姜黄素及其类似物抑制CCl4诱导大鼠肝纤维化的作用及机制研究
2020-04-27王子恺毛泳彬孔力
王子恺 毛泳彬 孔力


[摘要] 目的 研究姜黄素及其类似物抑制CCl4诱导大鼠肝纤维化的作用及机制。 方法 SD大鼠雄性40只,按体重随机分成模型组,对照组,姜黄素组,H8低、高剂量组,每组8只。模型组,姜黄素组和H8组腹腔注射40%CCl4橄榄油复制肝纤维化模型,8周后姜黄素组,H8低、高剂量组给予相应药物灌胃,同时每周腹腔注射40%CCl4橄榄油一次,共4周。处死大鼠后,检测血液生化指标,肝组织HE和Masson染色观察病理形态学变化,QPCR测Col1、纤连蛋白(FN)、转化生长因子-β(TGF-β)、α-平滑肌肌动蛋白(α-SMA)mRNA。 结果 模型组谷丙转氨酶(ALT)、谷草转氨酶(AST)、血清谷氨酰转肽酶(GGT)、肝脏系数、Col1、FN、TGF-β、α-SMA mRNA显著高于对照组,差异有高度统计学意义(P < 0.01)。姜黄素组,H8低、高剂量组血清ALT、AST、GGT、肝脏系数、Col1、FN、TGF-β、α-SMA mRNA显著低于模型组,差异有统计学意义(P < 0.05)。 结论 姜黄素及其类似物H8对CCl4诱导的肝纤维化大鼠有保护作用,且H8作用优于姜黄素。
[关键词] 姜黄素及其类似物;四氯化碳;肝纤维化;肝脏系数;病理形态学
[中图分类号] R575.2 [文献标识码] A [文章编号] 1673-7210(2020)03(b)-0013-04
[Abstract] Objective To study the effect and mechanism of curcumin and its analogues on CCl4 induced hepatic fibrosis in rats. Methods Forty male Wistar rats were randomly divided into model, normal curcumin, H8 low and high dose groups, with 8 rats in each group according to body weight. The model group, curcumin group and H8 group were intraperitoneally injected with 40% CCl4 olive oil to replicate the liver fibrosis model. After 8 weeks, the curcumin group, H8 low and high dose groups were given the corresponding drugs by gavage, and intraperitoneal injection of 40% CCl4 olive oil once for 4 weeks. After sacrificed the rats, blood biochemical indicators were measured, pathological morphological changes were observed by HE and Masson staining of liver tissue, while Col1, fibronectin (FN), transforming growth factor-β (TGF-β), and α-smooth muscle actin (Α-SMA) mRNA were measured by QPCR. Results The alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum glutamyl transpeptidase (GGT), liver coefficient, Col1, FN, TGF-β, α-SMA mRNA in the model group were significantly higher than those in the control group, and the differences were highly statistically significant (P < 0.01). In the curcumin group, the serum ALT, AST, GGT, liver coefficient, Col1, FN, TGF-β, and α-SMA mRNA in the H8 low and high-dose groups were significantly lower than those in the model group, and the differences were statistically significant (P < 0.05). Conclusion Curcumin and its analogue H8 have therapeutic and protective effects on CCl4 induced liver fibrosis in rats, and H8 has better effects than curcumin.
[Key words] Curcumin and its analogues; Carbon tetrachloride; Liver fibrosis; Liver coefficient; Pathomorphology
姜黃素(Curcumin)具有抗炎、抗癌、抗肝纤维化等药理作用[1-3],抗癌作用已二期临床[4-5]。但姜黄素溶解度差,血药浓度低,限制了应用[6]。课题组以姜黄素为母体进行结构修饰,合成稳定的姜黄素类似物[7-8]。本文探讨H8对CCl4诱导大鼠肝纤维化的作用及机制。
1 材料与方法
1.1 材料、仪器与试剂
实验动物及饲养SD大鼠[辽宁长生生物,SCXK(辽)2015-0001,合格号:211002300036102],雄性,体重300~400 g。于18~24℃、湿度50%~70%、12 h交替照明环境下,自由饮食。本研究经牡丹江医学院动物伦理委员会批准。
主要试剂与仪器H8(牡丹江医学院设计);RNA逆转录试剂盒(美国Invitrogen,货号:11904018);伊红、苏木精(北京索莱宝科技);Col1(GR3265108-1)、FN(GR3274504-1)、TGF-β(GR 3203762-3)、α-SMA(GR 3263275-5)抗体均购于美国Abcam;激光共聚焦显微镜(FV1000日本Olympus);凝胶成像分析系统(FluorChem M美国);荧光定量PCR仪(StepOne美国ABI)。
1.2 方法
SD大鼠40只,按体重随机分为模型组、对照组、姜黄素组(姜黄素5 mg/kg),H8低、高剂量组(H8 5、10 mg/kg)。对照组腹腔注射1.2 mL/kg橄榄油,2次/周×8周;其余4组按2 mL/kg的40%CCl4橄榄油腹腔注射,2次/周×8周。治疗组用1%CMC溶解姜黄素(5 mg/kg),H8(5、10 mg/kg),1次/d×28 d,模型组和对照组给予空白溶剂灌胃。
1.3 标本采集测定
12周后,禁食24 h,乙醚麻醉后处死大鼠。心脏取血,37℃孵育30 min,3000 × g离心15 min,生化分析仪测定谷丙转氨酶(ALT)、谷草转氨酶(AST)、血清中谷氨酰转肽酶(GGT)。肝脏重量除于体重得肝脏系数。部分肝组织用4%甲醛固定,其他放入-80℃冰箱中。
1.4 病理学检测
HE染色观察病理变化,Masson染色观察纤维化变化。HE染色:取甲醛固定的肝组织,石蜡包埋,切片5 μm,烘干、二甲苯,梯度酒精脱水,伊红-苏木精染色,在显微镜下观察大鼠肝组织病理变化。Masson染色:同上切片,Masson试剂盒染色,在显微镜下观察肝组织纤维化。
1.5 肝组织中纤维化相关基因mRNA表达检测
RNA提取试剂盒提取肝组织总RNA,逆转录成cDNA。取2 μL cDNA,9 μL SYBR Green Mix,分别加Col1、纤连蛋白(FN)、转化生长因子-β(TGF-β)、α-平滑肌肌动蛋白(α-SMA)、内参RPS16上下游引物各0.8 μL,ddH2O 7.4 μL。QPCR反应条件:95℃预变性5 min;94℃变性30 s,55~60℃退火30 s,40个循环,重复2个复孔,测Ct值,标准曲线法计算基因相对表达。引物:Col1正义3′-ACAGGCGAACAAGGTGACAGAG-5′,反义3′-GCCAGGAGAACCAGCAGA-GC-5′;TGF-β正义3′-CAACAATTCCTGGCGTTACCTTG-5′,反义3′-CGAAAGCCCTGTATTCCGTCTCC-5′;α-SMA正义3′-CACCATCGGGAATGAACGCTTC-5′,反义3′-CTGTCAGCAATGCCTGGGTA-5′;FN正义3′-GCCTGAACCAGCCTACGGAT-5′,反义ATGACC-ACTGCCAAAGCCCAAG-5′;RPS16正义3′-CGTG-CTTGTGCTCGGAGCTA-5′,反义3′-GCTCCTTGCCC-AGAAGCAAA-5′。
1.6 统计学方法
采用Graph Pad Prism 5.0对所得数据进行统计分析。计量资料以均值±标准差(x±s)表示,多组间采用One-way AVONA单因素方差分析,进一步两两比较采用LSD-t检查。以P < 0.05为差异有统计学意义。
2 结果
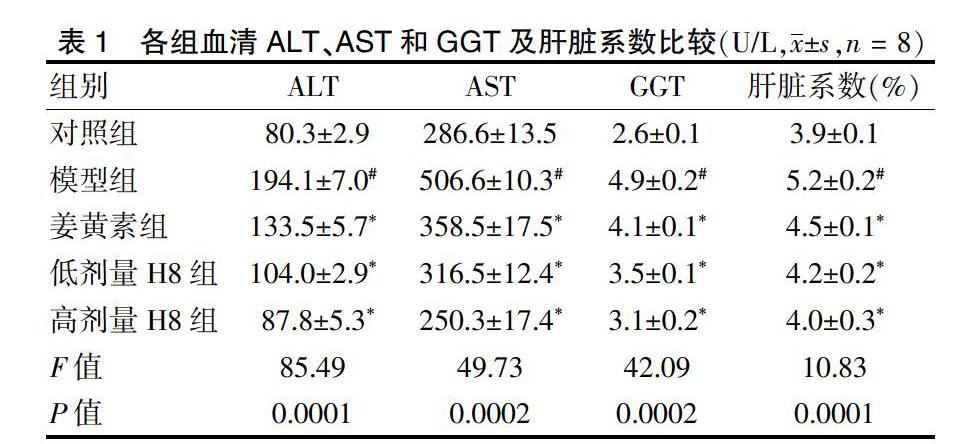
2.1 各组血清ALT、AST和GGT及肝脏系数比较
模型组血清ALT、AST和GGT显著高于对照组;姜黄素组,H8低、高剂量组血清ALT、AST和GGT水平显著低于模型组,差异有统计学意义(P < 0.05或P < 0.01)。模型组肝脏系数显著高于对照组;姜黄素组,H8低、高剂量组肝脏系数显著低于模型组,差异有统计学意义(P < 0.05或P < 0.01)。见表1。
2.2 肝臟组织病理学检测结果
与对照组比较,模型组肝索结构紊乱,肝小叶排列不规则,肝窦高度扩增,间质内有炎性浸润。与模型组比较,姜黄素组,H8低、高剂量组肝索排列规则,肝小叶结构较清晰,肝窦变窄,鲜见肝细胞变性和炎性浸润。HE染色见图1。
与对照组比较,模型组肝组织出现广泛纤维化。与模型组比较,姜黄素组大鼠纤维化减轻,H8低、高剂量组纤维化降低更明显。Masson染色见图2。
2.3 H8对大鼠肝纤维化基因的影响
模型组Col1、TGF-β、α-SMA、FN表达显著高于对照组,差异有高度统计学意义(P < 0.01);姜黄素组,H8低、高剂量Col1、TGF-β、α-SMA、FN表达显著低于模型组,差异有统计学意义(P < 0.05或P < 0.01)。见图3。
3 讨论
CCl4是经典肝纤维化模型的诱导剂,被机体吸收后被细胞色素氧化酶P450激活为CCl3和Cl自由基[9],与生物大分子结合,肝细胞坏死和胶原沉积,导致肝纤维化[10],可作为肝纤维化模型[11]。文献报道,肝纤维化动物血清中肝脏系数、ALT、AST、GGT增加[12-13],并且Col1、TGF-β、α-SMA、FN表达增多[14-15]。本实验在CCl4造模后,指标与相关文献一致。经过治疗这些指标均降低,提示姜黄素及H8能改善CCl4所致大鼠肝脏纤维化,其中H8效果更明显,具有应用前景[8]。
肝纖维化是复杂的病理生理过程,其特征为细胞外基质(ECM)增多,肝脏结构破坏,肝功能下降[16]。正常肝星状细胞(HSCs)维持静止表型[17],随着HSC活化和增殖,增加ECM相关蛋白α-SMA、Col1[18]。TGF-β/Smads信号通路激活,加快ECM生成与沉积[19-20]。本实验模型组Col1、TGF-β、α-SMA、FN增高,经治疗后又被抑制,结果与相关文献一致[15]。
综上,姜黄素及其类似物H8可抑制CCl4诱导的大鼠肝纤维化,机制与减少ECM相关蛋白有关,为开发保护肝脏纤维化的药物奠定基础。
[参考文献]
[1] Epstein J,Sanderson IR,Macdonald TT. Curcumin as a therapeutic agent:the evidence from in vitro,animal and human studies [J]. Br J Nutr,2010,103(11):1545-1557.
[2] Joseph AI,Edwards RL,Luis PB,et al. Stability and anti-inflammatory activity of the reduction-resistant curcumin analog,2,6-dimethyl-curcumin [J]. Org Biomol Chem,2018,16(17):3273-3281.
[3] Rajabi M,Farhadian S,Shareghi B,et al. Noncovalent interactions of bovine trypsin with curcumin and effect on stability,structure,and function [J]. Colloids Surf B Biointerfaces,2019,183:110287.
[4] Rezzani R,Franco C. Curcumin as a Therapeutic Strategy in Liver Diseases [J]. Nutrients,2019,11(10):pii:E2498.
[5] Mody D,Athamneh AIM,Seleem MN. Curcumin:A natural derivative with antibacterial activity against Clostridium difficile [J]. J Glob Antimicrob Resist,2019,pii:S2213-7165(19):30259-0.
[6] Aggarwal BB,Gupta SC,Sung B. Curcumin:an orally bioavailable blocker of TNF and otherpro-inflammatory biomarkers [J]. Br J Pharmacol,2013,169(8):1672-1692.
[7] Yuan X,Li H,Bai H,et al. The 11β-hydroxysteroid dehydrogenase type 1 inhibitor protects against the insulin resistance and hepatic steatosis in db/dbmice [J]. Eur J Pharmacol,2016,788:140-151.
[8] Yuan X,Li H,Bai H,et al. Synthesis of novel curcumin analogues for inhibition of 11β-Hydroxysteroid dehydrogenase type 1 with anti-diabetic properties [J]. Eur J Med Chem,2014,77:223-230.
[9] Gowri Shankar NL,Manavalan R,Venkappayya D,et al. Hepatoprotective and antioxidant effects of Commiphoraberryi(Arn)Engl bark extract against CCl4-induced oxidative damage in rats [J]. Food Chem Toxicol,2008,46(9):3182-3185.
[10] Weber LW,Boll M,Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes:carbon tetrachloride as a toxicological model [J]. Crit Rev Toxicol,2003,33(2):105-136.
[11] Jiang Y,Wang C,Li YY,et al. Mistletoe alkaloid fractions alleviates carbon tetrachloride-induced liver fibrosis through inhibition of hepatic stellate cell activation via TGF-β/Smadinterference [J]. J Ethnopharmacol,2014,158:230-238.
[12] Fan J,Chen CJ,Wang YC,et al. Hemodynamic changes in hepatic sinusoids of hepatic steatosis mice [J]. World J Gastroenterol,2019,25(11):1355-1365.
[13] Ge MX,Liu HT,Zhang N,et al. Costunolide represses hepatic fibrosis through WWP2-mediated Notch3 degradation [J]. Br J Pharmacol,2019,177(6):1453.
[14] Li L,Li H,Zhang Z,et al. Recombinant truncated TGF-β receptor Ⅱ attenuates carbon tetrachloride-induced epithelial-mesenchymal transition and liver fibrosis in rats [J]. Mol Med Rep,2018,17(1):315-321.
[15] Han F,Shu J,Wang S,et al. Metformin Inhibits the Expression of Biomarkers of Fibrosis of EPCs In Vitro [J]. Stem Cells Int,2019,2019:9019648.
[16] Gertz HJ,Kiefer M. Review about Ginkgo biloba special extract EGb 761(Ginkgo)[J]. Curr Pharm Des,2004,10(3):261-264.
[17] Ezhilarasan D,Sokal E,Najimi M. Hepatic fibrosis:It is time to go with hepatic stellate cell-specific therapeutic targets [J]. Hepatobiliary Pancreat Dis Int,2018,17(3):192-197.
[18] Hellerbrand C. Hepatic stellate cells—the pericytes in the liver [J]. Pflugers Arch,2013,465(6):775-778.
[19] Xu F,Liu C,Zhou D,et al. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis [J]. J Histochem Cytochem,2016,64(3):157-167.
[20] Hernández-Aquino E,Muriel P. Beneficial effects of naringenin in liver diseases:Molecular mechanisms [J]. World J Gastroenterol,2018,24(16):1679-1707.
(收稿日期:2019-08-28 本文編辑:王晓晔)
