sintered. Despite the fact that a separating boron nitrite (BN) barrier layer was used and the Mg–Fe phase diagram does not predict any significant solubility to each other. As a result of this study, a new bottom plate material without harming the sintering and the biodegradation performance of the as-sintered material,namely a carbon plate material, was suggested.
6.1.4. High efficiency corrosion inhibitors
Yang et al. [254] achieved a high corrosion inhibition efficiency for commercial purity Mg with the addition of sodium 2,5-pyridinedicarbolxylate, 3-methylsalicylate and fumarate into 0.5wt% neutral NaCl solution. Its corrosion efficiency increases with increasing concentration of inhibitors. They concluded that strong iron complexing agents are effective corrosion inhibitors to impede the micro-galvanic corrosion of Mg with a high amount of Fe impurities.
6.1.5. Bio corrosion behavior of magnesium alloys
Mei et al. [255] found that amino acids, vitamins and saccharides, do not critically influence the in-vitro corrosion of Mg and Mg–0.8Ca alloy.The presence of penicillin and streptomycin at a low concentration in MEM has no significant influence on the corrosion,but a higher concentration of streptomycin accelerates degradation. Promising corrosion inhibitors for Mg were proposed in their work, such as uric acid (for CP Mg) and ascorbic acid (for both Mg and Mg–Ca). The individual and synergetic influence of simulated body fluid(SBF) components on the corrosion of commercially pure Mg was investigated [256]. The results demonstrate that the corrosion acceleration in the presence of the synthetic pH buffer, Tris/HCl, is attributed to a combined effect of three factors: increased Cl−concentration, complexation of Mg2+and Ca2+and consumption of OH- needed for the formation of products. In Tris/HCl-free SBF, the synergy between Ca2+,Mg2+, HPO42−and HCO3−assures the slowest degradation.The effects of organic components, includingL-ascorbic acid(L-AA),L-glutamine (L-Gln),L-alanyl-L-glutamine (L-Ala-LGln) and fetal bovine serum (FBS), on the degradation of pure Mg under cell culture conditions were investigated[257].It is observed that they are time-dependent.Such organic components play an important part in influencing the formation of degradation layer. Hou et al. [258] also investigated the influence of bovine serum albumin(BSA),fibrinogen(Fib)or fetal bovine serum (FBS) on Mg degradation in Hanks’ balanced salt solution without (HBSS) or with Ca (HBSSCa) and Dulbecco’s modified eagle medium Glutamax-I (DMEM). The results demonstrate that the effect of BSA, Fib and FBS on the degradation rate of Mg is time- and media-dependent, as a result of the overlap of protein adsorption,binding/chelating to ions and interaction between organic molecules. They proposed that the addition of proteins to testing medium was necessary for in-vitro tests. DMEM plus 10% FBS was recommended as the in-vitro testing medium to present an in vivo-like degradation for Mg.
6.2. Surface treatment of structural magnesium alloys
Several surface treatment techniques were developed to enhance the corrosion resistance of magnesium alloy parts. For the practical application, some functions are also incorporated in the coatings.
6.2.1. Anti-corrosive coating
Na-EDTA is a hexadentate ("six-teeth") ligand and chelating agent with a strong ability to sequester metal ions such as Ca2+ and Mg2+. EDTA molecules may play a vital role in the nucleation and growth of Mg(OH)2. Li et al.[259] fabricated a dense Mg(OH)2 film on MAO-coated Mg alloy AZ31 in an alkaline electrolyte containing ethylenediamine tetraacetic acid disodium (EDTA-2Na). Fan et al.[260] prepared a hexagonal nanosheet Mg(OH)2coating through a one-step hydrothermal method using LiOH solution as mineralizer. The results EDTA-modified coating minimizes the rapid corrosion of AZ31 Mg alloy. Also,EDTA accelerates the nucleation and formation of Mg(OH)2.
Zhao et al. [261] used a novel spin-assisted Layer by Layer (LbL) assembling technology to fabricate the polyvinylpyrrolidone(PVP)/polyacrylic acid(PAA)multilayer film with excellent corrosion resistance and adhesion strength,which might be a reliable method to prepare a uniform and smooth coating due to the fact that the air shear force and centrifugal force caused by a high-speed spinning process. In addition to polyelectrolytes, nanoparticles can also be used as the assembly units of LbL.
Han et al. revealed that a rolled AZ31 alloy could be used without any treatment using the plasma electrolytic oxidation(PEO) coating [262]. The corrosion and wear resistance of PEO-coated AZ31 with clean and contaminated surfaces are basically the same. Jangde et al. compared the PEO coatings prepared using base silicate electrolyte (bPEO) and glycerol added silicate elecrolytes(gPEO)[263].It is shown that gPEO has a better corrosion resistance than bPEO. Mata et al. studied the early corrosion sensing poly ether imide(PEI)coatings with incorporated pH-sensing phenolphthalein loaded silica nano-sized capsules (SiNCs-PhPh) for an AZ31 alloy [264].The results indicated that SiNCs-PhPh/PEI coatings together with SiNCs-PhPh particles acted as effective pH-sensing.Yang et al. developed a hybrid PEO-epoxy coating for Mg[265].An efficient corrosion inhibitor(3-methysalicylate) was impregnated into the anodized layer, which was sealed by an epoxy layer through dip-coating process. They revealed that epoxy polymer could serve as a strong barrier to restrict the penetration of corrosive medium into the hybrid coating system.
6.2.2. Self-healing coating
Like other engineering materials, Mg alloy parts are inevitable to be crashed or scratched in the actual applications.Self-healing coatings, which can release corrosion inhibitors to the damaged areas, are capable of solving this problem.A duplex self-healing coating, loading interphase inhibitor Na3PO4into the pores of micro-arc oxidation (MAO) film and doping an organic inhibitor MBT into the top paint, is developed by Enhou Han’s Group in the Institute of Metal Research (IMR) [266]. When the duplex self-healing coating is damaged, Na3PO4and MBT show a synergistic effect to inhibit the corrosion of Mg substrate. On the one hand, abundant highly soluble Na3PO4is quickly released from the cross-section of scratch, and takes the main role in self-healing process. On the other hand, a small amount of MBT which is released from the top organic coating is absorbed in the defects of healing film, further enhancing the self-healing property [267]. This coating shows a superior self-healing property in the accelerated corrosion tests and atmospheric corrosion tests. Zhao et al. [13] fabricated a self-healing multilayer film composed of SiO2and CeO2nanoparticles on Mg alloys via spin-spray LbL assembly. In this multilayer film, SiO2could be used to build the physical barrier against corrosion solution, and CeO2was considered as a corrosion inhibitor to heal the physical damage. Swapnil H. Adsul et al. [268] found that Halloysite nanotubes loaded with cationic inhibitors Ce3+/Zr4+dispersed in hybrid matrix sol provide prolonged corrosion protection due to the controlled release of inhibitors, when exposed to a corrosive environment. G. Zhang et al. developed a composite coating on Mg alloys with layered double hydroxides (LDHs) (as shown in Fig. 29) exhibited excellent self-healing properties[269]. Because of the synergistic effect between Ce species and phosphate, the best self-healing ability was for PEO–Ce-LDH-P, where the compact insoluble precipitate covered the entire defect.
6.2.3. Gasochromic switchable films
Gasochromic switchable films, which can reversibly switch between hydrogenated and dehydrogenated states to achieve the switchable conversion of their optical states, have emerged in recent years with the development of hydrogen energy. An innovative composite system of fluorocarbon(FC)/Pd/MgeNb2O5switchable films was fabricated by Yue Liu et al. [270]. The FC/Pd/Mg-3 mol% Nb2O5film could effectively accelerate the switching response and improve such optical properties as transmittance at the transparent state and dynamic range. The microstructural analysis(Fig.30)reveals that the Nb2O5and an appropriate amount of ternary Mg–Nb–O phase in the Mg-based layer are supposed to facilitate rapid and sufficient hydrogen absorption and desorption. The excellent optical modulation performance and enhanced hydrophobicity of the FC/Pd/MgeNb2O5films signify an application prospect for gasochromic switchable mirror.
Fig. 29. The schematic representation of the entrapment of the aggressive chloride ions and the triggered release of anionic corrosion inhibitors from LDHs[269].
Fig. 30. The cross section of FC/Pd/Mg-3 mol% Nb2O5 film in the initial state [270].
6.2.4. Superhydrophobic coating
Superhydrophobic surfaces are generally defined as the surfaces on which water contact angles (θ) are greater than 150°and the sliding angles are smaller than 5°. It could exhibit self-cleaning, anti-icing, anti-bacterial, anti-reflection, fluidic drag reduction, anti-corrosion, and anti-fogging behavior of surfaces if the superhydrophobic properties are created in the textiles, glasses, polymers, and metals [3].
Zheng et al. [14] used a one-step electro-deposition method to fabricate a superhydrophobic coating on the surface of magnesium alloy. Using magnesium nitrate and ethanol solution of stearic acid as electrolyte, four groups of electrolytes with different ratios of stearic acid and magnesium nitrate were designed and the contact angles were 136.4±5.8° to 156.2±4.9° Khalifeh et al. have developed a super-hydrophobic coating on an anodized high purified magnesium for improving corrosion resistance [271]. The formation of the SA layer on the HP-Mg substrate provided super-hydrophobicity (165° and 164° for the anodized coated with DC and AC specimens, respectively).
6.3. Surface treatment of bio-magnesium alloys
Surface coating and surface modification with biomolecules are useful methods to improve the corrosion resistance of magnesium alloys.
6.3.1. Bioactive coatings for bone implantation
The traditional Mg–Zn–Y–Nd alloy has also been modified with MAO/PLLA/paclitaxel coating to design a drug-eluting stent (MPPS) aiming at the treatment of intestinal stricture[272]. Chen et al. [273] fabricated a citric acid/polydopamine(CA/PDA) composite coating on the Mg–Zn–Y–Nd alloy via layer by layer (LBL) assembly, which not only increased the growth of endothelial cells, but also inhibited the growth of smooth muscle cells and macrophages.
Li et al. [274] utilizes glucose as “catalyst” for boosting the formation of Ca–P barrier coating on Mg alloys to address their corrosion challenges. Liu et al. [275] obtained a refined and compact DNA-induced Ca–P coating on the surface of AZ31 alloy. The presence of DNA is beneficial to promote both corrosion resistance and bonding strength of the Ca-P coating. A novel SnO2-doped Ca–P coating has been fabricated on biomedical Mg–Li–Ca alloys [276]. The corrosion resistance of the Ca–P–Sn coating is increased by one-fold or higher in comparison with that of the substrate. The CFUs of E. coli implies that the Ca–P–Sn coating shows good antibacterial performance.
A zinc-loaded montmorillonite (Zn-MMT) coating has been hydrothermally prepared using Zn2+ion intercalated sodium montmorillonite (Na-MMT) upon magnesium alloy AZ31 as bone repairing materials [277]. The preparation procedure of Zn-MMT and Na-MMT coating and coating properties are shown in Fig. 31. Both Na-MMT and Zn-MMT coatings exhibited a better corrosion resistance than the bare Mg alloy AZ31 counterparts. In-vitro MTT tests and live-dead stain of osteoblast cells indicated a significant improvement in cytocompatibility of both Na-MMT and Zn-MMT coatings. The measured inhibitory zone and bacterial growth rate confirmed that Zn-MMT coatings exhibited higher suppression toward both E. coli and S. aureus than that of Na-MMT coatings.
6.3.2. Self-healing bioactive coatings
Zheng et al. [278] fabricated a self-healing coating, that is, bioactive Ca, Sr/P-containing silk firoin films, on Mg–1Ca alloy.It is observed that the Ca,Sr/P silk group showed excellent corrosion resistance, which is presumably due to the better retaining of the β-sheet silk conformation and ion-induced structural conversion to water-stable structure. Furthermore,Zheng et al. developed two kinds of self-healing coating systems(denoted as“silk-X”,X=phytic acid,K3PO4)[279,280].Both the phytic acid (PA) and K3PO4could act as the corrosion inhibitor, helping to regulate the secondary structure of silk fibroin. In the alkaline environment, the structure of silk fibroin could be transformed, leading to the rapid release of corrosion inhibitor and thus improving the corrosion resistance. Such self-healing coatings would not reduce the biocompatibility of alloys. Rather, it is proved that the “silk-X”systems had a positive effect on the proliferation, adherence,spreading and differentiation of MC3T3-E1 cells.
6.3.3. Antibacterial coating
Several coatings were designed to improve the antibacterial properties of magnesium alloys. Zhao et al. [281] developed a novel antibacterial AgNPs-containing multilayer film on the surface of Mg alloy through LbL assembly and siloxane self-condensation reaction. The composite coating possessed a self-healing ability against physical damages, resulting from the water swelling behavior of polysiloxane. A.D.Forero López also introduced silver in the coating to improve both the corrosion protection and antibacterial activity [282].The as-fabricated composite coating exhibits better corrosion protection properties because Ag particles seal porosity of the film and increase its conductivity. The Ag-modified bilayer exhibits also good antibacterial activity against E. coli.A MAO coating incorporated with phytic acid (PA) and tannic acid (TA) on an AZ31 Mg alloy shows good antibacterial properties [283], which can be ascribed to the ability of TA to interact with the bacterial cell wall and chelate metal ions to reduce the activity of metalloenzymes.
6.3.4. Other coatings
As depicted in Fig. 32(a)–(d), biomolecues adsorb on the Mg (0001) surface through the coordinate covalent bonds and flat adsorption configurations of biomolecules with more functional groups binding to surfaces were energetically stable[284,285]. The electronic structure of Mg alloy surfaces was changed by the addition of alloying elements (Zn, Y or Nd)due to different electronegativity as shown in Fig. 32(e) and(f), affecting the bonding ability between biomolecules and Mg alloy surfaces instead of the bonding nature.
Ji et al. [286] prepared a hydroxyapatite coating induced by ciprofloxacin-loaded and gentamicin-loaded polymeric multilayers on magnesium alloys, respectively. Both coatings exhibited favorable corrosion resistance, controlled antibiotic release, excellent antibacterial activity and suitable cytocompatibility.
Fig. 31. Schematic illustration of preparation of Zn-MMT and Na-MMT coating and coating properties [277].
Fig. 32. Simulated charge density difference with the value of the isosurface of 0.02 e/˚A3 and 0.005 e/˚A3.Mg1 and Mg2 represent Mg atoms in the first and second layer.
7. International standards on magnesium and magnesium alloys
Standards on magnesium and magnesium alloys have attracted more attention recently due to the rapid growth of magnesium market. Since he was elected as Chairman of ISO/TC 79/SC 5 (subcommittee on magnesium and magnesium alloys), Prof. Fusheng Pan chaired the plenary meetings of ISO/TC 79/SC 5 in Lisbon, Portugal in 2018 and in Chicago,United States in 2019,respectively.Important progress has been made on the development of ISO standards on magnesium and magnesium alloys.
As can be seen from Table 13, four ISO standards out of 26 were published in 2018–2019, which is quite active in the aspect of standard preparation. Dr. Zisheng Zhen from Magontec Xi’an Co., Ltd, Prof. Uemoto from Meisei University (Japan), Prof. Bin Jiang from Chongqing University,Prof. Ping Zeng from Guizhou Institute of Technology act as the project leader of ISO 26202:2019, ISO 20260:2019,ISO 3116:2019, and ISO 20258:2018, respectively. Twenty(20) wrought magnesium and magnesium alloys newly developed in China were collected in ISO 3116:2019, which were innovatively designated as MG9999, MAQ80, MAL32,MAT11,MAT61,MAJ31,MAZ31c,MVWZ641,MVWE751,MVW83, MVWZ842, MVK41, MLAZ433, MLAZ1033,MWEK711, MWEK911, MZAC811, MZC20, MZME210,MZM51. Fourteen (14) newly developed alloys from Japan and two from United Kingdom were also collected in ISO 3116:2019, which were MAX51,MAX61,MAX62,MAM60,MAZ91, MAXZ311, MAXZ611, MXAZ621, MAXZ811,MAXZ911, MAXZ921, MLZ91, MWZ73, MWZ75, and MWE43c, MVW76, respectively.
In addition, three standards are under development which are all proposed by China, as listed in Table 14. ISO/CD 8287 was proposed to revise ISO 8287:2011 (Ed. 4) due to the recent development of primary magnesium industry by Qinghai Salt Lake Co. Ltd. Prof. Xianhua Chen and Mr.Xinyu Gao served as projected leaders of ISO/WD 23700 and ISO/WD 23694, which were proposed to develop standards on the rolled plates and sheets and extruded rods, bars and tubes, respectively.

Table 13 Current published ISO standards on magnesium and magnesium alloys.

Table 14 Current ISO standards under development on magnesium and magnesium alloys.
However, there are still some issues in the development of ISO standards on magnesium and magnesium alloys. For instance, ISO standards are still not enough, and many standards are obsolete.The system of standards is lack of strategic design. The quality of some of ISO standards are not satisfied and are less powerful than ASTM standards. Besides, experts in the magnesium field are not actively participated in the development of ISO standards.
To solve the afore-mentioned problems, Chairman of ISO/TC 79/SC 5 proposed the following suggestions to further improve the impact of ISO standards.
(1) Present more on the standardization of Mg in the national and international Mg conferences.
(2) Provide more trainings on the preparation of Mg ISO standards.
(3) Establish an online real-time exchange platform for standard experts where they can express their thoughts faster and more easily.
(4) Intensify face-to-face communication and thorough discussion during the standards preparation.
(5) Publish a collection of national standards in English.
8. Summary and outlook
More and more scientists and researchers joined in the research and development of magnesium and its alloys in the world due to the increasing importance of magnesium and its alloys. A large number of new magnesium alloys have been developed. Among them, MG9999, MAQ80, MAL32,MAT11,MAT61,MAJ31,MAZ31c,MVWZ641,MVWE751,MVW83, MVWZ842, MVK41, MLAZ433, MLAZ1033,MWEK711, MWEK911, MZAC811, MZC20, MZME210,MZM51 from China, MWE43c, MVW76 from the United Kingdom,and MAX51,MAX61,MAX62,MAM60,MAZ91,MAXZ311, MAXZ611, MXAZ621, MAXZ811, MAXZ911,MAXZ921, MLZ91, MWZ73, MWZ75 from Japan were newly collected in the recently published ISO 3116:2019 (5th edition).
With further research on the precipitation strengthening and fine grain strengthening, the strength (especially the yield strength) of magnesium alloys is greatly improved in 2018–2019. For some high strength magnesium alloys, the ultimate tensile strength has exceeded 560MPa, and the yield strength has surpassed 540MPa. The development of rare earth free and low rare earth high strength magnesium alloys is more prominent,such as Mg–5Al–3Ca–0.3Mn alloy exhibits a yield strength of 420MPa, an ultimate tensile strength of 451MPa and an elongation to failure of 4.1%.
The proposed theory of solid solution strengthening and ductilizing (SSSD) provided an important theoretical basis for the development of high plasticity magnesium alloys,and provided a new solution to the balance between strength and plasticity of magnesium alloys. The development of high plasticity magnesium alloys has made an important breakthrough in 2018–2019 with comprehensive applications of the SSSD, grain refinement and controlling of long-period stacking ordered structure. A batch of high plasticity magnesium alloys exhibited great potential in industrial applications, such as low cost Mg–1Mn–0.5Al alloy showed an ultimate tensile strength of 263MPa and a high elongation of 33.4%.
The preparation and processing technologies of magnesium alloys have been further improved in 2018–2019. Among them, both the theory and technology of asymmetric processing technology have made a new breakthrough. Some of new preparation and processing technologies show important prospects,such as melt self-purification, magnetic assisted solidification,low temperature extrusion,on-line twist extrusion,hard pate rolling, composite extrusion, etc.
Magnesium matrix composites are expected to provide a new pathway for improving the strength and elastic modulus of magnesium alloys. In 2018–2019, the published results have shown that SiC-reinforced AZ91 alloy exhibited a high strength of ∼441MPa and a high elastic modulus of ∼60GPa.
Functional magnesium materials have demonstrated great potential and will become a hotspot in the breakthrough and application of magnesium and magnesium alloys. Among them, hydrogen storage Mg materials, battery Mg materials,bio-Mg materials, etc., have made rapid progresses, which have already exhibited advantages in competition with the traditional materials. Hence, functional magnesium materials are anticipated to become an important growth point in the field of new magnesium materials.
Compared with traditional materials such as steel and Al,the comprehensive properties of magnesium alloys need to be further improved. For example, the ultimate tensile strength and plasticity of ultra-high strength cast magnesium alloys must be increased to more than 400MPa,7–10%,respectively.The ultimate tensile strength and the plasticity of ultra-high strength wrought magnesium alloys must be increased to more than 600MPa and 7–10%, respectively. The elastic modulus of high elastic modulus magnesium alloys must be enhanced to the level close to that of aluminum alloys. The corrosion resistance of high corrosion resistant magnesium alloys must be improved to meet the application requirements in most occasions.
The fundamental research on magnesium and magnesium alloys needs to be further strengthened. For instance, accurate phase diagrams of magnesium alloys are awfully insufficient;fundamental data such as diffusion coefficient, thermal conductivity, activity coefficient, etc., of magnesium alloys are also scarce; the basic research on a variety of precipitates and their precipitation mechanisms including various phase relationships is limited; and the development of welding and joining technology in magnesium alloy parts especially more challenging dissimilar welding of magnesium alloys to other materials such as steels and aluminum alloys is also inadequate. It is hoped that most of these pressing issues can be solved in the next five years or so, so as to promote vigorously the widespread industrial applications of lightweight magnesium alloys.
Declaration of Competing Interest
None.
Acknowledgments
The authors would like to thank more than 50 experts from 25 universities and research institutes worldwide have contributed to this review through providing the important information and commenting on the preparation. The content in this review is financially supported by the National Key Research and Development Program of China (No.2016YFB0301100, 2017YFF0209100), the National Science Foundation for Scientists of China (No. 51531002, 51474043,51701027, 51971042, 51901028), the Chongqing Academician Special Fund (cstc2018jcyj-yszxX0007, cstc2019yszxjcyjX0004).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Advances in coatings on biodegradable magnesium alloys
- Stability of twins in Mg alloys – A short review
- A review on thermal conductivity of magnesium and its alloys
- Effect of Ca addition on the microstructure and the mechanical properties of asymmetric double-sided friction stir welded AZ61 magnesium alloy
- Effect of pre-deformation on microstructure and mechanical properties of WE43 magnesium alloy II: Aging at 250 and 300 °C
- Constitutive behavior and processing maps of a new wrought magnesium alloy ZE20 (Mg-2Zn-0.2Ce)
