Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation
2020-03-03LiZhungHiBoMouLnFngYuHengKiZhuZheYngQinLioShuSenZheng
Li Zhung , , # , Hi-Bo Mou , # , Ln-Fng Yu , Heng-Ki Zhu , Zhe Yng , Qin Lio , Shu-Sen Zheng , , *
a Zhejiang University School of Medicine, Hangzhou 310 0 0 0, China
b Department of Hepatobiliary Pancreatic Surgery, Shulan (Hangzhou) Hospital, Hangzhou 310022, China
c Department of Oncology, Shulan (Hangzhou) Hospital, Hangzhou 310022, China
d Division of Hepatobiliary and Pancreatic Surgery, Department of General Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
To the Editor:
Liver cancer is the fifth most common cancer and the second most frequent cause of cancer-related death globally. Hepatocel- lular carcinoma (HCC) accounts for 90% of primary liver cancers with the highest incidence in China (more than 50% of all cases worldwide) [1] . Liver transplantation (LT) is regarded as an opti- mal therapy for selected HCC patients. The Milan criteria are the benchmark for candidate selection that ensure excellent progno- sis for patients with HCC [2] . The Hangzhou criteria expand 51.5% more of Milan criteria for LT candidates with comparable post- transplant survivals [3] . However, LT recipients fulfilling Milan cri- teria or Hangzhou criteria are at the risk of up to 13% -18% HCC recurrence rate within five years [4] . Only 25% -50% of recurrent HCC patients post-LT are eligible for surgical treatment which have consistently presented favored survival benefit than systemic ther- apy [5] . Therefore, it worth exploring alternative treatments such as chemotherapy, targeted therapy and recently developed im- mune checkpoint inhibitor (ICI) treatment. Through blocking pro- grammed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) sig- naling pathway and restoring T cell activities, ICIs are promising in the treatment of many types of cancers [6] . Despite the eager demand for effective HCC treatment options, the present opinion considers immunosuppression a contraindication for ICIs in LT re- cipients, whom otherwise would be exposed to great risk of or- gan rejection. Here, we report a case of the longest survival benefit achieved by using ICIs in an immunosuppressed LT recipient with recurrent HCC.
A 54-year-old Chinese male was diagnosed as hepatitis B virus related decompensated cirrhosis and large recurrent HCC (10 cm diameter and portal vein tumor thrombosis) in December 2014 after hepatectomy and transcatheter arterial chemoembolization (TACE). In February 2015, he received orthotopic liver transplan- tation and individualized low-dose tacrolimus immunosuppressive protocol. In August 2015, he received video-assisted thoracoscopic wedge resection for 1.5 cm right pulmonary metastasis. Subse- quently, he received sorafenib, 3 times of mFolfox-6 chemother- apy, one gemcitabine plus S-1 therapy and one pulmonary TACE. All of these failed to preclude the progression of bilateral lung metastases. The serum level of AFP was increased to 680 ng/mL in October 2017. ICI was regarded as a salvage option in this situation for the patient. The patient and his family thoroughly understood the potential risks of an off-label therapy with ICIs, including graft rejection and fatal liver failure. The patient was given nivolumab injection 200 mg every 2 weeks for 31 times between October 2017 and April 2019. The first 12 cycles were nivolumab monotherapy and concomitant target drugs since 13rd cycle include regorafenib, anlotinib, apatinib and lenvatinib. Imag- ing showed stable pulmonary metastases. Progression of bone was observed in February, 2019. He passed away 3 weeks after brain metastasis in May 2019. The progression-free survival and over- all survival were 18 months and 20 months, respectively. During the course of immunotherapy, he was continued on tacrolimus for immunosuppression (trough level, 4-6 ng/mL) without steroids and mycophenolate mofetil. There was no severe adverse effect or rejection.
The PD-1/PD-L1 is a receptor-ligand system that plays an im- portant role not only in tumor progression but also in tolerance to solid organ transplantations. Rejection following PD-1/PD-L1 block- ade is associated with activation of cellular immunity through CD8 + effector cells and downregulation of regulatory T cells [7] . The application of immune suppression drugs conflict to the effi- cacy of ICIs whose effect requires an intact T cell response. In the other way, the reinvigoration of effector T cell by blocking PD-1 signal pathway probably will cause the life threatening organ re- jection.
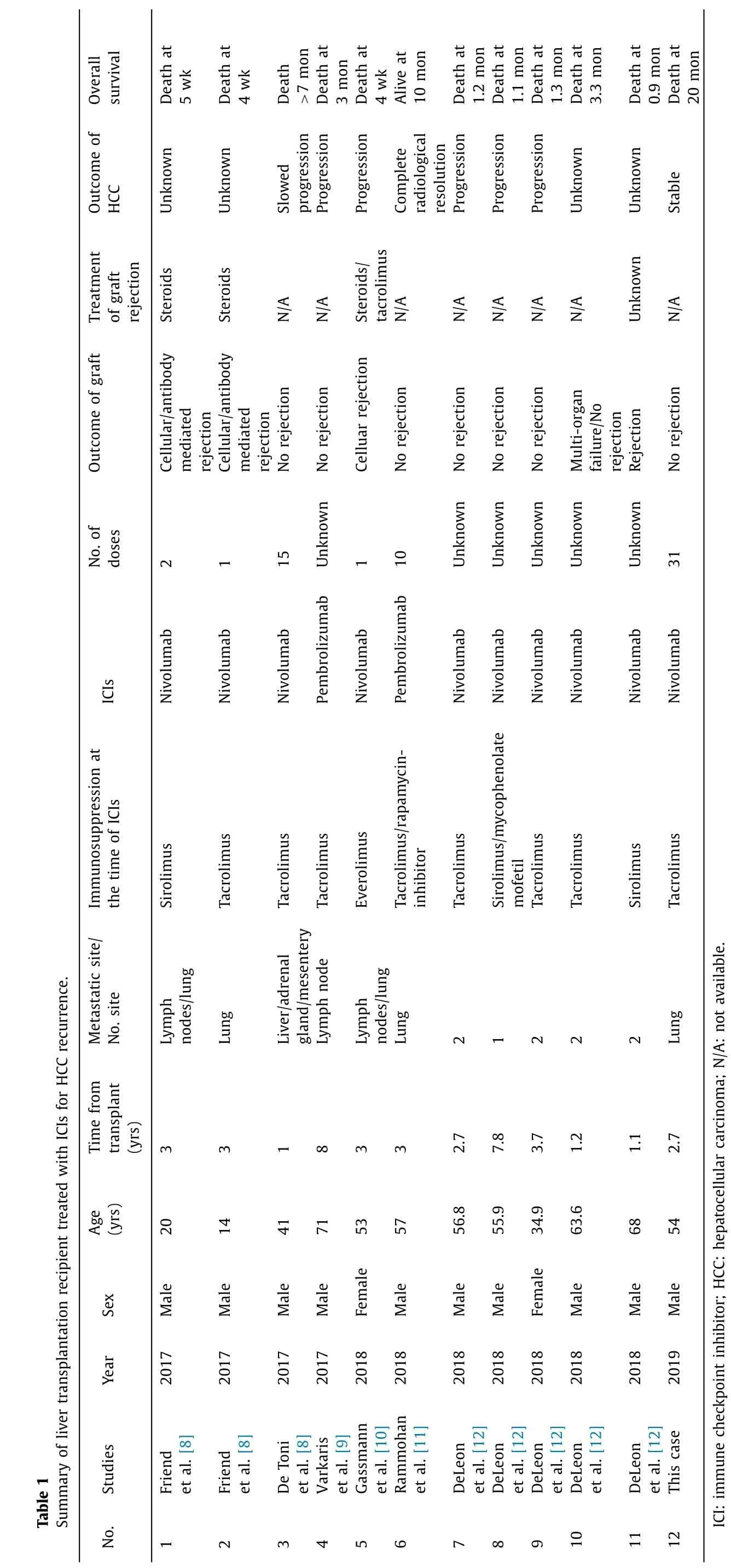
Overall survival Outcome of HCC Treatment of graft rejection Outcome of graft No. of doses ICIs Immunosuppression at the time of ICIs Summary of liver transplantation recipient treated with ICIs for HCC recurrence. Metastatic site/ No. site Time from transplant (yrs) Age (yrs) Sex Year Table 1 Studies No. Death at 5 wk Death at 4 wk Death > 7 mon Death at 3 mon Death at 4 wk Alive at 10 mon Death at 1.2 mon Death at 1.1 mon Death at 1.3 mon Death at 3.3 mon Death at 0.9 mon Death at 20 mon Unknown Unknown Slowed progression Progression Progression Complete radiological resolution Progression Progression Progression Unknown Unknown Stable Steroids Steroids N/A N/A Steroids/ tacrolimus N/A N/A N/A N/A N/A Unknown N/A Cellular/antibody mediated rejection Cellular/antibody mediated rejection No rejection No rejection Celluar rejection No rejection No rejection No rejection No rejection Multi-organ failure/No rejection Rejection No rejection 2 1 15 Unknown 1 10 Unknown Unknown Unknown Unknown Unknown 31 Nivolumab Nivolumab Nivolumab Pembrolizumab Nivolumab Pembrolizumab Nivolumab Nivolumab Nivolumab Nivolumab Nivolumab Nivolumab Sirolimus Tacrolimus Tacrolimus Tacrolimus Everolimus Tacrolimus/rapamycin- inhibitor Tacrolimus Sirolimus/mycophenolate mofetil Tacrolimus Tacrolimus Sirolimus Tacrolimus Lymph nodes/lung Lung Liver/adrenal gland/mesentery Lymph node Lymph nodes/lung Lung 2 1 2 2 2 Lung 3 3 1 8 3 3 2.7 7.8 3.7 1.2 1.1 2.7 20 14 41 71 53 57 56.8 55.9 34.9 63.6 68 54 Male Male Male Male Female Male Male Male Female Male Male Male 2017 2017 2017 2017 2018 2018 2018 2018 2018 2018 2018 2019 Friend et al. [8] Friend et al. [8] De Toni et al. [8] Varkaris et al. [9] Gassmann et al. [10] Rammohan et al. [11] DeLeon et al. [12] DeLeon et al. [12] DeLeon et al. [12] DeLeon et al. [12] DeLeon et al. [12] This case ICI: immune checkpoint inhibitor; HCC: hepatocellular carcinoma; N/A: not available. 1 2 3 4 5 6 7 8 9 10 11 12
In previous reports, graft rejection occurred in 4 out of 11 LT re- cipients (No. 1, 2, 5, 11) within 4 weeks after the initiation of anti- PD-1 antibody therapy for advanced recurrent HCC which eventu- ally led to lethal outcome ( Table 1 ) [8-13] . Meanwhile, only 1 case (No. 6) achieved complete radiological resolution and at least 10 months survival, and 1 case (No. 3) assumed to slow the tumor progression. The overall survival of other 5 cases (No. 4, 7, 8, 9, 10) ranged from 1.1 months to 3.3 months. Since definitive conclu- sions could not been drawn from the small size of patient cohort and limited medication experience, the pros and cons of ICI ap- plication to LT recipients on immunosuppression are controversial. Currently, there is no certain method to keep balance between ac- tive T cells recognizing tumor neoantigens and avoiding rejection. Patients shall be kept under close monitoring and timely interven- tion in case of acute rejection.
Our case demonstrates the positive effect of anti-PD-1 antibody treatment on HCC recurrence after LT. To the best of our knowl- edge, this case has demonstrated the longest sustained ICI cycles and obtained the longest survival without severe adverse effect or rejection. In conclusion, liver transplantation recipients could ben- efit from ICI treatment after the failure of conventional treatment with close follow-up to avoid organ rejection.
CRediT authorship contribution statement
Li Zhuang:Conceptualization, Writing - original draft.Hai-Bo Mou:Conceptualization, Writing - original draft.Lan-Fang Yu:Conceptualization, Writing - review & editing.Heng-Kai Zhu:Con- ceptualization, Writing - review & editing.Zhe Yang:Conceptual- ization, Writing - review & editing.Qin Liao:Conceptualization, Writing - review & editing.Shu-Sen Zheng:Conceptualization, Funding acquisition, Writing - review & editing.
Funding
This study was supported by grants from the National Science and Technology Major Project ( 2017ZX10203201 ) and Health Com- mission of Zhejiang Province (2016KYA073 and 2019KY540 ).
Ethical approval
This study was approved by Shulan (Hangzhou) Hospital In- stitutional Review Board and the informed consent was obtained from the relatives of the patient.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub- ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- MEETINGS AND COURSES
- Living-donor liver transplantation for patients with hepatocellular carcinoma in Japan: Current situations and challenge
- Clinicopathological features and prognosis of surgical resected cases of biliary cancer with pancreaticobiliary maljunction
- Deliberate external pancreatic fistula after pancreaticoduodenectomy performed in the setting of acute pancreatitis, and its internalization through fistula-jejunostomy
- Hypothermic oxygenated perfusion for a steatotic liver graft
- Postoperative negative-pressure drainage through a PEG tube can prevent pancreatic fistula after pancreatoduodenectomy
