Anastomosing hemangioma arising from the left renal vein: A case report
2020-04-08LiPingZhengWeiAiShenChunHuaWangChunDongHuXuJianChenYiYuShenJingWang
Li-Ping Zheng, Wei-Ai Shen, Chun-Hua Wang, Chun-Dong Hu, Xu-Jian Chen, Yi-Yu Shen, Jing Wang
Li-Ping Zheng, Chun-Dong Hu, Xu-Jian Chen, Yi-Yu Shen, Jing Wang, Department of General Surgery, The Second Affiliated Hospital of Jiaxing University, Jiaxing 314000, Zhejiang Province, China
Wei-Ai Shen, University of Ningbo, Ningbo 315000, Zhejiang Province, China
Chun-Hua Wang, Department of Pathology, The Second Affiliated Hospital of Jiaxing University, Jiaxing 314000, Zhejiang Province, China
Abstract BACKGROUND Anastomosing hemangioma (AH) is a rare subtype of benign hemangioma that is most commonly found in the genitourinary tract. Due to the lack of specific clinical and radiologic manifestations, it is easily misdiagnosed preoperatively.Here, we report a case of AH arising from the left renal vein that was discovered incidentally and confirmed pathologically, and then describe its imaging characteristics from a radiologic point of view and review its clinicopathologic features and treatment.CASE SUMMARY A 74-year-old woman was admitted to our department for a left retroperitoneal neoplasm measuring 2.6 cm × 2.0 cm. Her laboratory data showed no significant abnormalities. A non-contrast-enhanced computed tomography (CT) scan showed a heterogeneous density in the neoplasm. Non-contrast-enhanced magnetic resonance imaging (MRI) revealed a heterogeneous hypointensity on T1-weighed images and a heterogeneous hyperintensity on T2-weighed images. On contrastenhanced CT and MRI scans, the neoplasm presented marked septal enhancement in the arterial phase and persistent enhancement in the portal phase, and its boundary with the left renal vein was ill-defined. Based on these clinical and radiological manifestations, the neoplasm was initially considered to be a neurogenic neoplasm in the left retroperitoneum. Finally, the neoplasm was completely resected and pathologically diagnosed as AH.CONCLUSION AH is an uncommon benign hemangioma. Preoperative misdiagnoses are common not only because of a lack of specific clinical and radiologic manifestations but also because clinicians lack vigilance and diagnostic experience in identifying AH. AH is not exclusive to the urogenital parenchyma. We report the first case of this neoplasm in the left renal vein. Recognition of this entity in the left renal vein can be helpful in its diagnosis and distinction from other neoplasms.
Key Words: Anastomosing hemangioma; Angiosarcoma; Computed tomography; Magnetic resonance imaging; Case report; Pathology
INTRODUCTION
Hemangiomas are most common in the skin or subcutaneous soft tissue, while few occur in the liver, kidney, and other parenchymal organs. Morphologically, most are classic capillary hemangiomas or venous hemangiomas. In 2009, Montgomery and Epstein[1]reported a unique type of capillary hemangioma occurring in the kidney and testis. Its morphology consists of an ethmoid, sinusoid, anastomosing vascular pattern lined with hobnailed endothelial cells, which is similar to the red pulp of the spleen,and the mass was first named “anastomosing hemangioma” (AH). Subsequently, a few cases of AH were reported in the liver, gastrointestinal tract, retroperitoneum,ovary, bladder, adrenal gland, nasal cavity, and intracranial space[2-9].
Here we report the case of a 74-year-old woman who presented without any symptoms and was accidentally found to have a left retroperitoneal neoplasm by imaging examination. After surgical resection, the neoplasm was pathologically diagnosed as AH.
CASE PRESENTATION
Chief complaints
A 74-year-old woman was admitted to our department for a left retroperitoneal neoplasm that was found 1 mo prior during a routine checkup.
History of present illness
One month prior, abdominal non-contrast-enhanced computed tomography (CT) at a local hospital revealed a left retroperitoneal neoplasm. However, the patient had no symptoms.
History of past illness
The patient had a history of hypertension for 20 years and had been taking 0.15 g irbesartan once a day to control blood pressure to within the normal range. In addition, the patient had a history of auricular fibrillation for 1 mo and had been taking 0.1 g aspirin each night for anticoagulant therapy and 47.5 milligrams succinic metoprolol once a day to control the heart rate to approximately 90 beats/min.
Physical examination
The patient had no superficial lymph node enlargement. An abdominal physical examination showed that her abdomen was flat and soft without tenderness.
Laboratory examinations
Routine blood tests, biochemical function, coagulation function, and tumor markers, as well as the levels of cortisol (19.13 μg/dL, reference range 6.24-18 μg/dL), aldosterone(105.04 ng/L, reference range 50-313 ng/L), the three hypertension items [plasma renin activity, 1.03 μg/L/h, reference range 1.45-5 μg/L/h; angiotensin I, 0.96 μg/L;angiotensin II, 50.19 ng/L, reference range 32-90 ng/L) were all within normal ranges.
Imaging examinations
A CT scan of the abdomen was performed in our hospital, on which the neoplasm presented as a circular heterogeneous hypodense soft-tissue shadow (Figure 1A). On contrast-enhanced CT, the neoplasm presented heterogeneous septal enhancement in the arterial phase (Figure 1B) and persistent enhancement in the portal phase, and its boundary with the left renal vein was ill-defined (Figure 1C).
To further clarify the diagnosis, we also performed a contrast-enhanced magnetic resonance imaging (MRI) scan. On T1-weighted images, the neoplasm showed a circular heterogeneous hypointensity (Figure 2A). On T2-weighted images and diffusion-weighted images, the neoplasm showed a circular heterogeneous hyperintensity (Figure 2B and C). On arterial phase post-contrast T1-weighted images,the neoplasm showed obvious septal enhancement (Figure 2D). On axial and coronal portal venous phase post-contrast T1-weighted images, the neoplasm showed persistent enhancement and its boundary with the left renal vein was ill-defined(Figure 2E and F).
FINAL DIAGNOSIS
Based on these clinical and radiological manifestations, the neoplasm was initially diagnosed as a neurogenic neoplasm of the left retroperitoneum.
TREATMENT
The patient planned to undergo laparoscopic resection of the left retroperitoneal neoplasm, but due to the ill-defined boundary between the neoplasm and the left renal vein, massive bleeding occurred during the surgical separation, so the patient was transferred to open surgery. Finally, the neoplasm was completely resected in the case of renal vascular occlusion, and we reconstructed the left renal vein.
OUTCOME AND FOLLOW-UP
The patient recovered smoothly without any complications.In situ, the neoplasm was located in front of the left renal vein, and the boundary between them was ill-defined(Figure 3A). Macroscopically, the neoplasm presented as a mahogany brown lesion,with no capsule (Figure 3B). Microscopically, the neoplasm was composed of a clear ethmoid, sinusoid, and anastomosing vascular pattern, which was separated by fibers into lobules and had the appearance of the red pulp of the spleen. The vascular endothelial cells of the neoplasm looked like a hobnail, and the local vascular endothelial nuclei were enlarged, hyperchromatic, and slightly abnormal, but no mitotic figure was seen (Figure 3C and D). On immunohistochemical examination, the cytoplasm of the vascular endothelial cells were positive for CD31 (Figure 3E) and CD34 (Figure 3F), and the nuclei of the vascular endothelial cells were positive for ERG (Figure 3G), while only a few endothelial cells were positive for Ki 67 with a positive rate of 7% (Figure 3H). These characteristics were consistent with a diagnosis of AH. There were no signs of postoperative recurrence during the 2-mo CT follow-up.
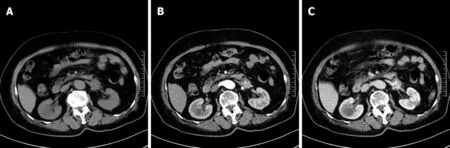
Figure 1 Anastomosing hemangioma of left renal vein on computed tomography. A: Non-contrast-enhanced scan; B: Arterial phase of contrastenhanced scan; C: Portal phase of contrast-enhanced scan.

Figure 2 Anastomosing hemangioma of left renal vein on magnetic resonance imaging. A: T1-weighted image; B: T2-weighted image; C: Diffusionweighted image; D: Arterial phase post-contrast T1-weighted image; E: Portal venous phase post-contrast T1-weighted image; F: Coronal portal venous phase postcontrast T1-weighted image. Orange arrow marks the anastomosing hemangioma, and blue arrow marks the left renal vein.
DISCUSSION
AH is extremely rare, and its etiology and pathogenesis remain unclear. It mainly occurs in the genitourinary tract; however, several cases have been reported in the liver, gastrointestinal tract, retroperitoneum, nasal cavity, and even the intracranial space[3,4,8,9]. Although AH involving the left renal vein branches has also been reported[10], we presented the first case of isolated anastomotic hemangioma arising from the left renal vein rather than the renal parenchyma.
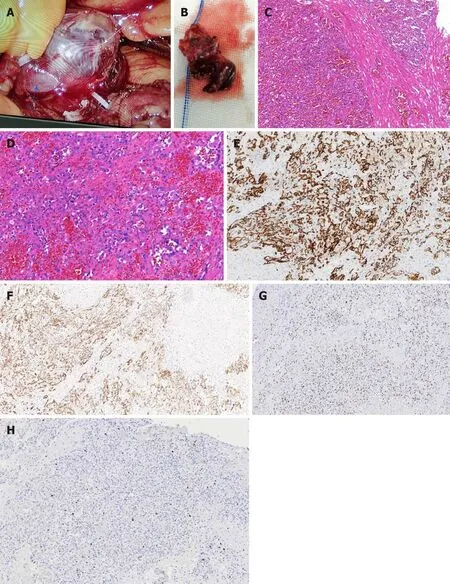
Figure 3 Histopathological findings of anastomosing hemangioma. A: In situ anastomosing hemangioma (AH, white arrow) and the left renal vein (blue arrow); B: Cut surface of the AH; C: Hematoxylin and eosin (H&E) staining (magnification: 100 ×); D: H&E staining (magnification: 200 ×); E-H: Immunohistochemical staining (magnification: 100 ×) for CD31 (E), CD34 (F), ERG (G), and Ki 67 (H).
AH is more common in middle-aged and elderly people, with a slight male predilection[11]. Generally, its diameter ranges from 0.1 cm to 6.0 cm[12]. AH has no special clinical symptoms or laboratory indictors, which is often found on accidental imaging examination. However, the imaging findings of AH show no specificity and are similar to most benign space-occupying lesions. On non-contrast-enhanced CT, the AH showed lobulated lesions, with soft-tissue attenuation, and on contrast-enhanced CT, it presented as a heterogeneous solid lesion with persistent enhancement[11,13]. On non-contrast-enhanced MRI, the AH presented as a round, well-defined T1-hypointense and T2-hyperintense lesion, and on contrast-enhanced MRI, it presented with avid peripheral enhancement in the arterial phase, which persisted in the delayed phase without central enhancement[14]. However, Merrittet al[15]also described the characteristics of their AH on MRI. In their report, the lesion presented as homogenous enhancement in the arterial phase, both peripherally and centrally, which persisted in the delayed phase. In our case, the lesion showed heterogeneous septal enhancement in the arterial phase, which persisted in the portal phase.
The diagnosis of AH mainly depends on histopathological examination.Macroscopically, AH is usually a spongy neoplasm with no capsule, but with a clear boundary and mahogany-brown in color[11,12,16]. Microscopically, AH is characterized by tightly packed capillary channels lined with hobnailed endothelial cells, which are similar in appearance to the red pulp of the spleen, have extramedullary hematopoiesis, and lack endothelial atypia[11,17]. Immunohistochemical staining revealed strong and diffuse CD31, CD34, and EGR positivity[17,18]. It is important to note that there is no mitotic activity, no or slight cellular atypia, and a low Ki-67 index[11,12,18].
AH should be mainly differentiated from angiosarcoma[19]. Angiosarcoma is a rare,invasive, malignant tumor and radiologic examination is unable to differentiate it from AH. Histologically, it also presents with hobnailed endothelial cells and can mimic AH[11]. However, angiosarcoma is characterized by high-grade cell atypia, multiple layers of endothelial cells, and obvious mitotic activity, none of which were present in our case. Therefore, based on these characteristic histopathological findings, our case was diagnosed as AH.
The treatment of AH is controversial because a diagnosis cannot be made from preoperative radiologic examination. When biopsy results are available, the treatment may vary depending on the lesion location, lesion size, and presence of symptoms,such as observation, embolization or radiofrequency ablation, and local or radical resection, to avoid overtreatment. However, some scholars are wary of percutaneous biopsies because of the risk of bleeding in some cases.
AH runs a benign clinical course. Previous studies have shown that there is no propensity for disease recurrence[11,20]. Yet recent research suggests that a recurrence of AH is indeed possible[21]and recurrent GNAQ mutations in the pathogenesis of AH[22].Although other capillary hemangiomas, especially congenital hemangioma, also have GNAQ mutations, the clinical setting (that is, the age and location of the patient)makes AH unique within this group[22]. Moreover, GNAQ mutations are not found in angiosarcoma, which may play an important role in distinguishing AH from angiosarcoma. Currently, due to the rarity of AH, there are no established guidelines for its follow-up.
CONCLUSION
We report a case of AH arising from the left renal vein, which, as far as we know, has never been reported before. In light of the lack of specific clinical and radiologic manifestations, AH is easily misdiagnosed preoperatively. Awareness of this entity occurring in the left renal vein will not only help improve doctors' vigilance and reduce the probability of misdiagnosis but also can aid in determining the most appropriate treatment for the patient.
ACKNOWLEDGEMENTS
We wish to acknowledge Gang Jin (Urinary Surgery, The Second Affiliated Hospital of Jiaxing University, Jiaxing) for his assistance in the surgical technique, Ya-Wei Yu(Department of Pathology, The Second Affiliated Hospital of Jiaxing University,Jiaxing) for her support on this case in pathology, and Xiao-feng Chen (Department of radiology, The Second Affiliated Hospital of Jiaxing University, Jiaxing) for her comments on this case in radiology.
杂志排行
World Journal of Clinical Cases的其它文章
- Relationship between non-alcoholic fatty liver disease and coronary heart disease
- Remission of hepatotoxicity in chronic pulmonary aspergillosis patients after lowering trough concentration of voriconazole
- Endoscopic submucosal dissection as alternative to surgery for complicated gastric heterotopic pancreas
- Observation of the effects of three methods for reducing perineal swelling in children with developmental hip dislocation
- Predictive value of serum cystatin C for risk of mortality in severe and critically ill patients with COVID-19
- Sleep quality of patients with postoperative glioma at home
