Effects of Facultative Anaerobic Cellulolytic Bacteria and Nitrogen-fixing Bacteria Isolated from Cow Rumen Fluid on Rumen Fermentation and Dry Matter Degradation in Vitro
2020-04-28ZhangMeimeiLiYanfangandLiuDasen
Zhang Mei-mei,Li Yan-fang,and Liu Da-sen,
1 College of Animal Sciences and Technology,Northeast Agricultural University,Harbin 150030,China
2 College of Science,Northeast Agricultural University,Harbin 150030,China
Abstract: The purpose of the paper was to study the effects of cellulolytic bacteria (CB) mixed with nitrogen-fixing bacteria (NFB)on fermentation in vitro. Nine CB strains and seven NFB strains were isolated from rumen fluid of three Holstein cows. Based on higher activity of cellulose or nitrogenase,three CB types [CB-2(KT725624),CB-5(KT725623) and CB-6(KT725622)] and one NFB type [NFB-3(KT735054)] were screened out,respectively. Two parts were included in this study. The first part explored the optimal mixed ratio of CB to NFB through inoculating filter paper medium with the bacteria of 2×105 cfu · mL-1. According to CMCase and FPase activities in the medium,the ratio of 4 to 1 was proven to be more effective. In the second part,rumen fermentation in vitro was conducted at 4 : 1 of CB to NFB,aiming at studying the effects of mixed bacteria. Six groups were classified,namely,control group(no bacteria),Group 1 (CB-2+NFB-3),Group 2 (CB-5+NFB-3),Group 3 (CB-6+NFB-3),Group 4 (NFB-3) and Group 5 (CB-6). All the experimental groups had the same amount of bacteria (4×106 cfu · mL-1) in the fermentation liquid. Samples were collected at 2,4,8,12 and 24 h of incubation. Compared with the groups with CB or NFB alone,gas production,dry matter degradability and bacterial protein expressions in the mixed groups increased. However,NH3-N concentration decreased and pH was stable. Meanwhile,related values among three mixed groups were significantly different; values in Group 2 were the best.
Key words: rumen,cellulolytic bacterium,nitrogen-fixing bacterium,mixed culture, in vitro fermentation
Introduction
The rumen is a complex microbial ecosystem,in which the dynamic balance is kept through the collaboration and constraint between rumen microorganisms and host,and among microbes (Dehority,2003). The rumen is evolved to digest various plant materials with highly complicated rumen microbial ecosystem (Theodorouet al.,1990; Kamra,2005)in which bacteria are the dominant microbes and make great contributions to digest and convert shortchain fatty acids and microbial proteins (Hobson and Stewart,1997). Most rumen bacteria are obligate anaerobes; however,some of them might be killed,due to their sensitivities to oxygen exposure (Kamra,2005). However,oxygen is consumed by facultative anaerobes in the rumen (Katoet al.,2004). Gonget al. (2008) isolated and identified facultative anaerobes from cattle rumen,in which seven strains with 16S rDNA genes are amplified by PCR; in the rumen fluid,facultative anaerobic cellulolytic bacteria(CB) are screened out to determine the activities of cellulose (Xu,2012). Further more,the effects of CB on rumen fermentation and cows' growth performance demonstrate that facultative anaerobe benefits from rumen environment by consuming oxygen swallowed along with diet and diffused through the rumen wall in the blood stream.
NFB is also found in animals; a majority of studies about nitrogen-fixing bacteria (NFB) isolated from the plant and soil are conducted (Hureket al.,1997;Bellengeret al.,2014). Nitrogenase genes are found in bacteria isolated from the gut of termites (Ohkumaet al.,1999). Moreover,NFB is found and studied in the ruminant. There are some aerobic bacteria in the sheep fix atmospheric nitrogen (Ellewayet al.,1971;Jones and Thomas,1974). However,only three strains of bacteria in the rumen need to fix nitrogen with oxygen.
In nature,cellulosic materials are degraded through the cooperation of many microorganisms. Some researchers reported that the mixed culture of one CB strain with non-cellulolytic bacterium is ideal for degradation of cellulose (Odom and Wall,1983;Lewiset al.,1988),proving that this "symbiosis"apparently promotes degradation. Harutaet al. (2002)found that high degradation ability and stability are achieved by a complex microbial community which enables cooperation between aerobic and anaerobic bacteria based on DGGE (PCR-denaturing gradient gel electrophoresis) and 16S rDNA sequence analyses.Non-cellulolytic bacteria are combined to degrade cellulose (straminisolvensCSK1) to supply anaerobic environment,consume metabolites,improve the cellulolytic activity,and neutralize pH (Katoet al.,2004). The cooperation between two kinds of microbes is observed in the experiments of treating household garbage (Puet al.,1999) and composition (Sun and Xu,2009).
For references,the effects of CB and NFB on rumen fermentation have not been reported; however,the live microbe in animals' feed supplement improves its microbial balance and activity (Mellenet al.,2017;Fon and Nsahlai,2013; Aydinet al.,2009 ). Indeed,using highly competitive active bacteria in the rumen is an effective approach of rumen fermentation (Reiset al.,2016). Inoculation of lactic acid bacteria (LAB,105-106·g-1) in wheat straw,concentrates or silage improves animal production performance (Khuntia and Chaudhary,2002; Weinberget al.,2004; Yuanet al.,2015); mixed yeast culture supplementation(1.5-2.0×109live cells · mL-1,1 mL · kg-1) improves the digestibility of poor quality forage,microbial CP synthesis,feed efficiency and growth performance(Bayatet al.,2015; Silva,2015; Tripathi and Karim,2010; Supratmanet al.,2018). In this paper,a fermentation experiment was conducted to explore the effects of CB and NFB on rumen fermentationin vitro.
Materials and Methods
Nitrogen-fixing bacteria (NFB) and cellulolytic bacteria (CB)
Collection of rumen fluid
Rumen fluid was collected from three Holstein cows that were fitted with rumen fistula; the animals were daily fed with an equal amount of meals at 6:00 a.m.and 6:00 p.m. (concentrate : forage=45 : 55,DM basis)and had free access to water. Ruminal content was obtained through the fistula before morning feeding and was then strained through four layers of surgical gauze to separate fluid. The fluid was put into a container pre-warmed (39℃) and filled with CO2;then the container was immediately transported to the laboratory for isolation of cellulolytic bacteria (CB)and nitrogen-fixing bacteria (NFB).
Isolation of the bacteria
Isolation of bacteria was performed on a clean bench to prevent contamination. The process was briefly described as the followings: 1 mL of fluid was added to 9 mL of sterilized 0.9% (w/v) saline solution for an initial dilution (10-1). Serial dilutions were conducted;0.1 mL of each dilution sample (10-2,10-3,10-4,10-5and 10-6) was spread on the defined medium agar plates (peptone 10g,agar 17 g,beef extract 3 g,NaCl 5 g,distilled water 1 L and pH 7.2) and incubated at 39℃ for 24-48 h under anaerobic conditions. Then cellulolytic bacteria (CB) and nitrogen-fixing bacteria(NFB) were isolated. For CB,colonies on plates were inoculated on new solid medium plates with carboxymethyl cellulose (CMC),which were taken as sole carbon source and incubated for 48 h,and then purified. For NFB,isolation was done on N-free solid medium. Eventually,two kinds of bacteria were purified for 48 h under aerobic conditions; the isolated strains of CB and NFB were facultative anaerobic bacteria.
Identification of bacteria
CB was identified by Congo red culture medium and then examined for its cellulose activity (Milleret al.,1960) with spectrophotometer (mini-1240 UV-Vis;SHIMADZU,Japan). In addition,nitrogenase activity of NFB was assayed by gas chromatography based on the acetylene reduction test (Limaet al.,2015).Furthermore,isolated CB and NFB were morphologically examined by Gram staining and malachite green spore staining (Cappuccino and Sherman,2008); physiological-biochemical identification was performed in accordance with Bergey's Manual of Systematic Bacteriology (Garrityet al.,2004).Molecules of the bacteria were identified by polymerase chain reaction (PCR),in which 16S ribosomal DNA was amplified with universal primers pair 27F(5'-AGAGTTTGATCCMTGGCTCAG-3') and 1492R(5'-TACGGTYTACCTTGTTACGACTT-3'). The purified PCR products were sequenced by Genwiz Biotech. Co.,Ltd (Beijing,China). The acquired data was retrieved for homologous sequences with EzTaxon in EzBioCloud (http://www.ezbiocloud.net).
Accession numbers
16S rDNA sequences for isolated strains (two CB strains and one NFB strain) were referred to GenBank(http://www.ncbi.nlm.nih.gov/genbank/) with the accession numbers: KT725624 (CB-2),KT725623(CB-5),KT725622 (CB-6) and KT735054 (NFB-3).
Mixed culture of nitrogen-fixing bacteria (NFB) with cellulolytic bacteria (CB)
Effects of mixed culture of NFB with CB were examined by cellulose enzyme activity and filter paper degradation rate. The total amount of CB+NFB among the experimental groups were 2×105cfu · mL-1and the ratio of CB to NFB from Group 1 to Group 5 were 4 : 1,3 : 2,2 : 3,5 : 0 and 0 : 5,respectively. The mixed bacteria were inoculated in 250 mL of flasks with 50 mL of filter paper medium per flask,and were incubated at 39℃ and shook at 150 r · min-1for 8 days.Samples were analyzed every 24 h. After incubation,the content in the flask was centrifuged at 4 500 r · min-1for 15 min. Precipitation was rinsed by deionized water and dried at 65℃ for 48 h. The decomposition rate of filter paper was measured by weight loss. Furthermore,pH of supernatant in the culture solution was determined by acidity meter (PB-21; Sartorius AG,GER) and activities of CMCase (Milleret al.,1960) and FPase.The measurement procedure of FPase activity could be briefly illustrated as the followings: the filter paper strip was mixed with 0.5 mL of 5-fold diluted clear supernatant and 2 mL of 0.1 mol · L-1citrate phosphate buffer (pH 4.8) in a test tube and then incubated at 50℃ for 1 h; subsequently,the operation process was applicable for assay of CMCase activity.Five replicates were included for each group.
Bath fermentationin vitro
Six groups were covered,including five experimental groups with the addition of the bacteria and one control group without the bacteria,with five replicates per group. In five mixed groups,the adding amount of the bacteria for each experimental group was 4×106cfu · mL-1at 4 : 1 of CB to NFB (based on the above experiments); Group 1,Group 2,Group 3,Group 4 and Group 5 were added with CB-2+NFB-3,CB-5+NFB-3,CB-6+NFB-3,NFB-3 and CB-6,respectively.The preliminary study results showed that during thein vitrofermentation process,results of groups adding CB-2,CB-5 or CB-6 alone were found to be not significantly different. Therefore,the effects of CB-2 and CB-5 on the fermentation were not covered in this study.
The rumen fluid for isolating NFB and CB came from the same cow rumen. Rumen fermentationin vitroconformed to the methods described by Menkeet al. (1979),but there were some differences. In brief,0.5 mL of mixed bacteria solution,1 g of substrate(consisting of 60% roughage with the addition of alfalfa and hay,and 40% concentrate with the addition of corn and soybean meal),27 mL of rumen fluid and 54 mL of buffer solution pre-prepared anaerobically were placed in a bottle,pre-warmed at 39℃ and flushed with CO2. The bottle was connected to a syringe through a tube; all the bottles were incubated in a shaking box (HZS-H; Dongyi Company,China) at 130 r · min-1. After 2,4,8,12 h or 24 h of incubation,gas productions in the syringes were recorded and the bottles were placed into ice water to stop fermentation; pH was measured by acidity meter (PB-21;Sartorius AG,GER). Then contents in the bottles were centrifuged at 8 000 r · min-1for 15 min,while the precipitate was dried at 65℃ overnight; the degradation rate of dry matter was measured (Salem,2005). Meanwhile,the supernatant was collected to analyze ammonia-N by phenol-hypochlorite (McKieet al.,2004; Soleimaniet al.,2015) and bacterial protein by purine content (Abdiet al.,2015). There were a total of 125 bottles (five mixed ratios×five replicates×five incubation time points).
Statistical analysis
Statistical analyses were carried out through SPSS version 17.0 software. All the experimental data were compared through one-way analysis of variance(ANOVA),followed by Duncan's multiple comparison tests;p<0.05 indicated the statistical significance.
Results
Characterization of CB and NFB
From anaerobic conditions to aerobic incubation conditions,nine strains of cellulolytic bacteria (CB)were isolated with CMC-Na,which were taken as sole carbon sources; seven stains of nitrogen-fixing bacteria(NFB) were obtained on N-free solid medium. All the bacteria were facultative anaerobic bacteria. Then CB was stained with Congo red method and tested for cellulose enzyme activity. Meanwhile,NFB were used for assay of nitrogenase activity. What's more,three CB strains and one NFB strain with higher enzyme activities were chosen for the further studies. Two kinds of bacteria were identified by Gram staining and malachite green spore staining (Table 1).

Table 1 Characterization of isolated bacteria
Ratio of NFB to CB
Effect of mixed culture on cellulose enzyme activity is shown in Fig. 1. During the incubation process(0-144 h),the highest activities of CMCase and FPase occurred at 72-96 h,but sharply decreased after 96-120 h of incubation,when the lowest activities of two enzymes were observed. At 72 h,results showed that CMCase activities in Groups 1 and 2 were higher than those of Groups 3 and 4 (p<0.05); FPase activity in Group 1 was 24.3% higher than that of Groups 3 and 4 (p<0.05). However,no significant difference was observed for FPase activities.
After 192 h of incubation,mixed culture remarkably increased the degradation rate of filter paper(Table 2). At the same time,Group 5 (NFB alone,no degradation) and Group 4 (CB alone) had lower values(only 15.6%) (p<0.05); but Group 1 had higher values than Group 2 (53.2% vs. 34.9%) (p<0.05). 1 : 4 of NFB to CB ratio was thought to be the best.
Values of Groups 1-4 were basically kept above 7;but values of Group 5 sharply declined to the lowest 48 h after incubation (about 3.5) from 120 h to168 h(Fig. 2).
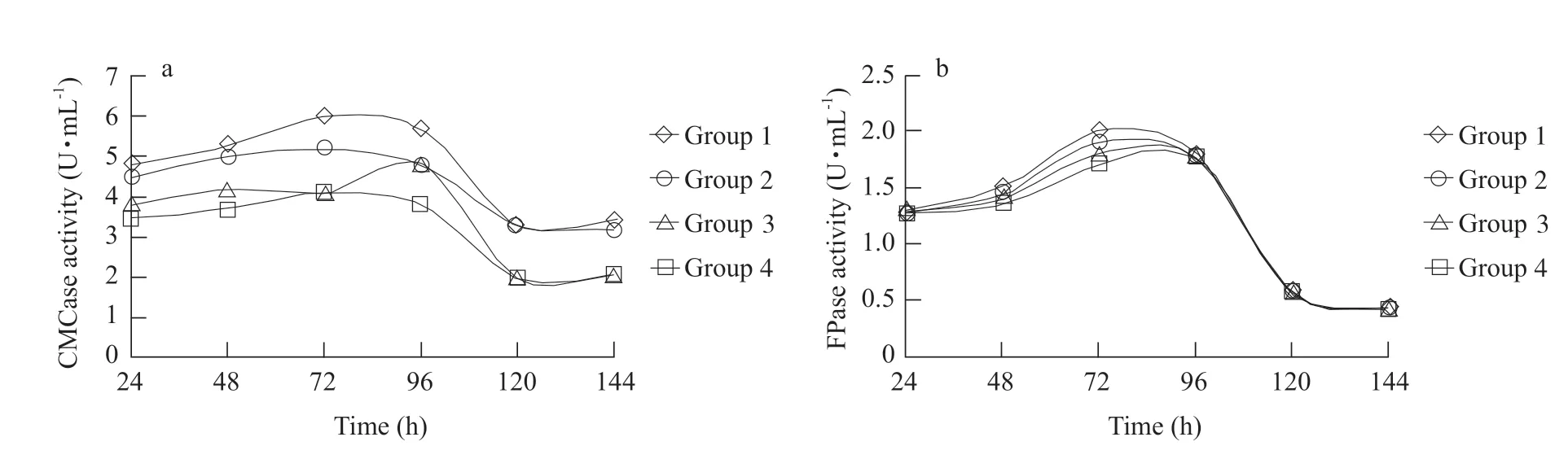
Fig. 1 CMCase and FPase in mixed culture

Table 2 Degradation rate of filter paper
In vitro fermentation
CB to NFB ratio of 4 : 1 was applicable forin vitrofermentation. Results in Groups 1,2 and 3 (adding cellulose of CB-2,CB-5 or CB-6) were similar (p>0.05).
The gas production among groups showed declining trends from 0 h to 8 h,but showed increasing trends from 12 h to 24 h. During 24 h of fermentation,pH in mixed groups (Groups 1,2 and 3) was higher than that in the control group and groups added with CB or NFB alone; related values in CB group were lower.However,no significant difference among groups was observed (p>0.05) (Fig. 3).
Compared with the control group,NH3-N concentration was affected by mixed treatment at 8,12 and 24 h (p<0.05). At 12 and 24 h,values of Group 3 were lower than those of other two mixed groups and groups with CB or NFB alone (p>0.05) (Table 3).
From 2 h to 8 h,productions of bacteria protein(BP) in the mixed groups were higher than those of the control group and groups with CB or NFB alone;but no significant difference was observed (p>0.05);at 12 h,productions of BP in Groups 1 and 2 were higher than those of the control group (p<0.05);Group 2 produced the highest BP at 24 h. However,BP contents in the treatment groups with CB or NFB alone were not different from those in the control group during 24 h of fermentation (p>0.05) (Table 4).
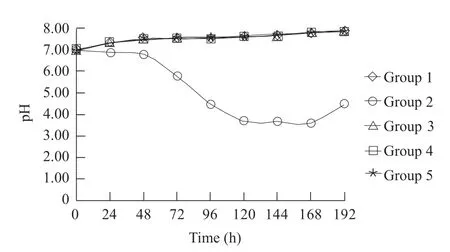
Fig. 2 pH in mixed culture

Fig. 3 Values of pH in culture solution during in vitro fermentation

Table 3 Concentration of NH3-N in culture solution (mg · 100 mL-1)

Table 4 Concentration of bacterial protein (mg · L-1)
Gas production
From 2 h to 24 h of fermentation,there was gas production in all the groups. At each time point,gas production amount in mixed groups (Groups 1 to 3)were higher than that in the control group (p<0.05); but no significant difference was observed among the three mixed groups (p>0.05). To sum up,it was inferred that there was an interaction between CB and NFB(Fig. 4).

Fig. 4 Gas production in culture solution during in vitro fermentation
Degradability of dry matter
Degradation rate of dry matter (DM) for all the groups are shown in Table 5. During 24 h of fermentation,no significant difference among groups was observed from 2 h to 8 h (p>0.05); at 12 and 24 h,the degradation rates of DM in Groups 2 and 3 were significantly increased compared with those in the control group(p<0.05); but no significant difference between groups was observed (p>0.05).
However,the degradation rate of DM with the treatment of CB or NFB alone was not significantly different from that of the control group at 12 and 24 h(p>0.05).

Table 5 Degradation rate of dry matter (%)
Discussion
Rumen cellulolytic bacteria and nitrogen-fixing bacteria
Many studies have reported that facultative anaerobe existed in the rumen and could be successfully isolated from rumen fluid (Hobson and Stewart,1997; Jones and Thomas,1974; Fanet al.,2012). In the rumen,oxygen was swallowed during feeding and drinking water was harmful to anaerobes. As a consequence,facultative anaerobes were used to generate and maintain anaerobic conditions (Kamra,2005). In this experiment,16 strains isolated from rumen fluid were facultative anaerobe. The reason was that it was failed to create an absolutely anaerobic condition,during the cultivation process. The determination for the enzyme activity of 16 strains showed that CB just had cellulose enzyme activity and NFB with nitrogenase activity. Besides,isolated CB and NFB strains were similar toBucillusby homologous sequences retrieved. Therefore,in vitrofermentation examination showed that the mixutre ofSonorensissp. andBacillustequilebsissp. was more effective by comparison with the mixture ofMethlotrophicussp. Three CB strains were classified asBacillusgenus. Research findings indicated that bacteria of the same genus had different effects; thus more researches about CB shall be further reported.
In vitro fermentation
pH in the rumen fluid reflect the rumen environment conditions and microbial fermentation in a comprehensive way. Earlier studies showed the effects of pH in the rumen fluid on the diet digestion and the amount of salivation (Tripathiet al.,2004; Emery and Brown,1961). The effect of pH on fiber digestibility in the rumen was extensively documented (Sariet al.,2015),in which few cases of cellulose degradation occurred under pH 6.0 (Brownet al.,2006). In this study,all the groups had pH of more than 6.5,which did not affect the cellulose degradation. From 0 h to 8 h,pH of all the groups decreased,which might be caused by the declining buffering capacity of artificial saliva and the accumulation of massive volatile fatty acids. Over time,more ammonia were released from feed proteins to regulate pH in the rumen fluid (Tripathiet al.,2004). Compared with single group,mixed groups with CB and NFB maintained pH at a higher level. This was because of the amide generated by nitrogen fixation; then pH in the rumen fluid decreased in a slow and stable manner. Ruminal NH3-N concentration was available to reflect the feed protein degradation ratein vivoandin vitro. The optimal NH3-N concentration was of great importance to bacterial protein synthesis (Czerkawsli,1976;Kroppet al.,1977); meanwhile,bacterial protein expression produced significant effects on the growth performance of ruminant. The extent of NH3-N in the rumen was primarily determined by the protein degradation rate in diets and nitrogen required by most ruminal bacteria strains. As mentioned above,the optimum pH in the mixed groups with the addition of CB and NFB resulted in the high microbial activity and quantity when a large amount of NH3-N was utilized. Inefficient utilization of NH3-N by the ruminal microorganisms was the major reason for intra ruminal nitrogen cycling (Hallet al.,2016). In this study,NH3-N concentrations of all the groups were kept in the suitable range for microbial growth(Wanapat and Pimpa,1999). The changing trends of NH3-N concentration were in agreement with those proposed by Pilajunet al(2016). Compared to the groups with CB or NFB alone,NH3-N concentration in the mixed groups was significantly decreased after 12 h of incubation; this was because the release of fermentation inhibition might result in the microbial fermentation again. Moreover,the decreased NH3-N concentration in the mixed groups was in accordance with the increased microbial protein contents owing to the outflow of microbial protein (Jouany,1991).
Gas production and dry matter degradability
During the microbial fermentation process,a lot of gas (CO2and CH4) were produced from the substrate in which ruminal nutrient degradation and the metabolizable energy of animal feed were predicted(Contreras-Goveaet al.,2011). This study showed that the more the dry matter was depredated,the more the gas was produced. The findings were in accordance with the positive correlation between dry matter degradation and gas production (Salem,2005).
On the one hand,dry matter (DM) degradation was applicable to evaluate the feed nutrition conditions,although it was not equal to the value in the digestive tube (El-Waziry and Ibrahim,2007); on the other hand,DM degradation largely reflected the digestive condition in the rumen. The experiment showed that DM degradation rate among the mixed groups was different; better degradation effects were found in Groups 2 and 3; the results were in agreement with the reports made by Katoet al(2004). Compared with the groups with CB or NFB alone,Groups 2 and 3 had higher gas production and DMD. Results indicated that consuming fermentation products and promoting microbial fermentation were good for cooperation between CB and NFB or between the two bacteria and original rumen microbes.
Interaction of two bacteria and its application prospects
The results had shown that CMCase and FPase activities in the mixed culture groups increased compared with the single culture group. This was a new finding for cellulose degradation in the rumen. Essential factors for cellulose degradation in the mixed culture system were expounded as the followings: firstly,NFB might supply the nitrogen source for CB,which the products of decomposing cellulose were taken as the carbon source for NFB (CB and NFB synergy);secondly,NFB might reduce the concentration of cellooligosaccharides in the culture solution and scavenge metabolites from cellulose,which might deteriorate cellulolytic activity (Katoet al.,2004);thirdly,pH in the mixed culture solution was about 7.0,which was the optimum value for microbial growth and cellulose degradation (Terryet al.,1969; Stewart,1977).
In this study,the addition of live CB and NFB based on pH,NH3-N concentration,bacterial protein,gas production and dry matter degradation was proven to be effective for rumen fermentationin vitro. The results showed that CB combined with NFB could achieve the high degradation ability and stability by the live microbial feed supplement,which was similar to the reports made by Yuanet al(2015). Since the antibiotics in the ruminants' feeds were banned,the direct-fed microbe (DFM) became the alternative choice. If these microbes could be colonized and established (gain stability) in the rumen,their availability would be recognized (Fon and Nsahlai,2013).In this research,both CB and NFB were isolated from cow rumen,which were adaptable and nonpathogenic to the ruminal environment. The additives might modify the rumen microbial composition,which selectively affected rumen symbionts by the introduction of foreign microbes in the rumen (Bello and Escobar,1997). Therefore,the bacteria used could be seen as the potential microbial ecological agent for the ruminants. In the future researches,the mixture of CB with NFB will subsequently be used in dairy cattle feeding,so as to investigate the effects of the two kinds of bacteria on fermentationin vivoand cow production.
Conclusions
During thein vitrofermentation process,cellulolytic bacteria (CB) mixed with nitrogen-fixing bacteria(NFB) had better effects than CB or NFB alone.According to gas production,dry matter degradation and bacterial protein content,the best ratio of CB to NFB was 4 : 1.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Genetic Diversity of Maturity Related Genes of Soybean During One Centurial Artificial Selection in Northeast China
- Effects of Lanthanum and Cerium on Chlorophyll Content,Yield and Components of Soybean in Northeast China
- Visualizing Patterns and Differences in Fine Particulate Matter(PM2.5) Research Between the USA and China over Last 25 Years: A Bibliometric Analysis
- Effects of Different Feeding Methods on Behaviors,Immunities and Growth Performances of Suckling Calves
- Effect of Zinc Acetate on Broiler Nutrient Metabolism and Skeleton Characteristic
- Prokaryotic Expression of IBV N Protein and Development of Indirect IBV N Protein-mediated ELISA
