接种物耐酸驯化对菌糠厌氧干发酵产气的影响
2020-04-24朱继英
朱继英,钟 慧,陆 玉,张 岐
接种物耐酸驯化对菌糠厌氧干发酵产气的影响
朱继英,钟 慧,陆 玉,张 岐
(山东理工大学农业工程与食品科学学院,淄博 255000)
为了提高厌氧干发酵体系运行稳定性,使中间产物酸及时转化为甲烷,避免出现酸抑制现象,该研究采用逐步提高乙酸浓度降低pH值的方式对接种物进行驯化,得到了在乙酸浓度10 200 mg/kg、pH值6.0条件下仍能快速产气的耐酸接种物,并以易水解酸化的菌糠为原料进行了厌氧干发酵试验。结果表明,驯化过程中产甲烷古菌类群的多样性下降,乙酸营养型甲烷八叠球菌丰度大幅提高,乙酸转化率和甲烷浓度逐渐提高;耐酸接种物的脱氢酶活性下降,辅酶420和纤维素酶活性升高;添加耐酸接种物可以加快菌糠厌氧干发酵启动速度,避免酸抑制现象的发生;接种物含有75%耐酸接种物的试验组甲烷产量提高了56.1%。该研究成果能够为有效解决厌氧干发酵过程酸抑制现象提供一定的理论指导。
甲烷;发酵;生物质
0 引 言
厌氧干发酵是指利用有机固体废弃物在干物质浓度高达20%~40%情况下生产沼气的发酵技术。与传统湿发酵相比,该技术具有原料适应性广泛,需水量低,耗能少,发酵残余物易处理等优点[1-4]。然而,由于干发酵有机质浓度高、含水量低,体系内能质传递过程受阻,易造成发酵不均匀、甲烷转化率低,中间产物挥发性脂肪酸(volatile fatty acids,VFAs)在局部积累的现象[5]。当VFAs浓度超过抑制阈时会影响产甲烷菌的活性,使发酵过程失衡,产气量降低,甚至因“过度酸化”而失败。乙酸是厌氧发酵过程中最重要的中间产物,约70%甲烷产自于乙酸[6],其浓度直接影响体系中产甲烷菌的活性和甲烷的产量。有研究表明,在两相厌氧发酵工艺中,酸化相的乙酸抑制阈明显高于产甲烷相[7],说明长期的高浓度VFA处理会得到具有良好酸耐受性的产甲烷菌群。Xu等[8]以餐厨垃圾为底物的厌氧发酵结果表明,乙酸是甲烷化阶段主要的抑制剂,高有机负荷下厌氧体系在发酵初始阶段的乙酸浓度也高,抑制了乙酸营养型产甲烷途径,但是某种未培养的甲烷鬃毛菌可以利用乙酸产生甲烷,并且在高乙酸浓度下生长良好。
菌糠为蘑菇采收后的废弃物,具有较丰富的营养物质[9]。蘑菇生长过程中,培养料中的有机质发生了巨大的变化,粗纤维和木质素被大量降解,粗蛋白和粗脂肪含量升高,可降解性增强,是沼气生产的良好原料[10]。但发酵料快速降解产生的VFAs如果不能被及时地转化为甲烷就容易在厌氧发酵体系中积累而导致酸抑制,因此菌糠较高的降解性对于厌氧干发酵的稳定性可能有消极的影响[11-12]。
为了使厌氧干发酵体系中产生的VFAs及时地转化为甲烷,提高发酵运行稳定性,本文通过耐酸驯化,改善接种物中的微生物群落结构和丰度,并以菌糠为原料,考察了耐酸接种物对厌氧干发酵产气特性的影响。
1 材料与方法
1.1 试验原料
接种物取自淄博市淄川区杨寨镇秸秆沼气站(主要发酵原料为玉米秸秆和牲畜粪便),为37 ℃下运行良好的液态沼气发酵后的残余物。菌糠取自淄博市淄川区杨寨镇的平菇养殖户,经过烘干使得水分含量低于10%,试验前研磨过5 mm筛。接种物和菌糠的特性如表1所示。

表1 接种物和菌糠特性
1.2 试验方法
1.2.1 接种物驯化方法
向25 L红泥塑料发酵袋中加入6 L的原始接种物(pH值为7.7),通过控制乙酸添加量使接种物pH值按梯度降低到6.0,整个驯化过程基于pH值分为4个阶段:7.2,6.7,6.2和6.0。驯化过程中,每天测定接种物实际pH值、产气量、甲烷含量,然后用乙酸调整至目标pH值,并记录乙酸溶液添加量。每个阶段的驯化直至在该pH值条件下接种物能快速稳定产气为止。4个驯化阶段分别于第12、22、32和40天结束,每个阶段结束时取样测定脱氢酶、纤维素酶和辅酶420的活性。整个驯化过程持续40 d,在37 ℃的恒温培养箱中进行。驯化最终阶段接种物产气量稳定,并且甲烷含量在50%以上,接种物中剩余的乙酸通过在培养箱中温育至无沼气产生来消除。
1.2.2 厌氧干发酵方法
厌氧干发酵采用批式发酵方式,菌糠和接种物按照5:1(基于可挥发性固形物)混合,以NH4Cl作为氮源调整发酵料的C/N比为20,以蒸馏水调节发酵料TS为25%。驯化后接种物添加量分别占总接种量的0%、25%、50%、75%,100%。采用手持电动搅拌器将物料(组成见表2)充分混合后装入5 L的广口玻璃反应器中,盖上连有乳胶管的橡胶瓶塞并用凡士林密封瓶口,通过真空泵反复抽真空检查装置气密性。发酵产生的气体通过与橡胶塞连接的4L聚氟乙烯气体采样袋收集。所有的反应器均放置在37 ℃的恒温培养箱中。在发酵期间,每1~2 d测定产气量、甲烷含量。每个处理3个重复。
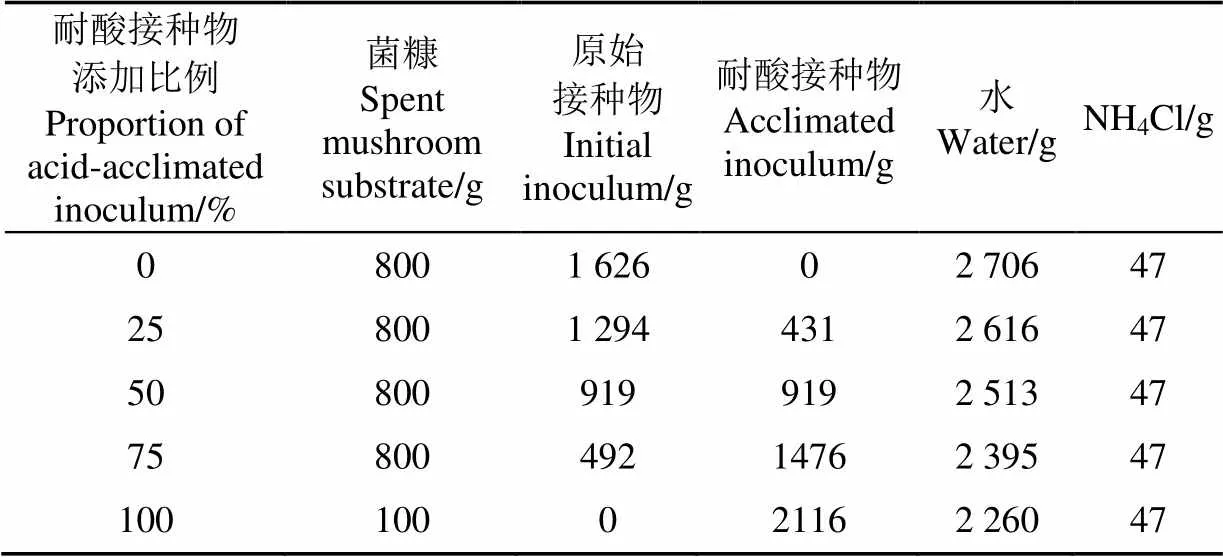
表2 发酵料的组成
1.2.3 厌氧发酵动力学分析
采用修正的Gompertz方程[13]对添加耐酸接种物反应器进行累积产甲烷量的拟合,拟合数据取添加发酵前40 d的累积甲烷产量。
=·exp{−exp[R·e·(−)/P+1]} (1)
式中为时刻对应的累积甲烷产量,L/kg;为发酵时间,d;P为发酵总累积产甲烷量,L;R为最大产甲烷率,L/(d·kg);为发酵滞后时间,d。
1.3 指标测定及分析方法
pH值采用雷磁pHS-3C型pH计(上海精科科学仪器有限公司雷磁仪器厂)测定;TS采用105 ℃烘24 h,差质量法测定;VS采用550 ℃灼烧4 h,差质量法测定;氮含量采用凯氏定氮法测定[14];有机碳含量采用重铬酸钾外源加热法测定;气体体积和甲烷体积分数分别采用湿式气体流量计(LML-1,长春汽车滤清器有限责任公司)和便携式红外沼气分析仪(Biogas Check,英国 Geotech)测定;乙酸转化率为投入乙酸的实际产气量与理论产气量的比值;脱氢酶酶活采用TTC-脱氢酶酶活法测定[15];纤维素酶酶活采用总酶活力测定法[15];辅酶F420酶活采用分光光度法[16]。古菌群落结构由生工生物工程(上海)股份有限公司通过Illumina MiSeq高通量测序平台进行分析。
采用SPSS18.0软件包进行方差统计和相关性分析,方差分析选择 Tukey-HSD 方法。
2 结果与分析
2.1 接种物驯化过程分析
微生物对环境胁迫有规律、逐步地适应可以提高其应对干扰的能力[17-19]。Xiao等[19]发现在7 400 mg/L高浓度乙酸冲击下,厌氧体系对乙酸降解存在短时延滞;而经过逐步提高乙酸浓度的厌氧体系并没有出现乙酸降解滞后,并且可以在8 200 mg/L乙酸浓度下产气良好。Jain等[20]也证明逐步降低厌氧体系的pH值可以驯化得到耐酸的产甲烷菌。杨莉丽等[21]研究发现通过8 g/L丁酸盐逐步驯化得到的接种物应用到餐厨垃圾的厌氧发酵中,可以获得良好的产气效果,见图1。

图1 接种物驯化过程
本研究通过逐步提高乙酸浓度降低pH值的策略对接种物持续驯化40 d,最终得到了能够在10 200 mg/L乙酸浓度和pH值为6.0条件下稳定产气的耐酸接种物。驯化过程中每天用6 mol/L的乙酸调节pH值1次至目标设定pH值。驯化期间由于添加的乙酸很快被转化成甲烷,导致pH值恢复,每天产气后的pH值差异不大,均在7.5左右(图1a)。驯化第1阶段的设定pH值为7.2,乙酸的每日添加量大约为1.0 g/L,共维持了12 d。前7 d的甲烷浓度保持在40%以下的低水平,上升较慢,第8天开始迅速升高,到本阶段末的第12天则升高至60.3%(图1c)。相应地,Hac的转化率也在本阶段末迅速升高至73.9%(图1b)。说明经过1周左右的驯化,接种物中的乙酸型产甲烷菌被逐渐富集并适应了pH 值7.2的环境。由于本阶段每日的乙酸添加量较低,甲烷日产量也维持在较低水平(图2)。第13天开始进入到驯化第2阶段,由于pH值突然降低至6.7和Hac浓度的突然增加(4.2 g/L),产甲烷菌的活性受到冲击,甲烷浓度和Hac的转化率分别降低至35.2%和62.7%,同时由于Hac积累产气后pH值也明显降低(图1a)。但随着产甲烷菌对驯化环境的快速适应,甲烷浓度和Hac转化率也迅速升高。驯化第3、4阶段调节的目标pH值已经超出产甲烷菌的最适生长范围,但除了每阶段的第1天外,甲烷浓度和Hac转化率均维持在较高水平,说明乙酸型产甲烷菌逐步适应了高酸性环境并在体系内得到了富集。Dogan等[22]以处理酒精厂废水的颗粒污泥为接种物,进行乙酸盐厌氧发酵产甲烷活性试验时得出乙酸盐浓度在4 000 mg/L时开始出现产甲烷抑制现象。而本试验研究范围内(1 000~11 000 mg/kg)并没有出现明显的酸抑制现象,可能是因为本研究是通过逐步提高系统中的乙酸浓度对接种物进行驯化,培养过程中乙酸营养型的甲烷八叠球菌丰度大幅提高(表3),系统中的乙酸能够被迅速转化。
脱氢酶作为物质氧化还原反应过程的催化剂,其活性可以反映出微生物对基质的降解能力[23]。由图2a所示,原始接种物(pH值7.7)的脱氢酶活性为137g/(h·g),目标pH值为7.2时脱氢酶活性增加到276g/(h·g),较初始提高了101.5%,这是由于此时pH值处于厌氧发酵各类微生物适宜pH值范围,在此驯化阶段各类微生物代谢活性强,并且接种物由室温(20 ℃)转移至37 ℃的恒温环境内,同时添加乙酸作为底物也诱导了乙酸降解微生物酶活的提高,因此整体脱氢酶活性较高。在随后的驯化阶段乙酸浓度抑制部分微生物的活性,使得脱氢酶酶活下降,最终驯化结束时脱氢酶酶活为50.7g/(h·g),较初始酶活降低了63%,这也是微生物多样性降低的结果(表3)。脱氢酶酶活在驯化周期内先上升后下降的趋势与杨莉丽[21]在测定不同浓度丁酸盐驯化方式下接种物脱氢酶活性的变化规律相似。
辅酶420广泛存在于产甲烷菌中,涉及甲烷形成过程的电子传递[24]。许多研究表明辅酶420可以定性反映污泥产甲烷能力[25]。原始接种物(pH值7.7)辅酶420的活性仅为0.49mol/L(图2b)。pH值7.2处于产甲烷菌最适pH值范围内,乙酸的添加诱导大量产甲烷生长代谢活跃,此时辅酶F420活性高为59.8mol/L。pH值6.7为产甲烷菌适宜pH值的下限,此阶段乙酸浓度提高,部分产甲烷菌的代谢受到了抑制,辅酶420活性下降至7.88,在之后的驯化阶段,乙酸浓度进一步提高,体系内乙酸型产甲烷菌富集并逐渐适应高酸低pH值的环境,最终420酶活性为16.9mol/L,为初始酶活的33倍。
原始接种物来源于秸秆沼气厂,因此纤维素酶初始活性较高,为371.7g/g(图2c)。由于驯化初期阶段(目标pH值为7.2)逐渐形成了厌氧环境,部分好氧纤维素降解菌失活,使得此时酶活降低了64.1%。随着驯化的进行,接种物中残留的纤维素类物质被降解,由于常见的纤维素降解菌产生的纤维素酶一般在酸性条件下有较高活性(pH值为5.5~6.0),因此在pH值为6.2时纤维素酶活性较高为669.7g/g,驯化最后阶段乙酸浓度的提高也抑制了纤维素酶的分泌[26],因此酶活略有降低。
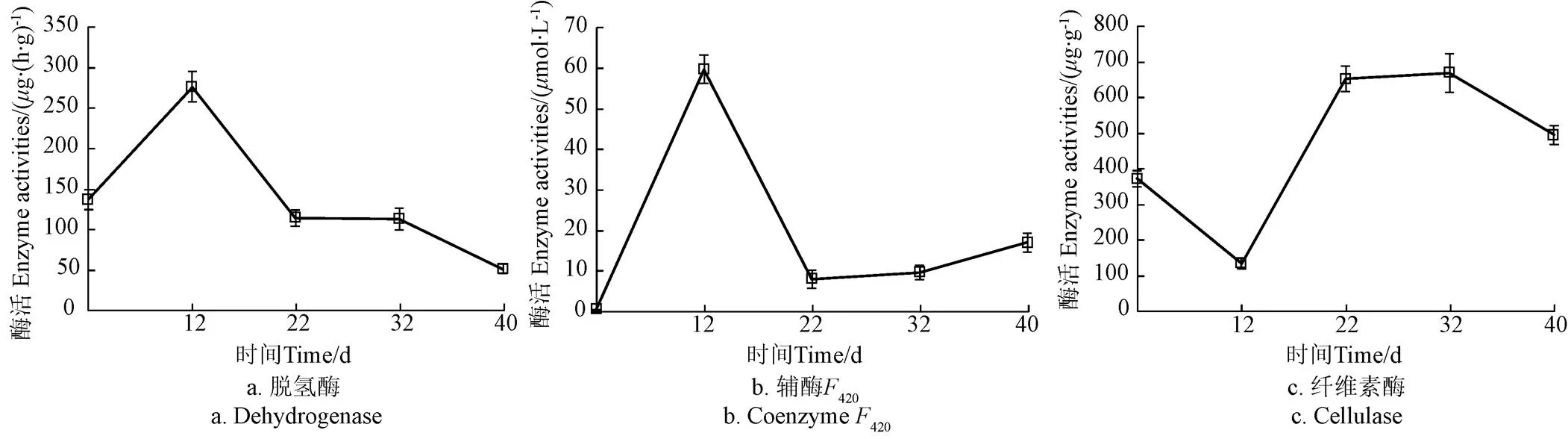
图2 驯化各阶段酶活变化
接种物驯化前后的主要古菌群落结构发生了明显变化,不同古菌类群的丰度分布如表3所示。原始接种物中的古菌以氢营养型为主,乙酸营养型的甲烷八叠球菌的丰度仅占4.20%。驯化后乙酸营养型的甲烷八叠球菌丰度则大幅提高至58.10%,而氢营养型的甲烷螺菌、甲烷短杆菌等的丰度则有不同程度的下降。驯化后未分类古菌的丰度由34.47%降低至6.76%,说明古菌类群的多样性有所降低。
2.2 添加耐酸接种物对厌氧干发酵产气的影响
添加不同比例耐酸接种物的反应器的产气情况如图3所示。与没有添加耐酸接种物的对照组添加质量分数0相比,添加耐酸接种物的反应器的甲烷浓度和日产气量在发酵初期均上升较快(图3a,3b)。对照组的第1个日产气量高峰延迟了2 d且持续时间较短,尽管随后有小范围上升但相较于添加耐酸接种物反应器明显偏低。添加不同比例耐酸接种物的反应器的产气趋势基本一致,有2个明显的产气高峰,高效产气时间持续较长且2个峰间隔较短。以上结果说明对照组出现了明显的酸抑制,而添加耐酸接种物能够显著改善酸抑制现象。

表3 驯化前后接种物中主要古菌类群相对丰度
甲烷的含量不仅会影响沼气的适用性和热值而且影响后续沼气净化和设备维护成本,因此监测沼气产量和甲烷浓度具有重要意义[27]。由图3b可以看出,除对照处理外,其他处理在发酵整个过程中甲烷含量均不超过50%,略低于以玉米秸秆为主要原料的厌氧发酵情况[28-29],这可能与原料特性以及本研究采用的接种量较低有关。邓媛方等以平菇菌糠为原料进行液态厌氧发酵时,在初始阶段甲烷浓度较高,发酵中后期大幅降低,维持在40%~50%之间[30]。由于菌糠中的有机物较易降解,厌氧干发酵过程中极易发生酸抑制现象。Lin等[31]的研究表明,以菌糠为唯一原料进行厌氧干发酵时,在菌糠与接种物可挥发性固形物之比/=3和/=4的条件下均发生了严重的酸抑制,导致了发酵过程的失败;即使提高接种量至/=2的情况下其甲烷含量仍然低于45%。本研究采用的接种量较低(/=5),虽然通过耐酸驯化提高了接种物中耐酸产甲烷菌的丰度,改善了接种物中的微生物群落结构,缓解了酸抑制程度,但可能仍然存在一定的酸抑制现象。
不同耐酸接种物添加量反应器的累积产气量和甲烷产量均有显著差异(<0.05)。随着耐酸接种物添加量的增加呈先增加后下降,添加75%耐酸接种物的反应器的累积产气量和甲烷产量均最高,比对照组分别提高42.8%和56.1%(图3c)。这可能是因为发酵体系中水解、酸化、甲烷化各类菌群代谢协调一致是高效产气的关键因素,而乙酸驯化过程较为单一的碳源使接种物的微生物群落结构发生了明显改变,降低了其发酵性能。同时,只接种耐酸接种物(pH值6.0)导致体系的初始pH值偏低且缓冲能力较弱,也影响到后续的发酵产气。

图3 添加耐酸接种物对产气的影响
修正的Gompertz方程可以较好的反映复杂有机物的厌氧降解过程,预测发酵趋势,得出评价厌氧发酵性能的关键参数延滞时间()和产气速率(R)[32]。对添加耐酸接种物反应器40 d的累计甲烷产量进行拟合的结果如图4所示。从表3可以看出,所有处理的方程拟合相关系数2均大于0.99,说明该方程能够较好地反映添加耐酸接种物厌氧干发酵的产甲烷过程。添加50%耐酸接种物的反应器有最短的延滞时间为4.28 d。添加75%耐酸接种物的反应器获得最高的产气速率为3.49 L/(d·kg),这与其最终较高的甲烷产量相符合。

图4 甲烷累积产量的修正Gompertz 方程拟合曲线

表3 修正的Gompertz 方程参数
注:为时刻对应的累积甲烷产量,L·kg-1;为发酵时间,d;P为发酵总累积产甲烷量,L;R为最大产甲烷率,L·(d·kg)-1;为发酵滞后时间,d。
Note:is timecumulative methane production, L·kg-1;is fermentation time, d;Pis total cumulative methane production by fermentation, L;Ris the maximum methane production rate, L·(d·kg)-1;is fermentation lag time, d.
3 结 论
本文通过对接种物进行耐酸驯化改善其菌群结构,并以易水解酸化的菌糠为原料进行了厌氧干发酵试验,探讨了耐酸驯化接种物添加量对厌氧干发酵产气特性的影响,得出如下主要结论:
1)乙酸驯化使接种物中产甲烷古菌类群的多样性下降,乙酸营养型甲烷八叠球菌丰度由4.20%提高至58.10%。
2)适量添加耐酸接种物,提高发酵体系中乙酸型产甲烷菌的初始丰度,可以加快菌糠厌氧干发酵过程的启动速度,避免酸抑制现象的发生,延长产气周期,提高甲烷产量。
3)过量添加耐酸接种物会降低发酵体系缓冲能力,导致酸抑制现象和产气量降低。
[1] Shi J, Xu F, Wang Z, et al. Effects of microbial and non-microbial factors of liquid anaerobic digestion effluent as inoculum on solid-state anaerobic digestion of corn stover[J]. Bioresource Technology, 2014, 157: 188-196.
[2] Andréa L, André Pauss, Ribeiro T. Solid anaerobic digestion: State-of-art, scientific and technological hurdles[J]. Bioresource Technology, 2018, 247: 1027-1037.
[3] Wang Z, Xu F, Karthik R, et al. Fractal-like kinetics of the solid-state anaerobic digestion[J]. Waste Management, 2016, 53: 55-61.
[4] Matheri A N, Sethunya V L, Belaid M, et al. Analysis of the biogas productivity from dry anaerobic digestion of organic fraction of municipal solid waste[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 2328-2334.
[5] Abbassi-Guendouz A, Brockmann D, Trably E, et al. Total solids content drives high solid anaerobic digestion via mass transfer limitation[J]. Bioresource Technology, 2012, 111(3): 55-61.
[6] Rincón B, Sánchez E, Raposo F, et al. Effect of the organic loading rate on the performance of anaerobic acidogenic fermentation of two-phase olive mill solid residue[J]. Waste Management, 2008, 28(5): 870-877.
[7] Xiao K K, Guo C H, Zhou Y, et al. Acetic acid inhibition on methanogens in a two-phase anaerobic process[J]. Biochemical Engineering Journal, 2013, 75(24): 1-7.
[8] Xu Z Y, Zhao M X, Miao H F, et al. In situ volatile fatty acids influence biogas generation from kitchen wastes by anaerobic digestion[J]. Bioresource Technology, 2014, 163: 186-192.
[9] 兰良程. 中国食用菌产业现状与发展[J]. 中国农学通报,2009,25(5):205-208.
Lan Liangcheng. The status quo and development of China’s edible mushroom industry[J]. Chinese Agricultural Science Bulletin, 2009, 25(5): 205-208. (in Chinese with English abstract)
[10] Phan C W, Sabaratnam V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes[J]. Appl Microbiol Biotechnol, 2012, 96(4): 863-873.
[11] Li Y B, Park S Y, Zhu J Y. Solid-state anaerobic digestion for methane production from organic waste[J]. Renewable and Sustainable Energy Reviews, 2011, 15(1): 821-826.
[12] Zhu J Y, Han M L, Zhang G K, et al. Co-digestion of spent mushroom substrate and corn stover for methane production via solid-state anaerobic digestion[J]. Journal of Renewable and Sustainable Energy, 2015, 7(2): 559-565.
[13] Zhai N, Tong Z, Yin D, et al. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure[J]. Waste Management, 2015, 38(2): 126-131.
[14] 刘福源. 沼气发酵常规分析[M]. 成都:中国科学院成都生物研究所,1984.
[15] 刘士清,张无敌,尹芳,等. 沼气发酵实验教程[M]. 北京:化学工业出版社,2013.
[16] 吴唯民,蒋青. 辅酶F(420)及其在厌氧处理中的作用[J]. 中国沼气,1984(2):3-11.
Wu Weimin, Jiang Qing. Properties of Coenzyme F420, its application in anaerobic digestion and its measurement[J]. China Biogas, 1984(2): 3-11. (in Chinese with English abstract)
[17] Carballa M, Regueiro L, Lema J M. Microbial management of anaerobic digestion: Exploiting the microbiome- functionality nexus[J]. Current Opinion in Biotechnology, 2015, 33: 103-111.
[18] Lins P, Reitschuler C, Illmer P. Methanosarcina spp., the key to relieve the start-up of a thermophilic anaerobic digestion suffering from high acetic acid loads[J]. Bioresource Technology, 2014, 152(1): 347-354.
[19] Xiao K K, Guo C H, Zhou Y, et al. Acetic acid effects on methanogens in the second stage of a two-stage anaerobic system[J]. Chemosphere, 2016, 144: 1498-1504.
[20] Jain S R, Mattiasson B. Acclimatization of methanogenic consortia for low pH biomethanation process[J]. Biotechnology Letters, 1998, 20(8): 771-775.
[21] 杨莉丽,赵明星,黄月,等. 产甲烷菌耐酸培养及其以餐厨为底物的产气特性[J]. 环境工程学报,2016,10(11):6673-6678.
Yang Lili, Zhao Mingxing, Huang Yue, et al. Acid-tolerant cultivation of methanogen and its biogas generation characteristics from food wastes[J]. Chinese Journal of Environmental Engineering, 2016, 10(11): 6673-6678. (in Chinese with English abstract)
[22] Dogan T, Ince O, Ince B K, et al. Inhibition of volatile fatty acid production in granular sludge from a UASB reactor[J]. Environmental Science and Health, 2005, 40(3): 633-644.
[23] 解军,祁峰,裴海燕,等. 脱氢酶活性检测方法及其在环境监测中的应用[J]. 中国环境监测,2006,22(5):13-18.
Xie Jun, Qi Feng, Pei Haiyan,et al. Determining method of dehydrogenase activity and its application in environmental monitoring[J]. Environmental Monitoring in China, 2006, 22(5): 13-18. (in Chinese with English abstract)
[24] 尹小波,连莉文,徐洁泉,等. 产甲烷过程的独特酶类及生化监测方法[J]. 中国沼气,1998(3):8-13.
Yin Xiaobo, Lian Liwen, Xu Jiequan, et al. Unique enzymes and biochemical monitoring methods in methanogenesis[J]. China Biogas, 1998(3): 8-13. (in Chinese with English abstract)
[25] 赵阳,李秀芬,堵国成,等. 钴的配合物对甲烷发酵和产甲烷过程中关键酶的影响[J]. 食品与生物技术学报,2007,26(5):71-74.
Zhao Yang, Li Xiufen, Du Guocheng, et al. Effect of cobalt and its complexation on methane fermentation and some unique coenzymes in methanogenesis[J]. Journal of Food Science and Biotechnology, 2007, 26(5): 71-74. (in Chinese with English abstract)
[26] 余益辉,黄振兴,高树梅,等. 固相餐厨垃圾厌氧发酵特性[J]. 环境工程学报,2015,9(1):355-361.
Yu Yihui, Huang Zhenxing, Gao Shumei, et al. Characteristics of anaerobic digestion with solid kitchen waste[J]. Chinese Journal of Environmental Engineering, 2015, 9(1): 355-361. (in Chinese with English abstract)
[27] 邓良伟. 沼气工程[M]. 北京:科学出版社,2015.
[28] Zhu J Y, Yang L C, Li Y B. Comparison of premixing methods for solid-state anaerobic digestion of corn stover[J]. Bioresource Technology, 2015, 175: 430-435.
[29] Li Y Y, Li Y, Zhang D F, et al. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production[J]. Bioresource Technology, 2016, 217: 50-55.
[30] 邓媛方,邱凌,孙全平,等. 蘑菇废弃菌棒及其与猪粪混合发酵对沼气产量及质量的影响[J]. 农业环境科学学报,2012,31(3):613-619.
Deng Yuangfang, Qiu Ling, Sun Quanping, et al. Influence of anaerobic co-digestion of mushroom cultivation wastes and pig manure on the biogas production and its quality[J]. Journal of Agro-Environment Science, 2012, 31(3): 613-619. (in Chinese with English abstract)
[31] Lin Y Q, Ge X M, Li Y B. Solid-state anaerobic co-digestion of spent mushroom substrate with yard trimmings and wheat straw for biogas production[J]. Bioresource Technology, 2014, 169: 468-474.
[32] Li L H, Kong X Y, Yang F Y, et al. Biogas production potential and kinetics of microwave and conventional thermal pretreatment of grass[J]. Applied Biochemistry and Biotechnology, 2012, 166(5): 1183-1191.
Effects of acid-acclimated inoculum on solid-state anaerobic digestion of spent mushroom substrate
Zhu Jiying, Zhong Hui, Lu Yu, Zhang Qi
(,,255000,)
Solid-state anaerobic digestion (SS-AD) generally operates at solid content between 20%-40%. Due to the low water content, mass transfer in the digestion material is slow and the intermediate acids are apt to accumulate locally. Excessive acid accumulation might inhibit the activity of methanogens, reduce bigas yield, and even result in failure of the SS-AD process. Acetic acid is the most important intermediate in anaerobic digestion, and its concentration directly affects the activity of methanogens. Spent mushroom substrate (SMS) is a feasible feedstock for anaerobic digestion, because it has high organic content and the fiber and lignin have been greatly degraded during mushroom growth. However, using spent mushroom substrate as feedstock for SS-AD is easy to result in acid inhibition due to the rapid hydrolysis and acidification of the organic matters. In order to avoid acid inhibition and improve the stability of SS-AD process, the inoculum was acclimated by gradually increasing the concentration of acetic acid in this study. The acclimation process was divided into four stages at the pH value of 7.2, 6.7, 6.2 and 6.0, respectively. During acclimation stage, the pH value of the inoculum was adapt to the set value with 6 mol/L acetic acid solution and the biogas production, methane content, additive ammout of acetic acid and conversion of actic acid were measured every day. The activities of dehydrogenase, CMCase and Co420were measured at the last day of each acclimation stage. The acclimation lasted for 40 days and a acid-acclimated inoculum that can prouduce biogas quickly under the condition of acetic acid concentration of 10 200 mg/kg and pH value 6.0 was obtained. Compared to the original inoculum, the diversity of methanogens in the acid-acclimated inoculum decreased and the abundance of acetic acid diauxotrophicwas obviously enriched. The activity of dehydrogenase increased 101.5% in the first acclimation stage (pH value 7.2) and then kept decreasing during the next 3 stages. The activity of dehydrogenase reflects the metabolic capacity of all the microorganisms in the digestion system. With the decrease of pH value during the acclimation, the activity of some microorganisms was inhibited. Coenzyme420is present in various methanogens. It often be used to reflect the methane production activity of sludge. The activty of coenzyme420also reached the highest in the first acclimation stage because the pH is optimum for most methanogenes. Although the abundance of the acetic acid diauxotrophicwas significantly enriched in the final acid-acclimated inoculum, the diversity of the archaea communities was reduced due to the low pH value. The activity of cellulase in the acclimation process reached the highest in the third stage (pH value 6.7). To investigate the effects of acid-acclimated inoculum, SS-AD experiments of SMS were carried out by adding different proportion of acid-acclimated inoculum. The results showed that adding acid-acclimated inoculum could avoid acid inhibition and accelerate the start-up of SS-AD. The methane yield increased 56.1% when acid-tolerant inoculum accounted to 75%. The results can provide some theoretical guidance for effectively solving acid inhibition in SS-AD process.
methane; fermentation; biomass
2019-10-30
2019-12-14
山东省重点研发计划(公益类)项目(2018GGX104014);山东省自然科学基金(ZR2015EM020)
朱继英,博士,教授,主要从事有机废弃物厌氧消化工艺及理论研究。Email:zhuying7711@sdut.edu.cn
10.11975/j.issn.1002-6819.2020.05.029
S216.4; X712
A
1002-6819(2020)-05-0249-06
朱继英,钟 慧,陆 玉,张 岐. 接种物耐酸驯化对菌糠厌氧干发酵产气的影响[J]. 农业工程学报,2020,36(5):249-254. doi:10.11975/j.issn.1002-6819.2020.05.029 http://www.tcsae.org
Zhu Jiying, Zhong Hui, Lu Yu, Zhang Qi. Effects of acid-acclimated inoculum on solid-state anaerobic digestion of spent mushroom substrate[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(5): 249-254. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2020.05.029 http://www.tcsae.org
