Preparation and electrochemical properties of modified PAN-based carbon fiber electrode materials with Nickel-plated polystyrene spheres
2020-03-24WANGHaoMingGUOLeiCAOZheWeiXUJiaLuZHANGDianYuLIUYiMingJINZheng
WANG Hao-Ming, GUO Lei, CAO Zhe-Wei, XU Jia-Lu, ZHANG Dian-Yu, LIU Yi-Ming, JIN Zheng
(School of Chemisty and Material Science, Heilongjiang University, Harbin 150080,China)
Abstract:The spheres prepared by inverse emulsion polymerization method, and then, in the form of chemical plating and the plating solution was coated nickel plating polystyrene ball, in the end, based on polyacrylonitrile carbon fiber, nickel-plated polystyrene spheres doped modified polyacrylonitrile activated carbon fiber composite material(Ni/PS/ACF)was prepared by electrostatic spinning, pre-oxidation and carbonization. After modification, the porosity between the fibers increases obviously, and the electrolyte ions diffused into the composites more easily. According to the electrochemical test, when the current density was 1 A·g-1, the specific capacitance value of Ni/PS/ACF-0.3 was the highest (1 457.6 F·g-1). When the current density is 1 A·g-1, the specific capacitance retention rate of Ni/PS/ACF-0.3 composite material is 93.85% after 5 000 times of constant current charging and discharging, showing good cyclic stability.
Key words:supercapacitor; modifier; polyacrylonitrile fibre; polystyrene spheres
Supercapacitors are now the new energy storage component. Compared with batteries and conventional capacitors which are used in shops and family and other places on the market, supercapacitors as today’s most promising replacement type of new energy storage device, are considered as a new type of valuable energy storage devices with high energy density, high power, high stability, fast charging and discharging and long cycle life[1-3]. Supercapacitors are widely used not only in consumer electronics, but also in solar power generation systems, smart grid systems, new energy vehicles, industrial energy saving systems and pulse power supply systems[4-7].
Although a great breakthrough has been made in various researches on supercapacitors at home and abroad in recent years, due to the increasing energy demand, supercapacitors are still faced with the defects of low energy density and general cycling stability[8]. The best way to improve the performance of supercapacitors is to try to find better electrode materials or to use better modifiers to change the structure of carbon-based materials to get a new structure and further optimize the performance of supercapacitors[9-10].
Polyacrylonitrile-based activated carbon fiber (PAN-ACF) is a new generation of fiber electrode material, which has the characteristics of good electrical conductivity and high strength[11]. In recent years, PAN-ACF has aroused people’s wide concern. Activated carbon fiber can be used as a supercapacitor electrode materials because there are uniform pore size distribution and the characteristics of a large number of microporous in the fiber structure of the channel, the electrolyte ions can pass the channel to the interior of the material, storage charge, to implement the ion exchange reaction[12-15]. Huang et al.[16]used polyacrylonitrile soluble in DMF and add a certain amount of allyl polyethylene glycol, got rich in nitrogen from electrostatic spinning and carbonization of porous activated carbon fibers, as a supercapacitor electrode materials, composite materials under the current density of 0.2 A·g-1, its specific capacitance values of 310 F·g-1, on the cycle stability of testing than capacitance retention rate is as high as 54.6%.
Polystyrene microspheres have the characteristics of polymer microspheres, such as large specific surface area, uniform and controllable particle size, etc.[17-19]. Polystyrene microspheres with good performance, volume resistivity, and surface resistivity of up to 1016~ 1018Ω·cm, and 1015~ 1018Ω[20]. Polystyrene microspheres are monodispersed, have a controllable size, and can be modified to functional polymer molecules on their surfaces. They can also be broken down at high temperatures for further pore making[21].
We used reverse emulsion polymerization preparation of polystyrene ball, then the nickel-plated polystyrene spheres were obtained by electroless plating and plating solution, in the end, based on polyacrylonitrile carbin fiber, nickel-plated polystyrene spheres doped modified polyacrylonitrile activated carbon fiber composite material(Ni/PS/ACF)was prepared by electrostatic spinning, pre-oxidation and carbonization.. The composite has a high porosity, which makes it easier for electrolyte ions to diffuse into the carbon fiber composite. According to the electrochemical test, when the current density was 1 A·g-1, the specific capacitance value of Ni/PS/ACF-0.3 was the highest (1 457.6 F·g-1). When the current density is 1 A·g-1, the specific capacitance retention rate of Ni/PS/ACF-0.3 composite material is 93.85% after 5 000 times of constant current charging and discharging, showing good cyclic stability.
1 Experimental
1.1 Preparation of polystyrene spheres
Prepare sodium hydroxide solution, remove polymerization inhibitor in styrene, wash four times, and then wash with water (about 100 mL) until neutral[22]. In this part of the experiment, a certain amount of Sp80, cyclohexane, and distilled water were mixed into solution by reverse emulsion polymerization and then added into a three-necked bottle. Under the condition of a constant temperature water bath, the set temperature was 72 ℃. The homogenized system was formed by stirring and nitrogen was continuously pumped for some time. Weight a certain amount of AIBN and styrene solution, a certain amount of AIBN is first slowly added to the styrene solution, add the mixture of both into the three-necked bottle and in a certain rate of nitrogen gas is piped in 36 min, stirring speed is about 120 r·min-1, after 12 h reaction, the polystyrene solution was removed and placed in the centrifugal tube, it was centrifuged for 5 min at a centrifugation rate of 8 000 r·min-1, take out after washing with ethanol, four times to remove the solvent. Dry for 12 h in a vacuum drying oven at 60 ℃.
1.2 Preparation of nickel-plated polystyrene spheres
Electroless plating was used to form a metal coating on the surface of polystyrene spheres. Step 1: Roughen the surface of the ball; After removing a certain amount of pellets and adding a certain amount of concentrated sulfuric acid into three-necked bottle for some time, ultrasonic treatment was performed for 60 min and centrifugation was carried out, washed with deionized water until the surface was free of sulfate ions (tested with barium chloride). Step 2: Add the coarsening ball to another beaker for sensitization, the configuration of a certain amount of 20 g·L-1stannous chloride and 20 mL·L-1hydrochloric acid mixture, mix after joining the small beaker to sensitizing the ball, ultrasonic 60 min after centrifugation, washing to ball surface without chloride (acid silver chloride test). Step 3: The balls sensitized in the previous step were added to another beaker and activated. A certain amount of 3 g·L-1palladium chloride and 20 mL·L-1hydrochloric acid were dispersed in a small amount of water, and the balls were activated. After ultrasound for 30 min, the surfaces of the balls were washed to be chlorine-free (acid silver chloride test). The activated polystyrene spheres were dried for 24 h in a vacuum drying oven with a set temperature of 60 ℃ for further use.
Plating solution for electroless plating: Will be a certain amount of 15 g·L-1sodium acetate, 20 g·L-1sodium dihydrogen phosphate, 18 g·L-1sodium citrate, 30 g·L-1nickel sulfate configured to mixed solution, diluted with deionized water, ammonia is used to adjust PH value of 6.0, constant temperature after some time, will have the activated polystyrene spheres join the plating solution, stir intermittently every 5 min until 30 min, then wash and dry to use.
1.3 The preparation of Ni/PS/ACF
At room temperature, a certain mass of Ni/PS was weighed and mixed with a solution composed of filamentous polyacrylonitrile and a certain volume of DMF. The spinning solution was poured into a three-mouth bottle and stirred in a 72 ℃ water bath for 12 h to obtain the fiber spinning solution. Pour the spinning liquid into a beaker and let cool to room temperature for further use. Use the electrostatic spinning device, use a syringe to draw 5 mL of fiber spinning solution, and use a 0.7 mm needle as spinning device output channel diameter and the bubble plate coated with aluminum foil, as receiving screen of spinning solution, the distance between the receiving screen and spinning device is 15 cm, the output voltage is 22 kV. Do electrostatic spinning. The preoxidation of polyacrylonitrile fiber composite material with 10 cm×10 cm of graphite paper parcel after into the small tube furnace, in a nitrogen atmosphere, with a heating rate of 5 ℃·min-1will tube furnace temperature to 800 ℃ and the high-temperature carbonization after 2 h, get different quality modifier modified by PAN-based carbon fiber composite materials, from dark brown to black, Ni/PS/ACF composite materials.
1.4 Electrochemical characterization
All electrochemical experiments were conducted with a three-electrode system in 6M KOH electrolyte using a CHI600D electrochemical workstation (Shanghai, Chenhua). The saturated calomel electrode (saturated KCl) and Pt electrode were used as reference electrode and counter electrode, respectively. Nickel foam was used as the current collector. The mixture of 85 wt% active materials, 5 wt% acetylene black, and 10 wt% PTFE (60 wt%) was fabricated using N-methylpyrrolidone as a solvent[23]. The slurry of the above mixture was pressed on the nickel foam at 20 MPa and dried. Five milligrams of active material was coated on the working electrode, and the coating area of the electrode active material was 1.5 cm×1.5 cm. The voltage range of the cyclic voltammogram (CVs) is from 0 to 0.65 V, with voltage scan rates ranging from 5 to 100 mV·s-1. At the current density of 1~10 A·g-1, the constant current charge-discharge curve (GCDs) was recorded. EIS was conducted by applying an AC voltage with an amplitude of 5 mV in a frequency range from 1 Hz to 100 Hz under open circuit potential conditions[24].
2 Results and discussion
2.1 Characterization
The scanning electron microscope (SEM) of polystyrene spheres synthesized by reverse emulsion polymerization is showed in Fig.1(a). As shown in Fig.1(a), the obtained polystyrene spheres have a uniform particle size, round surface, size of about 1 micron, close arrangement, and a stacked structure. Fig.1 (b) is the scanning electron microscope (SEM) of the nickel-plated polystyrene sphere synthesized by electroless plating. It can be seen that the surface of the polystyrene sphere is coated with a uniform nickel-metal coating. The surface of the sphere is full of “small burr”, and the coating has a certain thickness. And nickel-plated polystyrene spheres are hollow balls and white, having the slightly reflective phenomenon. It can be concluded that nickel-plated polystyrene spheres have good electrical conductivity, and uniform nickel metal plating on the surface of the spheres, can be used as a modifier to increase the capacitance value of the fiber. As can be seen from Fig.1 (c) of the scanning electron microscope of PAN-ACF, the crosslinking degree of fibers is slightly higher, which makes the porosity between fibers in PAN-ACF low and affects the internal velocity of electrolyte ion diffusion in the material. Fig.1 (d) for nickel-plated polystyrene spheres doped into the polyacrylonitrile fiber composite material can be seen from the picture, when the amount of polystyrene spheres doped nickel plating is 0.1 g, the composite material to form a core-shell type of bead chain structure, the thermal decomposition of polystyrene sphere after high-temperature carbonization, left a nickel-metal coating, fiber wrapped in metal shell-forming droplets of core-shell structure,which can be seen from the actual electron micrograph of a carbon fiber composite materials, composite materials with good electric conductivity. Fig.1 (e, f) are respectively the doping amount of 0.2 g and 0.3 g of plating nickel spheres, carbon fiber composite materials, you can see from the photos by doping metal plating, there are electrostatic repulsion and coulomb force between the fiber and fiber. Due to the electrostatic repulsion between fiber, the distance between the fiber increases, and with the addition of more plating nickel spheres, the form of modifier in the fiber is also slightly different. And Fig.1 (f) of the nickel-plated polystyrene spheres in fiber have round balls shape, the distance between the fiber is bigger, hence, the fiber specific surface area has increased. This special porous loose bead chain structure also exists in a large number of carbon fiber composites, which increases the porosity between the fibers and the conductivity and specific capacitance of the carbon fiber composites.
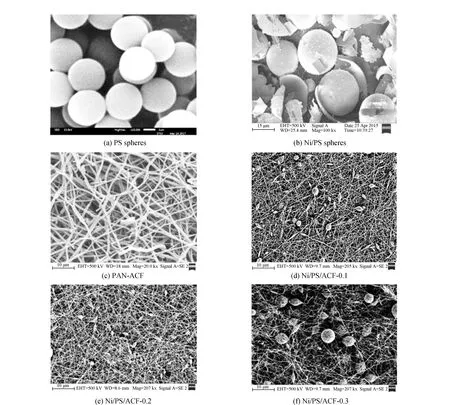
Fig.1 SEM images
To study the surface functional groups and chemical states of polyacrylonitrile fiber composites doped by nickel-plated polystyrene spheres, this section uses the CasaXPS peaking software to peak the elements of the fiber composite. The XPS full spectrum of Ni/PS/ACF composite materials is showed in Fig.2, carbon fiber composite material is composed of carbon, oxygen, and nickel, and the peak value of carbon is highest can be seen from the full XPS spectrum. After the carbon fiber composite is carbonized at high temperature in the tubular furnace, the nickel-plated polystyrene spheres in the composite material are decomposed by heat, leaving the nickel-metal coating as a hollow core-shell structure. After carbonization at high temperature, many oxygen-containing functional groups in carbon fiber composites are also burned off, so carbon is the main element in carbon fiber composites.

Fig.2 XPS full spectrum curve of PAN-based fiber composite
To further study the existence form of nickel ions in carbon fiber composite materials, we carried out peak fitting for nickel elements in the composite materials. It can be seen from Fig.3 that the peak of nickel elements appeared at the positions of 859.6 eV and 862.6 eV respectively, corresponding to Ni2p3/2and Ni2p. It can be concluded that the nickel element is mixed in carbon fiber composite material, which proves that the nickel element can be plated on the surface of polystyrene spheres by the electroless plating method. The reductive agent in the plating solution is used to provide electrons, so that the metal ions in the solution can be reduced to metal and deposited on the surface of the matrix, forming nickel-metal coating.

Fig.3 Ni2p and Ni2p3/2 peak fitting curve
The wide diffraction peak of the composite at 2θ=18.71°-26.5° corresponds to the surface of graphite microcrystalline (002),which can be seen in Fig.4, due to the doping of nickel-plated polystyrene spheres, the XRD patterns of the carbon fiber composites Ni/PS/ACF-0.2 and Ni/PS/ACF-0.3 show a small convex and narrow diffraction peak corresponding to the nickel (111) crystal surface around 2θ=43°, this indicates that Ni metal was doped into the carbon fiber composites of Ni/PS/ACF-0.2 and Ni/PS/ACF-0.3, and XRD spectra at 2θ=44° no characteristic peaks of graphite and this indicates that there are irregular graphitized structures in carbon fiber composites. Because the introduction of nickel-plated polystyrene spheres changed the structure of the fiber, the fiber appeared porous loose bead chain structure.
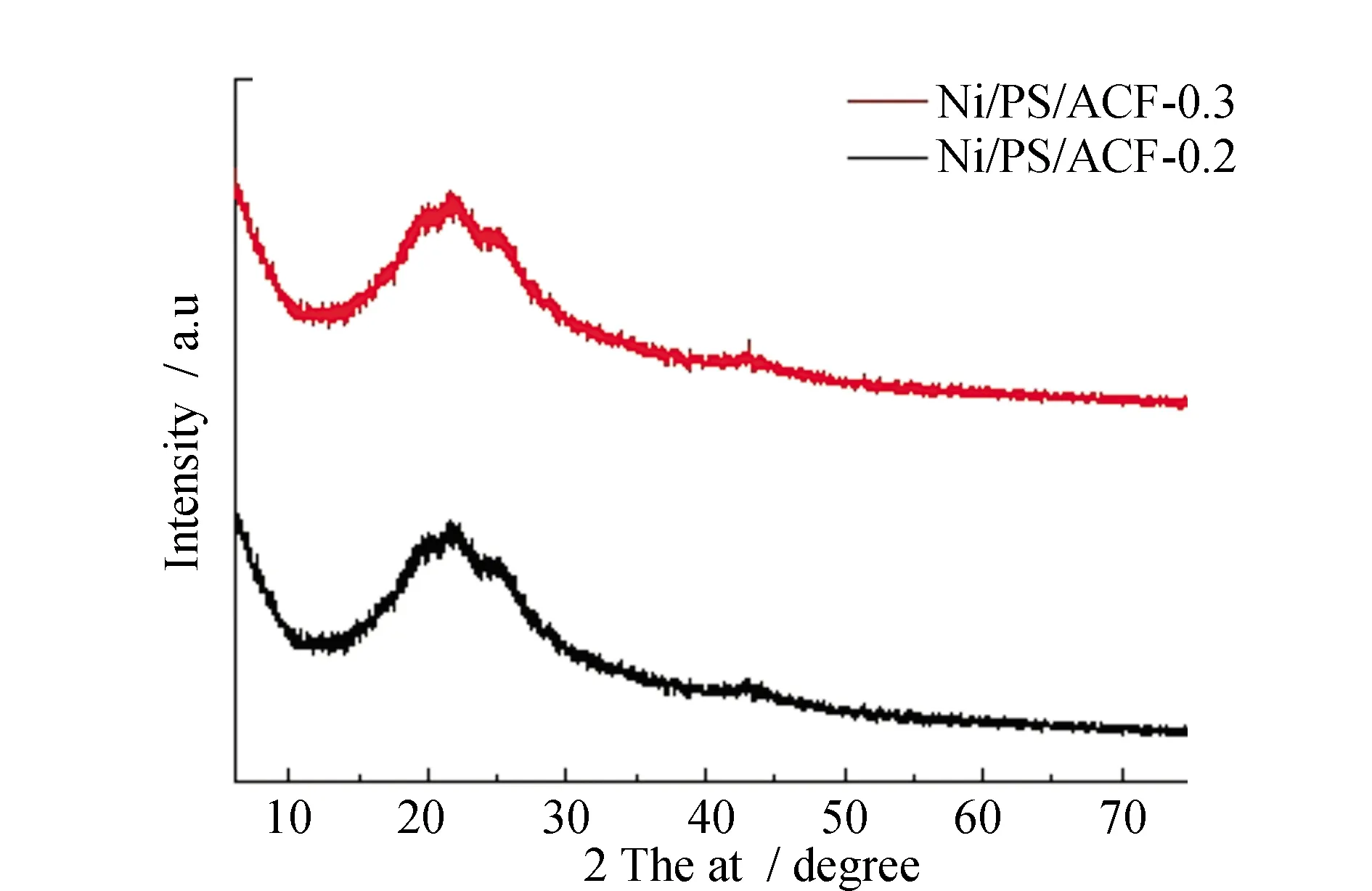
Fig.4 XRD patterns of Ni/PS/ACF-0.2 and Ni/PS/ACF-0.3
2.2 Electrochemical analysis
The cyclic voltammogram obtained by the Ni/PS/ACF fiber composite at a scanning rate of 5 mV/s is showed in Fig.5. As can be seen from Fig.5, all carbon fiber composite electrode materials show a very symmetric REDOX peak[25]. The carbon fiber composite materials as Ni/PS/ACF-0.1, Ni/PS/ACF-0.2, and Ni/PS/ACF-0.3 show good pseudocapacitance and have the characteristics of a pseudo capacitor. In general, the charging process of materials is the storage energy, it related to oxidation peak, while the discharge process of materials is the released energy, it related to reduction peak. Compared with Ni/PS/ACF-0.1 and Ni/PS/ACF-0.2 composites, Ni/PS/ACF-0.3 has the highest oxidation peak and the lowest reduction peak, indicating that Ni/PS/ACF-0.3 has the highest specific capacitance. The capacitance value of composites are higher than pure fiber because the nickel-plated polystyrene spheres doped into the fiber composite material, change the structure of the carbon fiber, left a nickel-metal hollow coating, forming porous loose type of bead chain structure, reduce the degree of crosslinking between the fiber and fiber, improve the utilization rate of the fiber specific surface area, the electrolyte ion fully to the interior materials, increase the specific capacitance values of carbon fiber composite materials. After calculation, when the scanning rate was 5 mV/s, the Ni/PS/ACF-0.1, Ni/PS/ACF-0.2, Ni/PS/ACF-0.3 were 842.4 F·g-1, 1 409 F·g-1, and 1 457.6 F·g-1, respectively.

Fig.5 Cyclic voltammetry curves of Ni/PS/ACF composites at 5 mV/s
The cyclic voltammogram curves of Ni/PS/ACF-0.3 at different scanning rates from 5~50 mV·s-1. As you can see from Fig.6, all curve presents the REDOX peak. In addition, the shape change was not obvious at a high scanning rate of 50 mV·s-1, and the oxidation peak and reduction peak did not move significantly, which indicated that the Ni/PS/ACF-0.3 composite material had good reversibility, and the diffusion and transfer of electrolyte ions in the fiber composites can still take place at a high sweep rate. The specific capacitance of Ni/PS/ACF-0.3 composites from 5~50 mV·s-1is 1 457.6 F·g-1, 704.2 F·g-1, 565.7 F·g-1and 379.52 F·g-1, respectively. This may be because the structure of the material itself, bead chain structure of the porous fibers with loose type, and with the nickel-metal coating, hollow holes of core-shell structure and hierarchical structure can make the electrolyte ion fully spread to the interior materials, complete ion transmission and electron transfer, to make the Ni/PS/ACF-0.3 composite material maintains a high specific capacitance at a higher scanning rate. The capacitance values of PS/ACF electrode materials doped with different contents of nickel-plated polystyrene spheres is showed in Table 1.
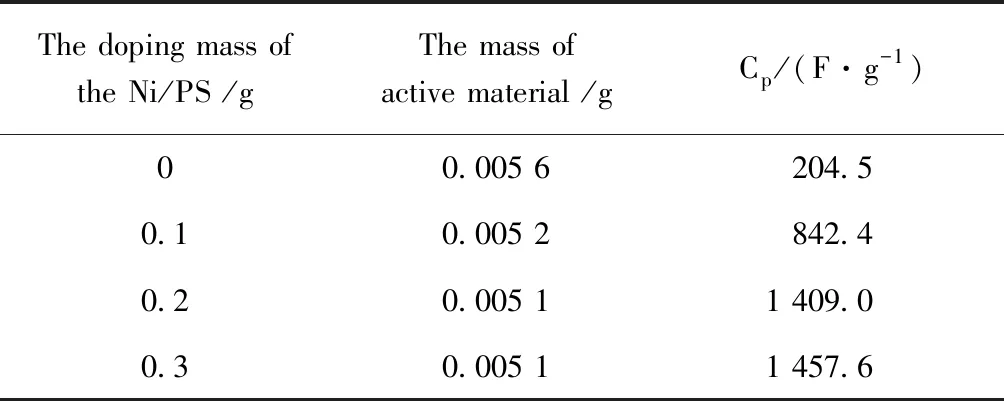
Table 1 Capacitance values of PS/ACF electrode materials doped with different contents of nickel plated polystyrene spheres
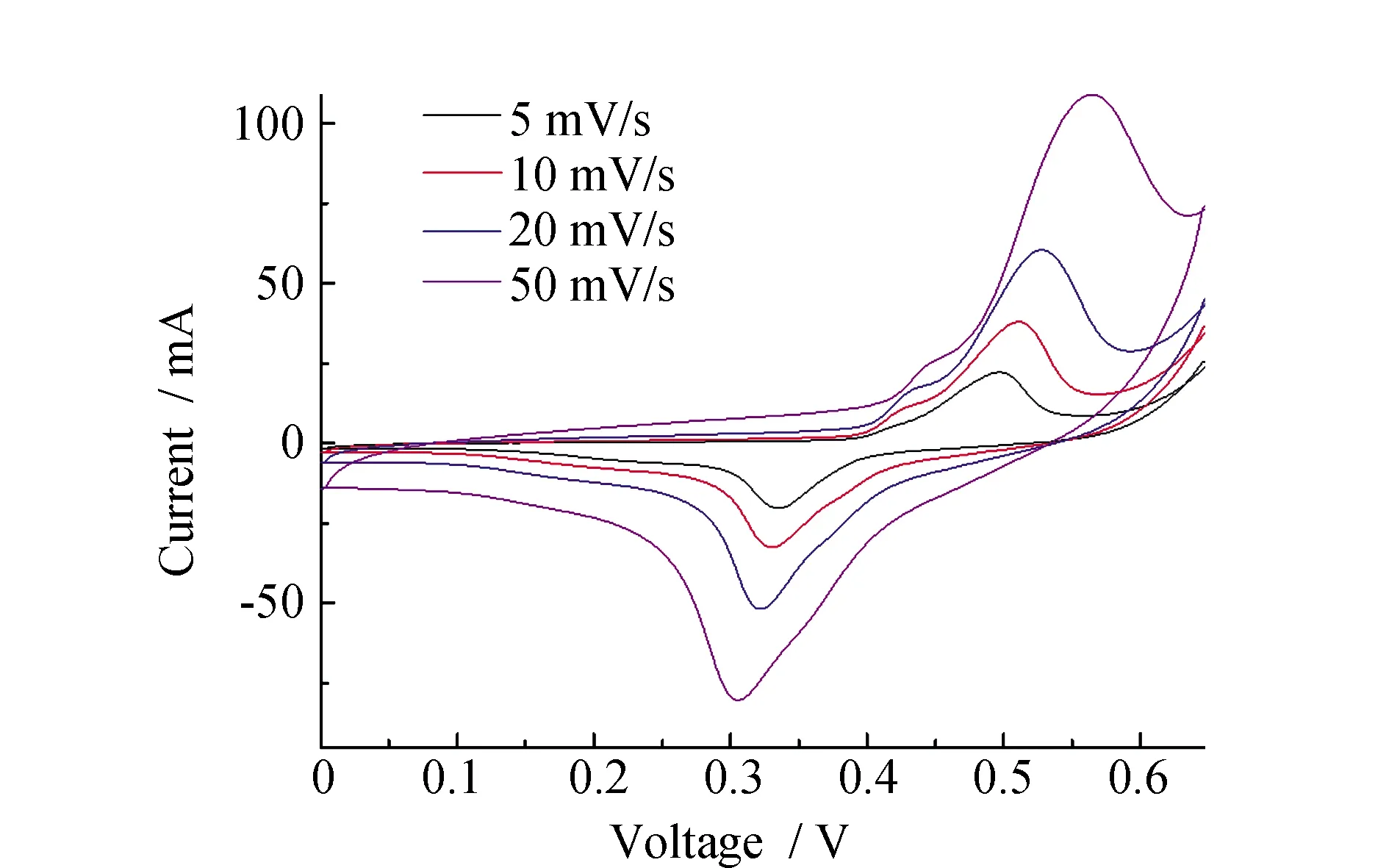
Fig.6 Cyclic voltammetry curves of Ni/PS/ACF-0.3 at different scanning rates
The constant current charge-discharge curves of asymmetric capacitor assembled with Ni/PS/ACF carbon fiber composite doped with different contents is showed in Fig.7 as the positive electrode material and activated carbon and graphite (9∶1) as the negative electrode material under the current density of 1 A·g-1, which can be seen from the Fig.7, and Ni/PS/ACF-0.1, Ni/PS/ACF-0.2, Ni/PS/ACF-0.3 there was no obvious drop, all curves appear concave irregularly. This is because the REDOX reaction between special structure of the electrode material and the electrolyte ions generated pseudo-capacitance, all curves show the same trend, Ni/PS/ACF-0.1 composites compared with Ni/PS/ACF-0.3 composite, the charging time of Ni/PS/ACF-0.1 and Ni/PS/ACF-0.3 materials is similar. The charging time of Ni/PS/ACF-0.2 composite materials is longer, but the discharge time of Ni/PS/ACF-0.1 and Ni/PS/ACF-0.2 compared with that of Ni/PS/ACF-0.3 composite materials is reduced. This indicates that the Ni/PS/ACF-0.3 composite has the minimum internal resistance, and its formed structure also promotes the diffusion of electrolyte ions.
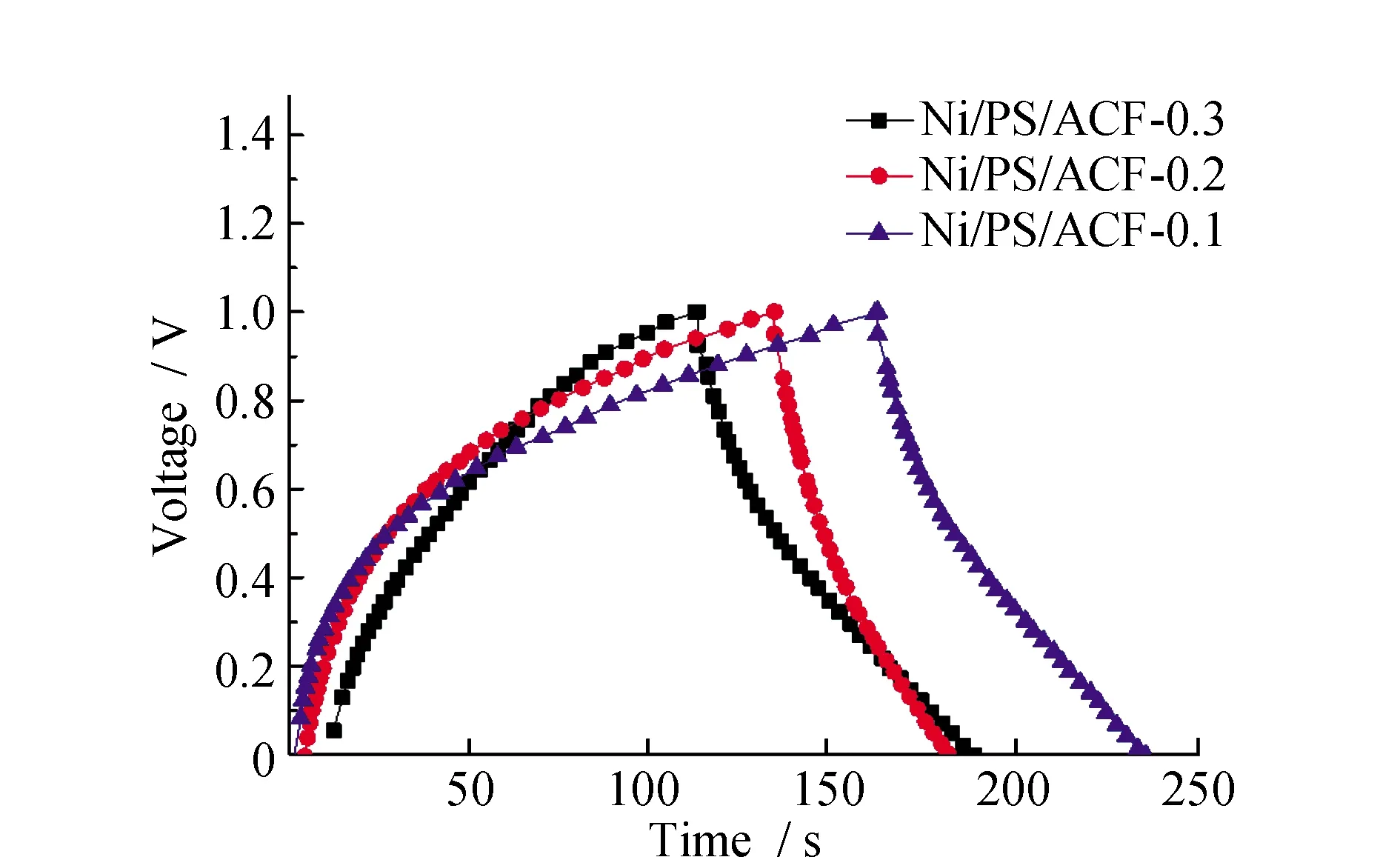
Fig.7 Constant current charge-discharge curves of Ni/PS/ACF-0.1, Ni/PS/ACF-0.2, and Ni/PS/ACF-0.3 composites under the current density of 1 A·g-1
Ni/PS/ACF-0.1, Ni/PS/ACF-0.2, and Ni/PS/ACF-0.3 have a unique pore structure that is conducive to the transmission of electrolyte ions. Constitute asymmetric capacitors, according to the specific capacitance calculation formula, the specific capacitance values at a current density of 1 A·g-1are 48 F·g-1, 60 F·g-1, and 72 F·g-1, respectively. Thus, the Ni/PS/ACF-0.3 composite material has the higher specific capacitance. The specific capacitance of the material is higher than that of PS/ACF composite because of the special structure of the nickel-metal coating left at high-temperature carbonization. Ni/PS/ACF-0.3 composite material under a different current density of constant current charge and discharge curve can be seen from the Fig.8, the material in different current density test, it is concluded that the constant current charge and discharge curves show roughly the same trend, no obvious drop, its internal resistance is small, the spread of the electrolyte ion fully to the interior of composite material. As shown in the Fig.8, the specific capacitance values of Ni/PS/ACF-0.3 composites at current densities of 1, 2, 3, 5, 10 A·g-1are respectively 72, 30, 20.2, 12, 6 F·g-1.

Fig.8 Constant current charge-discharge curves of Ni/PS/ACF-0.3 composites at different current densities
As you can see from Fig.9 impedance spectra, The curve consists of a half arc in the high frequency region and a straight line in the low frequency region, high-frequency semicircle diameter on behalf of the charge transfer resistance, is inherent resistance and materials and electrolyte interface contact resistance, it is related to the diffusion ability of ions at the interface between electrolyte and carbon fiber composite electrode materials. The high-frequency region of nickel-plated polystyrene spheres shows a linear curve. Because when nickel-plated polystyrene spheres doped into the polyacrylonitrile fiber, changed the structure of the fiber itself, after high-temperature carbonization, carbon fiber composite material to form a porous loose bead chain structure, increase the distance between the fiber and fiber, increase the porosity of fibers, specific surface area of the carbon fiber composite materials also increases, making the electrolyte ions are more likely to spread to the interior of the carbon fiber composite materials.
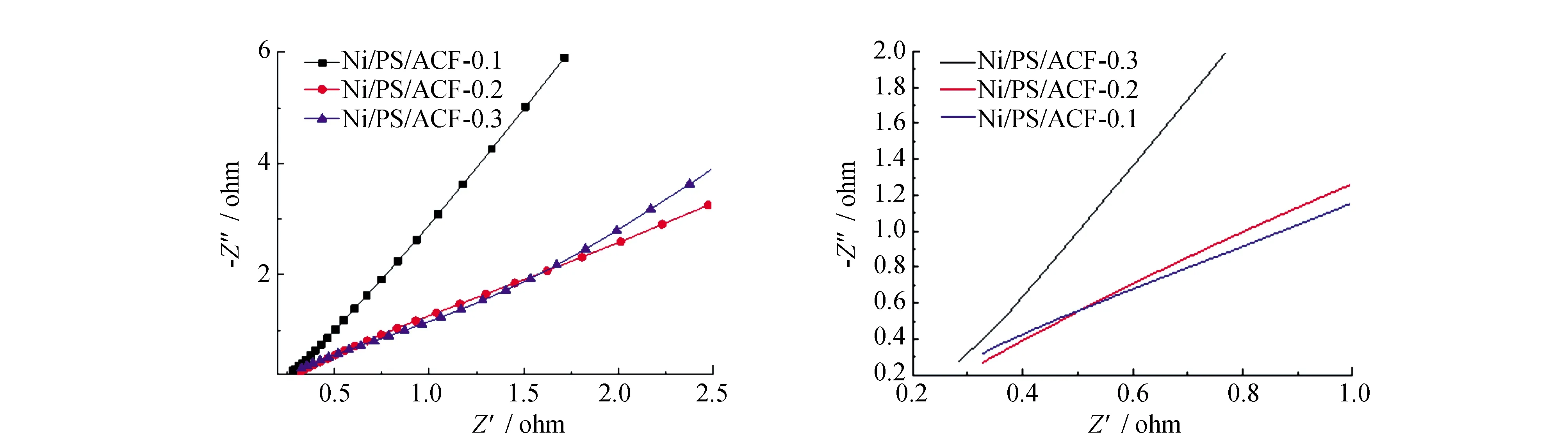
Fig.9 Ac impedance curves of Ni/PS/ACF composite material
In Fig.10, Ni/PS/ACF carbon fiber composite as the positive electrode material and activated carbon and graphite (9∶1) as the negative electrode material were assembled into the cyclic stability curve of an asymmetric capacitor with a current density of 1 A·g-1. It can be seen from the figure that after 5 000 times of constant current charging and discharging, the specific capacitance value of Ni/PS/ACF-0.3 composite material still retains 67.56 F·g-1, the initial value of specific capacitance is 72 F·g-1, and the retention rate of the specific capacitance value of the material is 93.85%, showing good cyclic stability. This is mainly because the nickel-plated polystyrene spheres doped into the fiber material formed a bead chain structure with holes, which greatly promoted the diffusion of electrolyte ions in the composite material, and the nickel-metal coating provided pseudocapacitor, thus improving the specific capacitance value and cycling stability of the material.
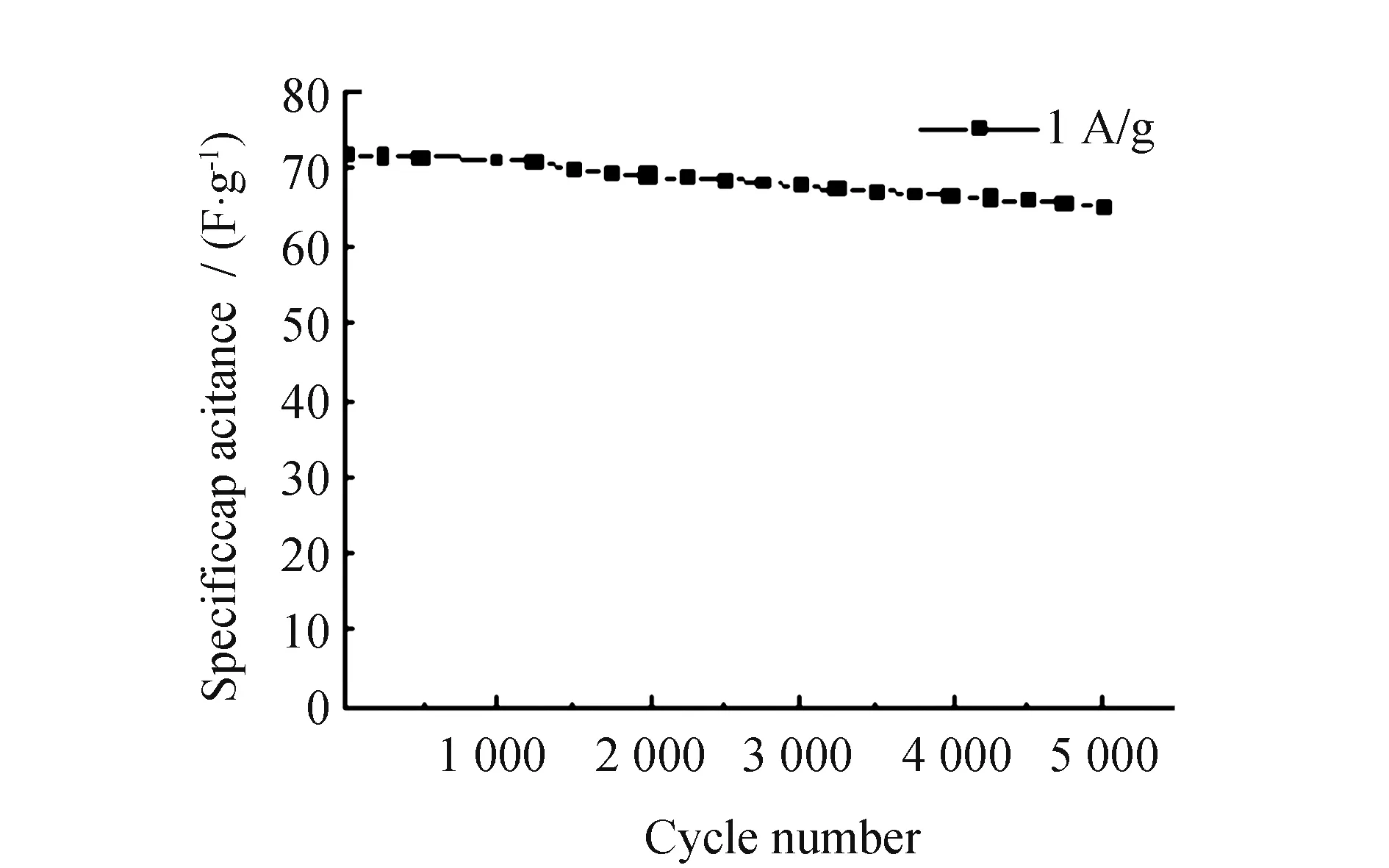
Fig.10 Cycle life test diagram of Ni/PS/ACF-0.3 composite materials
3 Conclusions
In summary, the nickel-plated polystyrene spheres were doped into the polyacrylonitrile spinning liquid during the preparation of the spinning liquid, and then the nickel-plated polystyrene spheres/polyacrylonitrile fiber composites were prepared by electrostatic spinning. Then Ni/PS/ACF composite materials were prepared by the stage heating preoxidation of the oven and the high-temperature carbonization of the tube furnace. Because in the interior of the fiber material with nickel-plated polystyrene spheres, thus changing the structure of the fiber, make originally crosslinking tubular fiber into unconsolidated porous bead chain structure, this kind of hierarchical hollow hole structure reduces the crosslinking degree of fiber, increase the distance between the fiber and the fiber, which increases the porosity between the fiber and fiber, improve the specific surface area of carbon fiber composites. The test results show that the Ni/PS/ACF-0.3 composite material shows hollow nickel-metal coating when the content of nickel-plated polystyrene spheres doping into polyacrylonitrile fiber is 0.3 g, forming a loose bead chain structure, which is distributed in the polyacrylonitrile fiber composite material. When the current density is 1 A·g-1, the specific capacitance value is as high as 72.0 F·g-1, and the specific capacitance retention rate of the device is 93.85% after 5 000 constant current charging and discharging cycles. Therefore, the Ni/PS/ACF composite material is expected to be the electrode material of supercapacitors in the future.
