Endoscopic submucosal dissection as alternative to surgery for complicated gastric heterotopic pancreas
2020-04-08JinHeeNohDoHoonKimSoWoonKimYoungSooParkHeeKyongNaJiYongAhnKeeWookJungJeongHoonLeeKeeDonChoiHoJuneSongGinHyugLeeHwoonYongJung
Jin Hee Noh, Do Hoon Kim, So-Woon Kim, Young Soo Park, Hee Kyong Na, Ji Yong Ahn, Kee Wook Jung,Jeong Hoon Lee, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung
Jin Hee Noh, Do Hoon Kim, Hee Kyong Na, Ji Yong Ahn, Kee Wook Jung, Jeong Hoon Lee, Kee Don Choi, Ho June Song, Gin Hyug Lee, Hwoon-Yong Jung, Department of Gastroenterology,University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, South Korea
So-Woon Kim, Young Soo Park, Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, South Korea
Abstract BACKGROUND Gastric heterotopic pancreas (GHP) is generally asymptomatic and rarely features complications such as pancreatitis, pseudocysts, gastric outlet obstruction,bleeding, obstructive jaundice, or intussusception. However, the treatment of complicated GHP is challenging and often requires surgical resection.AIM To investigate the clinical outcomes of endoscopic submucosal dissection (ESD) as alternative to surgical resection for complicated GHP.METHODS This is a single-center, retrospective study. Between January 2013 and December 2017, a total of 5 patients underwent ESD for complicated GHP at Asan Medical Center. Patients who were diagnosed with complicated GHP were treated conservatively as with general practice for acute pancreatitis. After conservative management for resolving the acute phase of pancreatitis, ESD was performed as definitive treatment for complicated GHP. ESD was performed using the conventional method under conscious sedation. The clinical features of patients and tumors, procedure-related characteristics, and long-term outcomes were investigated.RESULTS The age of the 5 patients ranged from 28-43 years. Two of the patients were males.All lesions were located in the greater curvature of the antrum. On endoscopic ultrasonography during the pain episode, all lesions were located across the muscularis mucosa, submucosa, and proper muscle layers. The median lesion size was 20 [interquartile range (IQR), 18-35] during the pain episode at the time of the diagnosis of complicated GHP, and 15 mm (IQR, 9-33) at the time of ESD after conservative treatment. The procedure time ranged from 15-120 min. There were no procedure-related adverse events such as perforation or bleeding. The length of hospital stay after the procedure ranged from 2-4 d. All patients were symptom free during the median follow-up period of 46.0 mo (IQR, 39-60).CONCLUSION ESD appears to be a feasible and effective treatment option for complicated GHP based on the favorable clinical outcomes.
Key Words: Endoscopic submucosal dissection; Gastric; Heterotopic pancreas; Pancreatitis
INTRODUCTION
Heterotopic pancreas is a congenital anomaly defined as an abnormally located pancreatic tissue without anatomic or vascular connections with the pancreas. Its incidence is reportedly 0.55%-14% on autopsy[1,2]. Heterotopic pancreas is primarily found in the stomach, duodenum, or proximal part of the jejunum but may occur in the esophagus, gallbladder, bile duct, liver, spleen, or mesentery[3,4]. Gastric heterotopic pancreas (GHP) appears as a subepithelial lesion and is usually localized in the greater curvature or posterior wall of the antrum. It has a central indentation as an opening of a large excretory tube[5]. Its histological diagnosis is usually difficult because adequate tissue specimens cannot be obtained on conventional endoscopic forceps biopsy.Endoscopic ultrasonography (EUS) is useful for diagnosing heterotopic pancreas[6].EUS features of heterotopic pancreas include a heterogeneous hypoechoic echo pattern[7].
Most cases of GHP are asymptomatic, but a minority may present with nonspecific symptoms such as epigastric pain, abdominal discomfort, nausea, and vomiting[8].GHP is rarely associated with complications such as pancreatitis, pseudocysts, abscess,hemorrhages, and necrosis;i.e., conditions similar to those that may occur in normal pancreases. In addition to gastrointestinal obstruction, stenosis or intussusception can occur depending on the location and size of the lesion[3,9,10]. Asymptomatic GHP is generally considered benign and can be periodically follow-up by endoscopy without intervention, whereas complicated GHP often requires treatment for symptom relief,including surgical resection[11-14]. We previously reported a case of GHP with pancreatitis and pseudocyst that was treated successfully with endoscopic submucosal dissection (ESD); our findings suggested that ESD can be a feasible alternative to surgical resection with minimal invasiveness[15]. In this study, we investigated the clinical outcomes to determine the feasibility and effectiveness of ESD for complicated GHP.
MATERIALS AND METHODS
Between January 2013 and December 2017, subjects underwent ESD for complicated GHP at Asan Medical Center. The diagnosis of complicated GHP was made when a patient with GHP had a recurrent severe abdominal pain and met at least one of the following criteria: (1) Morphological change of the GHP on endoscopy, abdominal computed tomography (CT), or magnetic resonance imaging (MRI) during the pain episode; and (2) Elevated serum pancreatic enzyme level with normal pancreas on imaging. All patients who were diagnosed with complicated GHP underwent EUS for the evaluation of lesion size, sonographic layer, echogenicity, homogeneity, and accompanied complications.
Patients who were diagnosed with complicated GHP were treated conservatively with intravenous hydration, pain control, and restricted oral intake as with general practice for acute pancreatitis[16]. After conservative management for resolving the acute phase of pancreatitis, ESD was performed as definitive treatment for complicated GHP.
ESD was performed using the conventional method under conscious sedation with intravenous midazolam (0.05-0.1 mg/kg) and pethidine (25-50 mg), with continuous monitoring of cardiorespiratory function (Figure 1). After marking outside the lesion,normal saline containing epinephrine and indigo carmine was submucosally injected with a 23-gauge needle (Finemedix Co., LTD., Daegu, South Korea). A circumferential incision was made with a needle (MTW Endoskopie, Wesel, Germany) or O type knife(Finemedix Co., LTD.). The submucosal connective tissue and part of the muscularis propria was dissected with an O-type knife. Hemostasis was performed during or after the dissection using hemostatic forceps (FD-410LR; Olympus). After the procedure, patients were monitored in an outpatient clinic to assess symptom recurrence. The endoscopy was performed 6 mo after the procedure, and was performed annually thereafter.
Informed consent was obtained from all patients before the procedure. This study was approved by the institutional review board of Asan Medical Center (IRB No. 2019-0963).
Descriptive variables are summarized as median (interquartile range, IQR) or mean± standard deviation. All statistical analyses were performed using SPSS version 24(IBM Corporation, Somers, NY, United States).
RESULTS
Five patients were diagnosed with complicated GHP and underwent ESD during the study period. The characteristics of each patients and lesions, and procedure-related outcomes are shown in Table 1 and Figure 2. The age of the 5 patients ranged from 28-43 years. Two of the patients were male. All lesions were located in the greater curvature of the antrum and appeared to be subepithelial. Three lesions showed a typical central indentation, while 2 lesions were accompanied by surface ulcerations.All lesions involved the muscularis mucosa, submucosa, and muscularis propria layer on EUS. The median lesion size was 20 mm (IQR, 18-35) at the time of the diagnosis of complicated GHP and decreased after conservative treatment [median, 15 mm, (IQR,9-33)].
The procedure time ranged from 15-120 min. There were no procedure-related adverse events such as perforation or bleeding. The length of hospital stay after the procedure ranged from 2-4 d. During the median follow-up period of 46.0 mo (IQR,39-60), no patients experienced symptom recurrence.
The diagnosis and treatment of each patient is summarized as follows. In patient 1,the laboratory test was normal and there were no specific findings except for gastric subepithelial tumor on CT at the time of the presentation of abdominal pain. The abdominal pain was localized to the epigastric and right upper quadrant areas. The size of GHP was 18.6 mm on EUS, larger than that at the initial diagnosis (10 mm).After conservative management, lesion size decreased to 15 mm, but symptoms persisted. The patient eventually underwent therapeutic ESD. The clinical course of lesion size according to abdominal pain are shown in Figure 3A. According to the histopathological report, the pancreatic tissue was within the proper muscle layer, and the peripheral resection margin was negative and the deep resection margin was positive for tumor involvement (Figure 4A).
Patient 2 complained of epigastric and right upper quadrant pain and had elevated pancreatic enzyme levels during pain (amylase, 236 U/L; lipase, 361 U/L). Theabdominal CT revealed irregular wall thickening of the gastric antrum and fluid collection along the greater curvature of the stomach. The GHP was 28 mm on EUS.After one month of conservative treatment, no symptom improvement was noted;rather, severe inflammatory changes around the gastric antrum and cystic lesions presumed to be pseudocysts were observed on follow-up abdominal CT. Lesion size was increased to 50 mm. The patient underwent therapeutic ESD[15]. According to the histopathological report, the pancreatic tissue was extended to the proper muscle layer, and there was no involvement of the peripheral resection margin; however, the deep resection margin was positive.
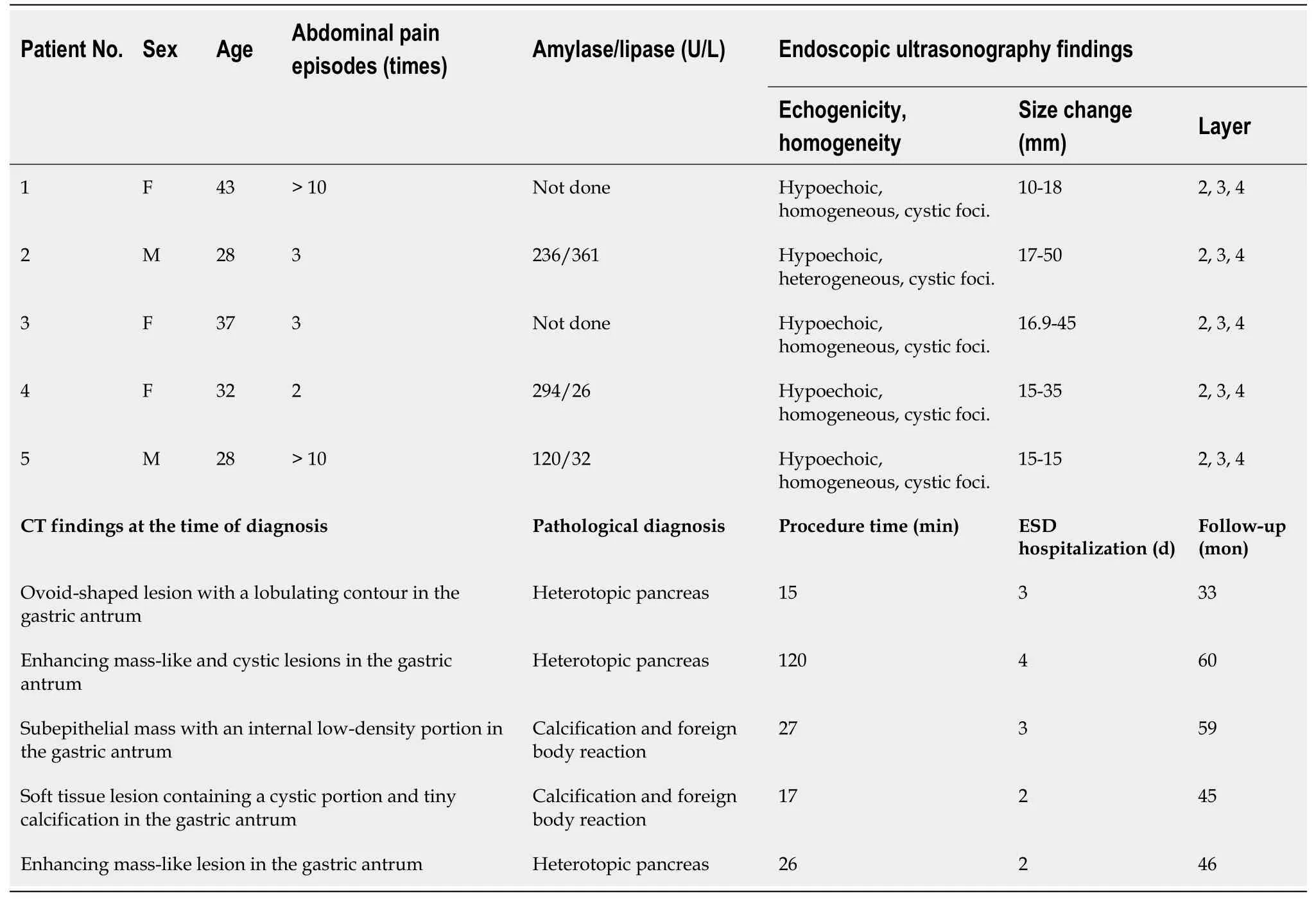
Table 1 Patient and lesion characteristics and procedure-related outcomes
In patient 3, GHP with pancreatitis and suspected pseudocyst was observed on abdominal CT, and the lesion size on EUS (45 mm) was larger than ever (17 mm). The symptoms were improved and the lesion size was decreased to 10 mm after conservative treatment; thereafter, the patient requested outpatient follow-up only.One month later, the patient complained of recurrent epigastric pain. She eventually underwent ESD to prevent recurrence of symptom and complications (Figure 3B). On the histopathologic report, the lesion was dissected to the submucosal layer, and only foreign body reactions and calcification were noted (Figure 4B). After the endoscopic therapy, she did not complain of abdominal pain during follow-up period.
In patient 4, the GHP size increased from 15 mm to 35 mm on EUS. She complained of epigastric pain and had elevated pancreatic enzyme levels during times of abdominal pain (amylase, 294 U/L; lipase, 26 U/L). A soft tissue lesion containing a cystic portion in the gastric antral wall was observed on CT. After conservative treatment, the lesion size decreased to less than 10 mm on EUS, and the symptoms were improved. The patient requested to be followed in the outpatient clinic, but abdominal pain similar to previous pain recurred during the follow-up period.Therefore, she underwent ESD for GHP (Figure 3C). On the histopathologic report, the lesion was dissected to the submucosal layer, and only foreign body reaction and calcification were noted similar to patient 3 (Figure 4C). The abdominal pain improved after the procedure.
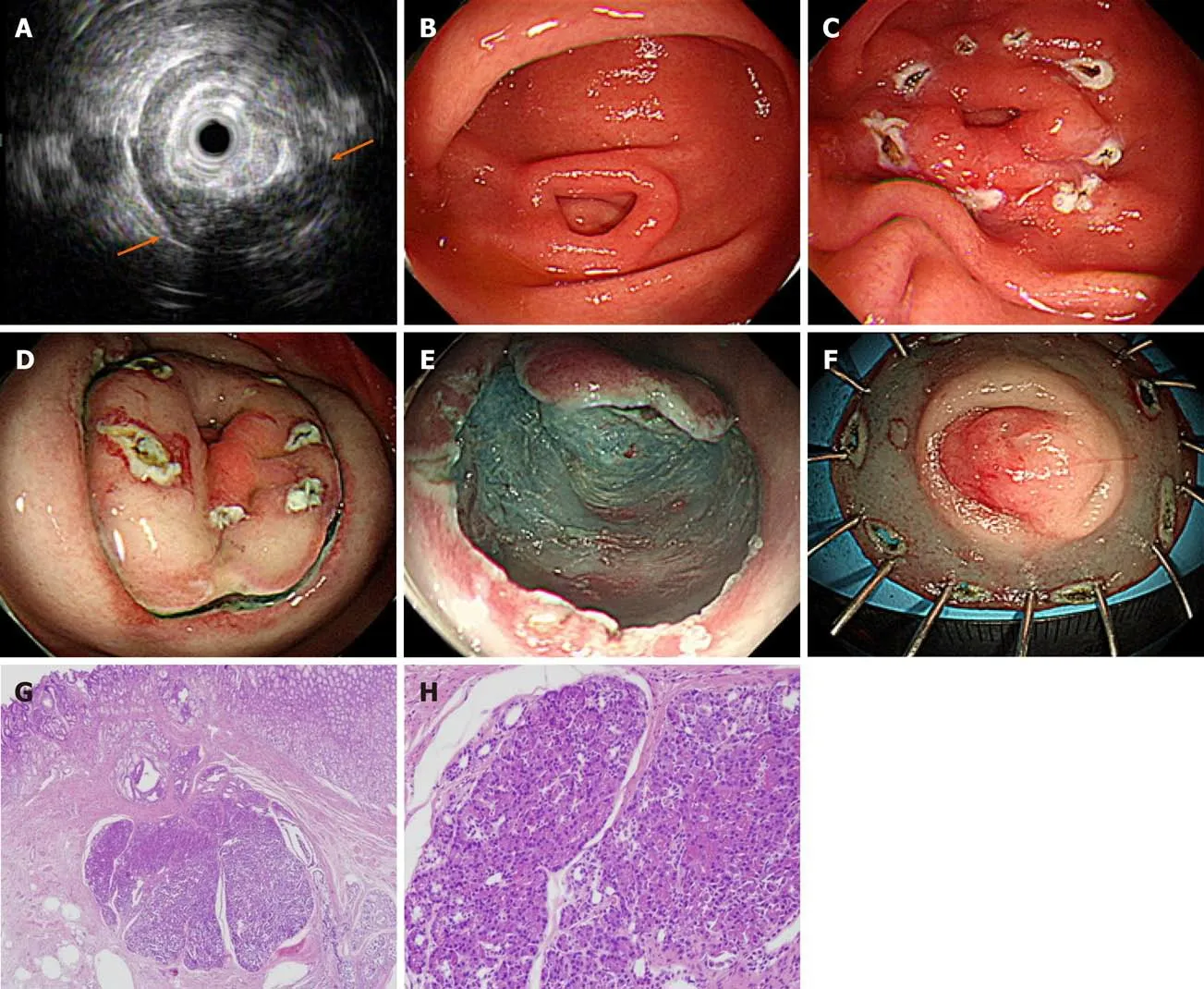
Figure 1 Endoscopic submucosal dissection of complicated gastric heterotopic pancreas (patient 5). A: Endoscopic ultrasonography image showing a homogeneous hypoechoic lesion with cystic (hypoechoic) foci that was located within the 2nd, 3rd, and 4th layers; B: A 1.5 cm subepithelial tumor with umbilication was observed on the greater curvature of the antrum; C: The lesion borders were marked; D: Saline with epinephrine was submucosally injected, and a circumferential mucosal incision was made; E: The submucosal connective tissue and part of the muscularis propria were dissected; F: The resected specimen was fixed; G: Heterotopic pancreas is located in the submucosa underlying intact gastric mucosa (hematoxylin-eosin; original magnification, × 40); H: Normal pancreatic acini with ducts are noted (× 200).
In patient 5, the GHP was 12 mm, and the pancreas appeared normal on MRI. He complained of epigastric pain and showed no GHP size changes on EUS during the pain. However, the abdominal pain continued, and the pancreatic enzymes were elevated (amylase, 120 U/L; lipase, 32 U/L). We suspected that the symptoms might be related with complicated GHP; thus, the patient underwent therapeutic ESD.Thereafter, the symptoms were relieved. The histopathological report indicated that the pancreatic tissue was located in the submucosa and there was no involvement of the peripheral or deep resection margins (Figure 4D).
DISCUSSION
ESD appears to be a feasible and effective treatment option for complicated GHP that shows favorable clinical outcomes. The conservative treatment for resolving the acute phase of pancreatitis was helpful to perform ESD, since parenchymal inflammation,edema, and necrosis make ESD difficult.
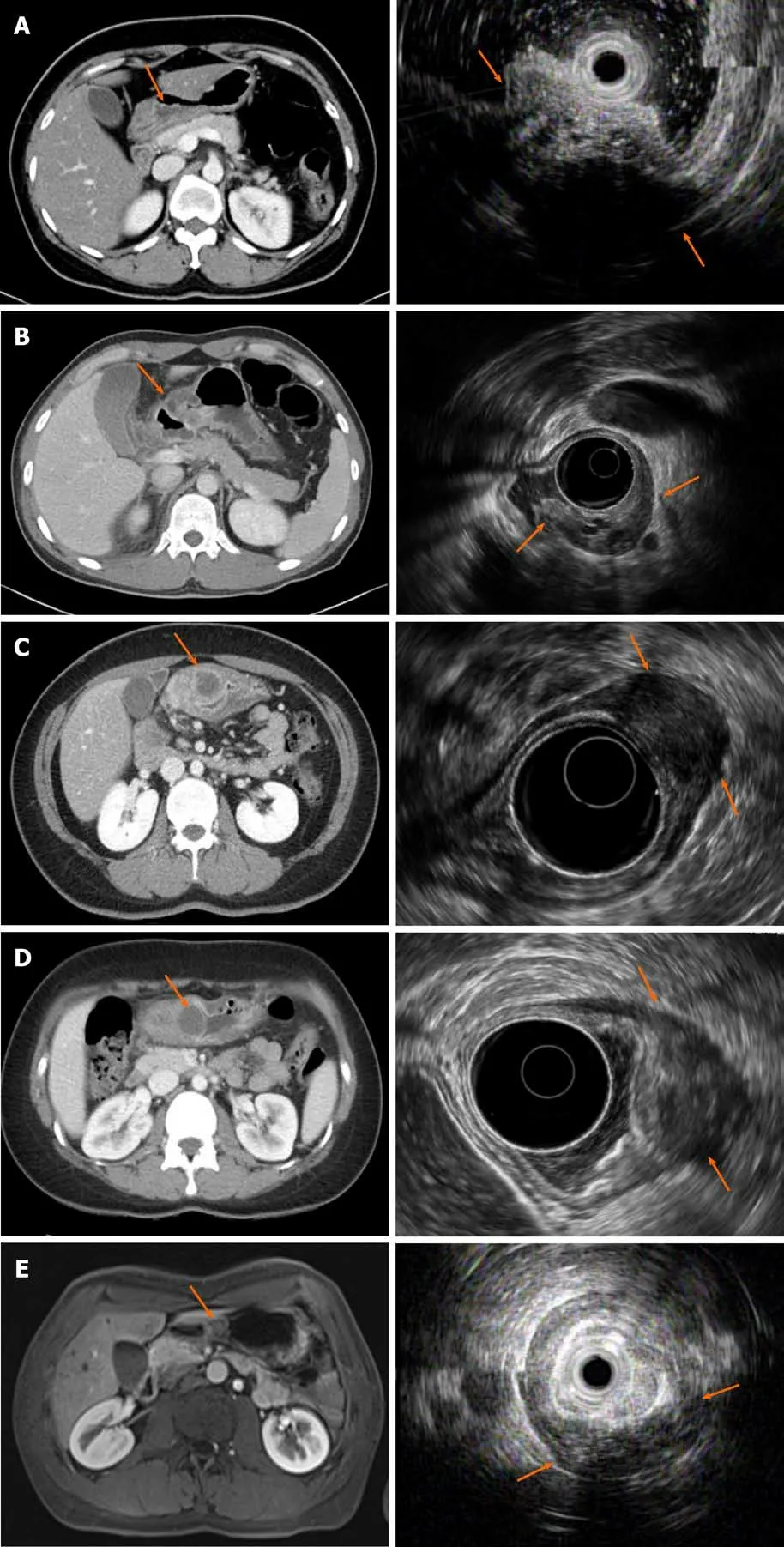
Figure 2 Computed tomography and endoscopic ultrasonography images of each patient at the time of diagnosis of complicated gastric heterotopic pancreas. A-E: Correspond to patient 1, 2, 3, 4, and 5, respectively. The computed tomography and endoscopic ultrasonography findings of each patient are summarized in Table 1.
Diagnostic criteria for complicated GHP are not established. Most cases of GHP are asymptomatic, but a few patients have nonspecific symptoms such as abdominal pain,indigestion, nausea, and vomiting. Only a few patients may experience complications,such as pancreatitis, hemorrhage, necrosis, and malignant transformation that could occur in normal pancreatic tissue[9,17]. Therefore, further clues are needed in addition to abdominal pain to diagnose complicated GHP. According to a previous report, some cases of complicated GHP can elevate the pancreatic enzymes[18]. In the present study,three patients had elevated pancreatic enzymes. Chemical irritation or inflammation caused by the endocrine and exocrine function of the heterotopic pancreas tissue can cause symptoms[19]. In pancreatitis, the pancreas is enlarged diffusely or locally by the interstitial or inflammatory edema; in fact, four of the five patients in our study showed GHP enlargement[20]. Based on the above results, we diagnosed complicated GHP when the patient had recurrent severe abdominal pain and met at least one of the following criteria: (1) Morphologic change in GHP on endoscopy, CT, or MRI during the pain episode; and (2) Elevated serum pancreatic enzymes with a normal pancreas on imaging.
The heterotopic pancreas on CT reportedly shows similar enhancement to that of normal pancreatic tissue. The common CT feature is that of an oval-shaped intramural mass with microlobulated margins[21]. However, the role of CT in the diagnosis of GHP without complications is insignificant[22]. In our cases, all patients showed a normal pancreas and a small irregular enhancing intramural mass in the gastric antrum. They showed inflammatory changes on GHP and fluid collection along the greater curvature of the stomach. Two patients also had cystic lesions in the gastric antrum,which suggests a pseudocyst on GHP.
In asymptomatic GHP, close observation with serial follow-up endoscopy is sufficient because the risk of complications or malignant changes is extremely low.However, if symptoms or complications occur, complete resection should be considered[15,23]. In a previous study, ESD was safe and feasible for curative treatment of GHP. This procedure was performed for asymptomatic or symptomatic GHP with a nonspecific abdominal pain, and approximately 65% of lesions originated from the mucosa or the submucosa layer[24]. The complete resection of complicated GHP by ESD is challenging, because the lesion is usually located in the deep submucosal and proper muscle layers.
When GHP is accompanied by complications including pancreatitis like in a normal pancreas, the lesion enlarges because of tissue inflammation and edema. In this case,each layer is difficult to distinguish because of the inflammatory changes. Even if the inflammation has improved, endoscopic dissection is difficult because the tumor boundary is not clear owing to the tissue changes caused by the inflammation.Therefore, dissection as close as possible to the proper muscle based on the easily identifiable layer, rather than on the tumor with an unclear border, would be a better tip for complete resection. In this study, there were two cases of endoscopically complete but pathologically incomplete resection of the deep resection margin. The possibility of recurrence from remnant tissues was closely followed up, but neither symptoms nor tumors recurred during the median follow-up period of 46.0 mo. It might suggest that the significant cauterization effect at the deep resection margin of the lesion during ESD generally ablates any remnant cells. And other two cases were confirmed only foreign body reactions and calcification on histopathologic report. We suggested the reasons for this situation as follows. First, the pancreas tissue was eliminated because of the ulcer. Second, the severe inflammation resulted in the necrosis of normal pancreatic tissue, and eventually, there are no normal pancreatic tissues after conservative treatment. Third, significant cauterization effect at the deep resection margin of the lesion during ESD generally ablates any remnant cells.
Although further discussions are needed to determine the optimal removal timing and method, early removal is recommended for patients with complicated GHP given that severe recurrent abdominal pain occurred 2-10 times before lesion removal in our study. These patients were first treated conservatively with the aim of reducing inflammation and relieving symptoms, while ESD was performed later. None of the patients who underwent ESD for complicated GHP experienced procedure-related adverse events or symptom recurrence during the follow-up period.
Our study had several limitations. First, because it was retrospective and conducted in a single center, there were no definitive criteria for patient selection, optimal resection timing, or treatment method. Second, the assessment of severe pain required for the diagnosis of complicated GHP is subjective and may vary from person to person. However, we used the numeric rating scale to assess pain intensity for persons able to self-report. We attempted to evaluate pain as objectively as possible by documenting the patient’s history of requiring narcotic analgesics to improve pain or visiting the emergency room when the pain occurred. We also tried to differentiate between abdominal pain caused by pancreatitis and that due to other reasons. Despite these limitations, the present study examined the largest number of cases to date reported in the literature, and our findings strongly support the current strategy of ESD for complicated GHP.
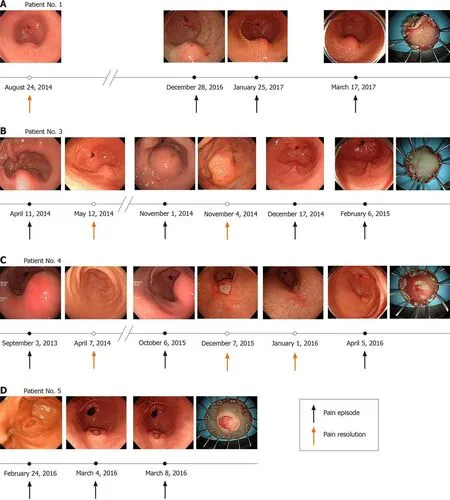
Figure 3 The clinical course of lesion size according to abdominal pain are summarized. A: Patient 1; B: Patient 3; C: Patient 4; D: Patient 5.
CONCLUSION
In conclusion, when patients with GHP experience recurrent severe acute abdominal pain, complicated GHP should be considered as a differential diagnosis. Conservative treatment followed by ESD can be a feasible minimally invasive alternative to surgical resection.
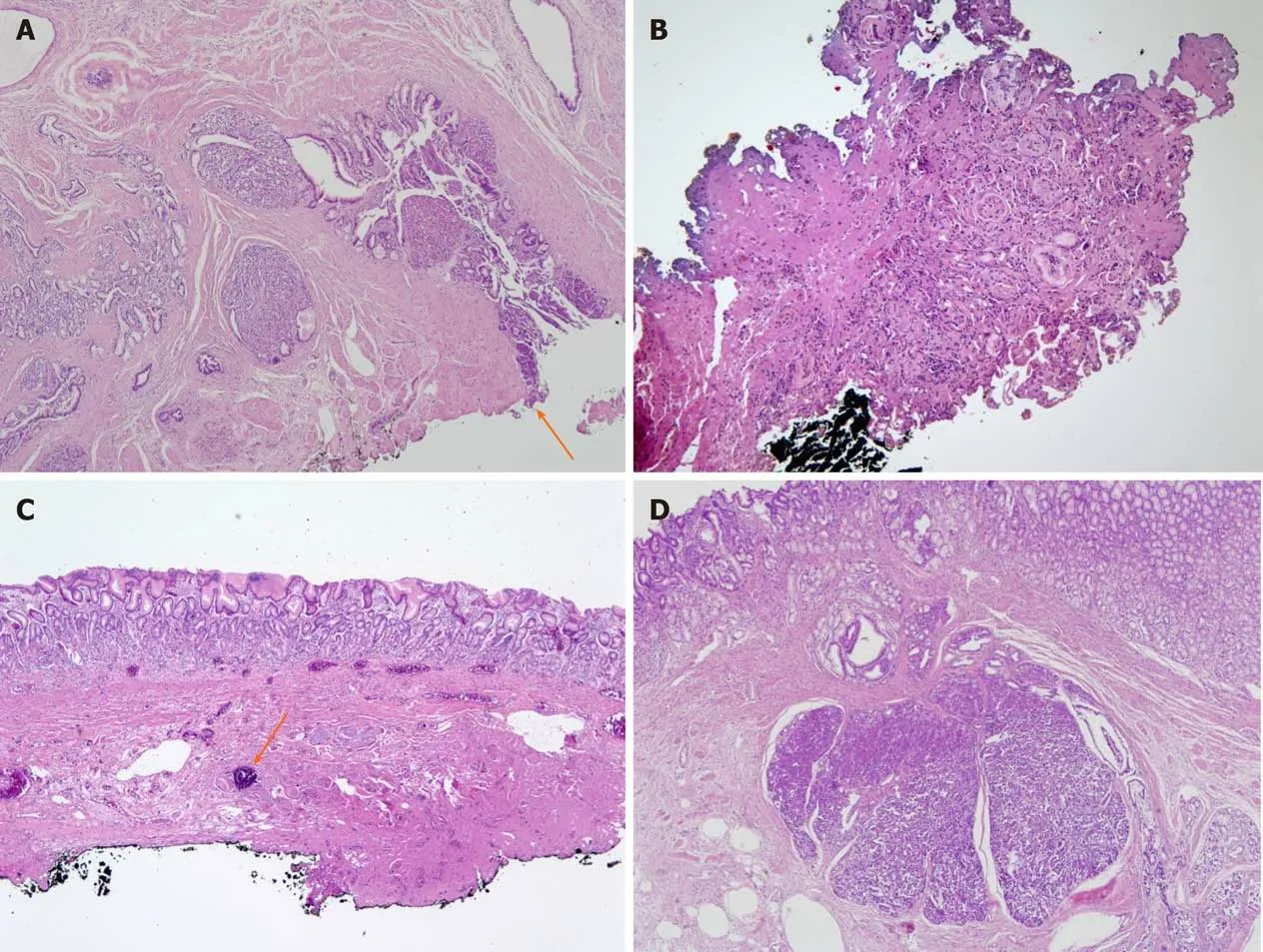
Figure 4 Representative histologic images of gastric heterotopic pancreas. A: Patient 1: Pancreatic tissue is in proper muscle with involvement of resection margin (arrow) (hematoxylin-eosin; original magnification, × 40); B: Patient 3: There is focal nest of cells and bluish material with fibrosis and severe cautery artifact (× 100); C: Patient 4: Submucosal fibrosis with foreign body reaction and dystrophic calcification (arrow) was noted (× 40); D: Patient 5: Pancreatic tissue is in submucosa overlying gastric mucosa (× 40).
ARTICLE HIGHLIGHTS
Research background
Gastric heterotopic pancreas (GHP) is generally asymptomatic and rarely features complications. However, the treatment of complicated GHP is challenging and often requires surgical resection.
Research motivation
This retrospective study describes clinical outcomes in patients who underwent endoscopic submucosal dissection (ESD) for complicated GHP.
Research objectives
This study aimed to investigate the clinical outcomes of ESD as alternative to surgical resection for complicated GHP.
Research methods
Patients who were diagnosed with complicated GHP were treated conservatively as with general practice for acute pancreatitis. After conservative management for resolving the acute phase of pancreatitis, ESD was performed as definitive treatment for complicated GHP. The clinical features of patients and tumors, procedure-related characteristics, and long-term outcomes were investigated.
Research results
All lesions were located in the greater curvature of the antrum. On endoscopic ultrasonography during the pain episode, all lesions were located across the muscularis mucosa, submucosa, and proper muscle layers. The median lesion size was 20 during the pain episode at the time of the diagnosis of complicated GHP, and 15 mm at the time of ESD after conservative treatment. The procedure time ranged from 15-120 min. There were no procedure-related adverse events such as perforation or bleeding. The length of hospital stay after the procedure ranged from 2-4 d. All patients were symptom free during the median follow-up period of 46.0 mo.
Research conclusions
When patients with GHP experience recurrent severe acute abdominal pain,complicated GHP should be considered as a differential diagnosis.
Research perspectives
Conservative treatment followed by ESD can be a feasible minimally invasive alternative to surgical resection.
杂志排行
World Journal of Clinical Cases的其它文章
- Relationship between non-alcoholic fatty liver disease and coronary heart disease
- Remission of hepatotoxicity in chronic pulmonary aspergillosis patients after lowering trough concentration of voriconazole
- Observation of the effects of three methods for reducing perineal swelling in children with developmental hip dislocation
- Predictive value of serum cystatin C for risk of mortality in severe and critically ill patients with COVID-19
- Sleep quality of patients with postoperative glioma at home
- Early complications of preoperative external traction fixation in the staged treatment of tibial fractures: A series of 402 cases
