Hypothermic oxygenated perfusion for a steatotic liver graft
2020-03-03QuirinoLiFrncoRuertoFioMelndroZoeLrghiLureiroMssimoRossiGinlucMennini
Quirino Li , , Frnco Ruerto , Fio Melndro , Zoe Lrghi Lureiro , Mssimo Rossi , Ginluc Mennini
a Department of General Surgery and Organ Transplantation, Sapienza University of Rome, Rome, Italy
b Department of Anaesthesiology, Critical Care Medicine and Pain Therapy, Sapienza University of Rome, Rome, Italy
To the Editor:
Donor steatosis represents a well-known risk factor for primary non-function, early allograft dysfunction, and biliary complications after liver transplantation (LT) [1,2] . Recently, machine perfusion (MP) technology has been implemented in the clinical practice, with the primary intent to assess the graft quality and to optimize the organ selection process [3] . A limited number of articles has been published specifically investigated the role of MP in steatotic livers [4-10] , with few of them looking at the role of hypothermic MP.
We report our initial clinical experience on the use of a hy- pothermic MP for reconditioning a steatotic liver for LT. We pre- liminarily obtained the approval from our Local Ethical Committee for using MP technology in the setting of solid organ transplanta- tion (Ref. 50 0 0, 24/05/2018).
A liver graft offer from a 65-year-old Caucasian deceased-brain donor was accepted for transplantation by our center. The cause of death was blunt trauma. The donor body mass index was 30 kg/m2. During the procurement, the liver was described as steatotic. A core biopsy showed a moderate macro- and microvesicular steato- sis (both 40%), mild fibrosis (F1), moderate cholestasis, and no signs of inflammation, necrosis or arteriolosclerosis. After standard organ procurement, the graft was transported to our center us- ing static cold storage. Hypothermic oxygenated perfusion (HOPE) was started immediately after the bench surgery. Cold ischemic time before HOPE was 330 min. Perfusion with Belzer perfusion machine fluid at 10 °C using the Liver Assist device (OrganAssist, Groningen, the Netherlands) was done according to the HOPE pro- tocol. The portal vein was cannulated and perfused using a non- pulsatile low-pressure flow. Immediately after the beginning of the perfusion, the graft showed homogeneous perfusion. Portal pres- sure was stably maintained at 3-4 mmHg during the entire pro- cedure. Portal venous flow rate passed from 280 to 500 mL/min after the first perfusion hour: the last value was 500 mL/min. To- tal HOPE duration was 170 min, while the cumulative time from donor cross-clamp to graft reperfusion in the recipient was 9.2 h.
The selected recipient was a 45-year-old male with HCV-related cirrhosis complicated by hepatocellular cancer. MELD score was 16. Informed consent was preoperatively obtained. The total operative time was 390 min, with two units of packed red cells and ten units of fresh frozen plasma required during the operation. Reperfusion was homogeneous. The different perfusion phases are shown in Fig. 1 . No evidence of post-reperfusion syndrome or fibrinolysis was observed. Postoperative course was uneventful. The peak post- transplant aspartate aminotransferase (AST), alanine aminotrans- ferase (ALT) were 1013 IU/L (LT day) and 605 IU/L (postoperative day 2), respectively. International normalized ratio (INR) and total bilirubin raised to 1.82 (LT day) and 17.5 mg/dL (postoperative day 10), rapidly returning to average values ( Fig. 2 ). Intensive care unit stay was five days. The patient was discharged home on postop- erative day 16, with total bilirubin values of 2.7 mg/dL. After six months of follow-up, the recipient has normal liver and kidney function, without any evidence of intrahepatic biliary ischemia.
According to the results of the small series reported in the lit- erature, the use of MP consents to obtain excellent results, also in the case of a hypothermic approach. Our results are in line with the previous literatures [4-10] . More studies explicitly investigat- ing the relationship between MP and steatosis are needed.
CRediT authorship contribution statement
Quirino Lai:Conceptualization, Formal analysis, Investigation, Software, Writing - original draft, Writing - review & editing.Franco Ruberto:Data curation, Methodology, Writing - review & editing.Fabio Melandro:Data curation, Methodology, Writing - re- view & editing.Zoe Larghi Laureiro:Data curation, Methodology, Writing - review & editing.Massimo Rossi:Project administration, Resources, Supervision, Visualization, Writing - review & editing.Gianluca Mennini:Supervision, Validation, Visualization, Writing - review & editing.
Funding
None.
Ethical approval
This study was approved by our Local Ethical Committee (Ref. 50 0 0, 24/05/2018). Consent for publication was obtained from the patient.
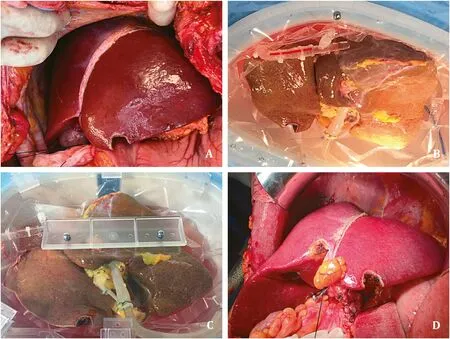
Fig. 1. A: Donor liver during procurement; B: Beginning of the HOPE procedure; C: Parenchymal perfusion during HOPE; D: Homogeneous reperfusion after liver transplan- tation.

Fig. 2. Blood test analyses after LT and during the first 12 postoperative days. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpep- tidase (GGT), and alkaline phosphatases (ALP) were reported in IU/L. Total bilirubin, direct bilirubin, and serum creatinine were reported in mg/dL. INR: international nor- malized ratio; POD: postoperative day.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the sub- ject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- MEETINGS AND COURSES
- Living-donor liver transplantation for patients with hepatocellular carcinoma in Japan: Current situations and challenge
- Clinicopathological features and prognosis of surgical resected cases of biliary cancer with pancreaticobiliary maljunction
- Deliberate external pancreatic fistula after pancreaticoduodenectomy performed in the setting of acute pancreatitis, and its internalization through fistula-jejunostomy
- Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation
- Postoperative negative-pressure drainage through a PEG tube can prevent pancreatic fistula after pancreatoduodenectomy
