Determination of L-norvaline and L-tryptophan in dietary supplements by nano-LC using an O-[2-(methacryloyloxy)-ethylcarbamoyl]-10,11-dihydroquinidine-silica hybrid monolithic column
2020-02-28DongshengXuElenanchezpezQiqinWangZhengjinJiangMarLuisaMarina
Dongsheng Xu ,Elena Sánchez-López ,Qiqin Wang ,Zhengjin Jiang ,**,María Luisa Marina ,*
a Departamento de Química Analítica,Química Física e Ingeniería Química,Universidad de Alcalá,Ctra.Madrid-Barcelona Km.33.600,28871,Alcalá de Henares(Madrid),Spain
b Institute of Pharmaceutical Analysis,College of Pharmacy,Jinan University,Guangzhou,510632,China
c Department of Pharmacy and Guangdong Province Key Laboratory of Pharmacodynamic Constituents of Traditional Chinese Medicine & New Drug Research,Jinan University,Guangzhou,510632,China
d Instituto de Investigación Química“Andrés M.del Río”(IQAR),Universidad de Alcalá,Ctra.Madrid-Barcelona Km.33.600,28871,Alcalá de Henares(Madrid),Spain
Keywords:
ABSTRACT
1.Introduction
Amino acids are essential and ubiquitous compounds playing a vital role in many living organisms.Differences in the properties of D-and L-enantiomers have been widely reported,both for protein and non-protein amino acids[1].For instance,the L-enantiomer of the non-protein amino acid norvaline enhances the nitric oxide production,which is an important regulator and mediator in physiological and pathophysiological events such as vasodilatation[2].Moreover,it can be used to treat artificial metabolic syndrome in a rat model[3],and it has been proven to be effective in Alzheimer’s disease(AD)[4,5].However,to the best of our knowledge,D-norvaline has not been reported as an effective substance.On the other hand,L-tryptophan is a protein amino acid which is the precursor of important neurotransmitters,hormones and other relevant biomolecules[6].The level of L-tryptophan in humans has been reported to act as a biomarker of certain diseases[7,8].At the pharmacological level,L-tryptophan is used as antidepressant agent[9],whereas D-tryptophan is considered as an impurity because of its low biological activity[10,11].In addition,only the L-enantiomer of tryptophan is involved in the synthesis of proteins and,additionally,it can cross the blood-brain,being the precursor of the important neurotransmitter serotonin[12].Amino acids can be used as ingredients in dietary supplements where the presence of the D-enantiomer is not allowed by legal regulations[13].Therefore,the enantiomeric determination of DL-amino acids in real samples remains a very important challenge.
In order to obtain satisfactory chiral separation of amino acids,various chiral stationary phases(CSPs)have been developed,including those based on cyclodextrins[14],polysaccharides[15],3,5-dinitrobenzamide-naphthylglycine derivatives[16],macrocyclic antibiotics[17]and cinchona alkaloids[18-21].Cinchona alkaloids,especially quinine and quinidine have attracted much attention owing to their excellent enantioselectivity for amino acids.Usually,because of the low detection sensitivity and weak interaction between the chiralselectorandanalytes,different derivatization groups were used to react with the amino acids,such as 4-fluoro-7-nitro-2,1,3-benzoxadiazole(NBD-F)[22-24],9-fluorenylmethoxycarbonyl(FMOC)fluoride[25,26],2,4-dinitrofluorobenzene(DNFB)[27],dansyl chloride(DNS-Cl)[28],and carbazole-9-carbonyl chloride(CC-Cl)[18].Among the different derivatization reagents,FMOC not onlyallows the amino acids to achieve satisfactory enantioresolution,but also has the advantage of reacting with primary and secondary amines in just 3 min.
Over the past several decades,monolithic columns have exhibited high separation efficiency and sensitivity,low sample and elution solvent consumption,as well as the ease of coupling to MS[29-32].So far,the use of quinine or quinidine functionalized monolithic columns has been reported in the enantioseparation of amino acids in capillary electrochromatography(CEC)and nanoliquid chromatography(nano-LC).For instance,Lämmerhofer et al.[18,33]separated some N-derivatized amino acid standards with high efficiency and enantioresolution on the quinine and quinidine silica-based monolithic column by CEC.On the other hand,Wang et al.[25,29]developed several quinidine polymerbased monolithic columns which were also successful in enantioseparating N-derivatized amino acid standards by nano-LC.However,the silica or polymer based monolithic column was limited by the cumbersome and multi-step preparation method,or low mechanical strength and poor stability.On the other hand,they focused on the development of the novel monolithic columns,but their application in the real sample was not reported.
Silica-hybrid monolithic columns offer great advantages such as high surface,little shrinkage,excellent pH stability and good permeability[34-36].However,they have scarcely been employed for the enantioseparation of amino acids.Thus,Tran et al.[19]only separated dinitrobenzoyl-leucine on the quinidine-silica/zirconia hybrid monolithic column by CEC and Kato et al.[37]tried to enantioseparate DL-tryptophan on a new-type bovine serum albumin(BSA)-encapsulated hybrid monolithic column.Unfortunately,the enantioresolution was very limited.In a previous work,our research group prepared an O-[2-(methacryloyloxy)-ethylcarbamoyl]-10,11-dihydroquinidine(MQD)-silica hybrid monolithic column and the enantiomeric separation of protein and nonprotein amino acids was investigated using up to 8 different derivatization reagents including 3,5-dinitrobenzoyl chloride,3,5-dichlorobenzoyl chloride, p-nitrobenzoyl chloride, 3,5-dimethoxybenzoyl chloride,m-chlorobenzoyl chloride,p-chlorobenzoyl chloride,benzoyl chloride,and FMOC chloride[38].Nevertheless,from the 8 FMOC-amino acids tested,only 2 of them were baseline separated in the reversed phase mode(valine(Rs 1.55)and isoleucine(Rs 1.71))and none of them was baseline separated in the polar organic phase mode[38].Moreover,the applicability of quinidine silica-hybrid monolithic column to the quantitative analysis of amino acids in real samples has not been demonstrated yet.In this work,the enantiomeric separation of protein and non-protein FMOC derivatized amino acids was achieved by nano-LC using the above-mentioned MQD-silica hybrid monolithic column.In order to obtain satisfactory enantioresolution performance in the reversed phased mode,the mobile phase was systematically optimized,including the apparent pH,content of ACN,and buffer concentration.Under the optimized conditions,27 FMOC-derivatized amino acids consisting of 19 protein and 8 non-protein amino acids were tested.Finally,the analytical characteristics of the developed method were evaluated for norvaline and tryptophan and the method was applied to the quantitation of L-norvaline and L-tryptophan in dietary supplements.
2.Materials and methods
2.1.Reagents and samples
All reagents were of analytical grade.Methanol(MeOH),acetonitrile(ACN),and acetic acid(HAc)were acquired from Scharlau Chemie(Barcelona,Spain).Triethylamine(TEA)and 9-fluorenylmethoxycarbonyl(FMOC)chloride were obtained from Fluka(Buchs,Switzerland).Ammonium hydroxide(NH3·H2O),boric acid(H3BO3)and pentane were from Sigma(St.Louis,Missouri,USA),and ammonium acetate was from Merck(Darmstadt,Germany).10,11-dihydroquinidine,2,2’-azobisisobutyronitrile(AIBN),3-(trimethoxysilyl)-propylmethacrylate(γ-MAPS),vinyltrimethoxysilane(VTMS),cetyltrimethylammonium bromide(CTAB),tetramethoxysilane(TMOS)and ethylene glycol(EG)were acquired from Aladdin Chemicals(Shanghai,China).DLarginine,DL-histidine,DL-lysine,DL-serine,DL-threonine,DL-asparagine,DL-glutamine,DL-cysteine,DL-proline,DL-alanine,DL-valine,DL-leucine,DL-methionine,DL-phenylalanine,DL-tyrosine,D-tryptophan,L-tryptophan,DL-ornithine,DL-citrulline standards were from Fluka(Buchs,Switzerland),while DL-isoleucine,DL-carnitine,DL-aspartic acid,DL-glutamic acid,DL-norvaline,L-norvaline,DLnorleucine,DL-DOPA,DL-pyroglutamic acid and DL-methionine sulfone were obtained from Sigma(St.Louis,Missouri,USA).FMOC-amino acids were synthesized as reported previously[29,30].The dietary supplements were obtained in capsule form from online sources.
2.2.Instrumentation
All nano-LC experiments were conducted on a laboratory selfassembled nano-LC instrument.The system consisted of a Shimadzu LC-20AD pump(Kyoto,Japan),a Linear Instruments UV-Vis 200 detector(California,USA),and a Valco four-port injection valve with 20 nL internal loop(Houston,USA).In order to reduce the flow and pressure,a stainless-steel tee(Cheminert,Valco Instruments Houston,Texas,USA)with flow split capillary(150 mm×25μm I.D.)was employed before the injection valve.The data acquisition and data handling were performed using the software Chromatostation N200(Zhejiang University,China).All chromatograms were converted to a text file and redrawn using Microcal Origin 8.5.pH values of buffer solutions were measured in a 744 pH meter(Herisau,Switzerland).
2.3.Chromatographic conditions
Mobile phase was prepared by mixing the 3 mM ammonium acetate solution with ACN(35/65,v/v),and adjusting the apparent pH to the desired value(pH 4.8)with acetic acid.In the beginning,the mobile phase was subjected to filtration through a 0.22μm membrane and sonication degas prior to be employed.The total flow rate was 10μL/min,total backpressure was 23 bar,injection volume was 20 nL,and the UV detection wavelength was 254 nm.
2.4.Preparation of the MQD-silica hybrid monolithic column
Synthesis of the MQD-silica hybrid monolithic column was conducted as previously reported[38].Briefly,anchoring sites for the bulk polymer on the inner capillary walls were generated using γ-MAPS/MeOH(50/50,v/v).The pre-polymerizable mix for the MQD column was prepared as follows:MQD(6.0 mg),MeOH(100μL),EG(30μL),CTAB(1.6 mg),H2O(30μL),NH3·H2O(0.02 M,30μL),TMOS(60μL),VTMS(80μL),and AIBN(1 mg)were mixed in a 2mL vial and sonicated during 5 min at room temperature.The obtained homogeneous solution was introduced into the 30 cm pre-treated capillary.Both ends of the capillary column were sealed using GC septa and capillary was placed in a water bath at 40℃ during 12 h first,then at 60℃ for another 12 h.Non-reacted CTAB and other waste products were rinsed out by flushing the column with MeOH.The monolithic column was cut to 15 cm for further use.
(设计意图:“智慧珠”的“胚珠”颜色各异、数量多样、形状不同,丰富的结构势必会刺激学生的思维起点。分类材料的结构直接影响了学生的思维。实验表明,三、四年级学生能够较好地进行“胚珠”分类。这个年龄阶段的学生的形象思维、抽象逻辑思维能力的发展已经形成。学生只有对思维对象的属性进行全面了解,才能促成其思维的广阔性和灵活性,使他们可能进行多种组合分析的分类。思维需要“静观”才能“深虑”,通过“近思”才能“远谋”。以静态的方式呈现智慧珠,有利于学生冷静地思考,理性地分析。关注“智慧珠”的类别不同,有利于学生分类、对应、数形等思想的形成,为实践操作打下理性的思维基础。)
2.5.Preparation of the standard and dietary supplements solutions
FMOC-amino acids standard solutions were prepared by dissolving the corresponding amino acid standards into 1 mL of 200 mM H3BO3(pH 9.0)to obtain a 10 mM solution,and weresonicated for 2 min.Then,1 mL of a 220 mM FMOC-Cl solution in acetonitrile was added and it was made to react at room temperature for 3 min.Afterwards,2 mL of pentane was added into the solution and it was vortexed.After 5 min of resting,the lower layer solution was diluted at the desired concentration using the mobile phase.
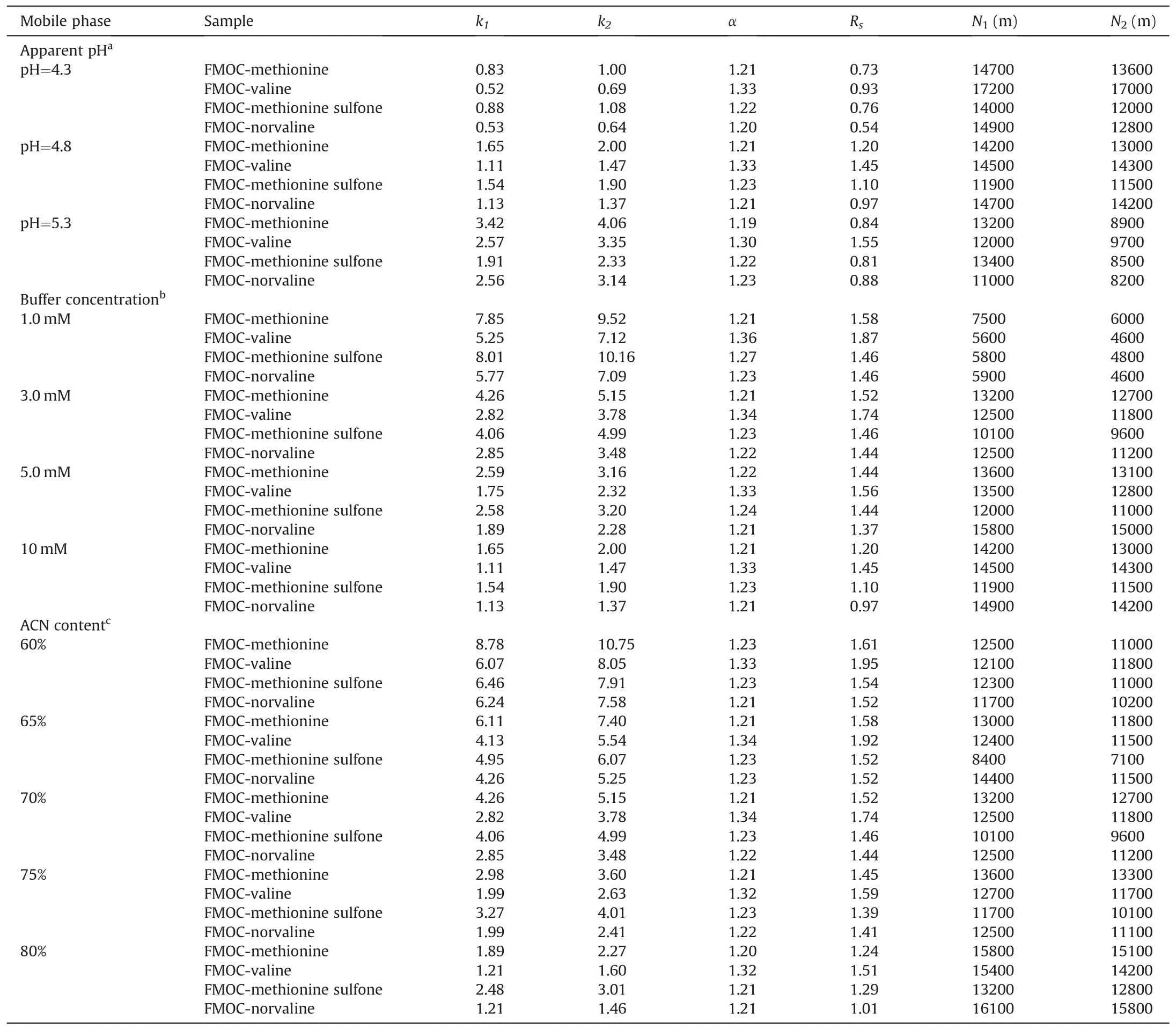
Table 1Effect of the mobile phase composition and pH on the enantioseparation data for FMOC derivatized amino acids.
FMOC-dietary supplement solutions were prepared by dissolving the powder within the capsules(200 mg norvaline or 2 mg tryptophan)into 1 mL of 200 mM H3BO3(pH 9.0)and were sonicated for 2 min,then they were filtered and 1 mL of 220 mM FMOCCl in acetonitrile was then added into the filtrate.Reaction took place for 3 min at room temperature.Afterwards,2 mL of pentane was added into the solution and it was vortexed.Finally,after 5 min of resting,the lower layer solution was diluted at the desired concentration using the mobile phase.
3.Results and discussion
3.1.Enantioseparation of FMOC-amino acids by nano-LC using a MQD-silica hybrid monolithic column
The MQD-silica hybrid monolithic column was prepared as detailed in Section 2.4.In a previous work from our research group,the enantiomeric separation of protein and non-protein amino acids was investigated using up to 8 different derivatization reagents[38].However,when using FMOC as derivatizing reagent,from the eight amino acids tested,only two of them were baseline separated in the reversed phase mode(10 mM ammonium acetate/ACN(30/70,v/v)(apparent pH 5.3))(valine(Rs 1.55)and isoleucine(Rs 1.71))and none of them was baseline separated in the polar organic phase mode(0.055%HAc,0.005%TEA in ACN/MeOH(60/40,v/v))[38].As shown in Fig.S1,when comparing the polar organicphase and reversed-phase modes for some FMOC-amino acids,the latter enabled obtaining better peak shapes[38].Thus,the reversed phase mode was selected in order to obtain a good enantioresolution for the FMOC derivatized amino acids.With this aim,in this work,the mobile phase was optimized using four FMOC derivatized amino acids,i.e.two protein amino acids(methionine and valine)and two non-protein amino acids(methionine sulfone and norvaline).
First,the effect of apparent pH on the retention factors(k1and k2),enantioselectivity(α),enantioresolution(Rs)and efficiency(N1and N2).As shown in Table 1,the apparent pH values were evaluated from 4.3 to 5.3 in the mobile phase 10 mM ammonium acetate/ACN(30/70,v/v),while other conditions were kept constant.On the one hand,when increasing the apparent pH value from 4.3 to 5.3,the enantioselectivity kept constant but the retention factors increased for the four analytes.This can be explained that the chiral selector is positively charged when the pH of the mobile phase is between 4.3 and 5.3,while the four FMOC-derivatized amino acids are negatively charged,thus leading to a stronger electrostatic interaction between the analytes and the CSP.On the other hand,the enantioresolution significantly increased when the apparent pH value changed from 4.3 to 4.8,while the efficiency gradually decreased.Further increasing in the apparent pH value to 5.3 led to similar values of enantioresolution,while the efficiency decreased significantly.Considering the retention factors,enantioresolution and efficiency,an apparent pH of 4.8 was selected for the following experiments.
Second,the influence of the buffer concentration was investigated while keeping constant the mobile phase apparent pH at 4.8 and composition as ammonium acetate/ACN(30/70,v/v).As shown in Table 1,the retention factors,analysis time and enantioresolution values decreased when increasing the concentration of the ammonium acetate buffer from 1 to 10 mM,meanwhile the efficiency increased.Comparing the efficiency,analysis time,and enantioresolution obtained in 1 and 3 mM of ammonium acetate buffer,the efficiency was significantly higher when the buffer concentration increased,but the analysis time increased from 35 min to 60 min(such as in the case of methionine and methionine sulfone),whereas the enantioresolution did not significantly change.Hence,a buffer concentration of 3 mM was selected as a compromise between the analysis time and efficiency.

Fig.1.Enantioseparation of some FMOC-derivatized amino acids.Experimental conditions:column dimensions:15 cm×100μm I.D.;mobile phase:ACN/3 mM ammonium acetate(65/35,v/v)(apparent pH=4.8);UV detection wavelength:254 nm;flow rate:10μL/min;backpressure:23 bar;injection volume:20 nL.
Third,the ACN content was investigated by varying its percentage from 60 to 80%.As can be seen in Table 1,the retention factor and enantioresolution decreased when increasing the ACN content from 60 to 80%,while apparent pH=4.8 and 3 mM ammonium acetate were kept constant.The hydrophobic interaction between the FMOC derivatized amino acids and MQD-silica hybrid monolithic column is weakened when the organic solvent content in the mobile phase is increased because the elution ability of the mobile phase increases.Considering the analysis time,enantioresolution and efficiency,65% ACN was selected as the optimum condition in the mobile phase.

Table 2Analytical characteristics of the developed method for the enantiomeric determination of norvaline in dietary supplements.
3.2.Application of the developed method to the enantiomeric determination of tryptophan and norvaline in dietary supplements
Based on the interest of tryptophan and norvaline as ingredients in dietary supplements,the developed method was applied to the enantiomeric determination of these two amino acids in different dietary supplements.With this aim,the analytical characteristics of the method were evaluated in terms of selectivity,linearity,precision,accuracy and limits of detection(LOD)and quantitation(LOQ)both for norvaline(Table 2)and for tryptophan(Table 3).The selectivity was suitable for tryptophan and norvaline enantiomers because they were separated and there were no interfering peaks which originated from the sample matrices.The linearity results were obtained from six standard solutions at different concentration levels,injected in triplicate.As shown in Tables 2 and 3,the linearity for the two analytes was demonstrated to be adequate in all cases as R2values were≥99.0%,and confidence intervals for the slope did not include the zero value,meanwhile confidence interval for the intercept included the zero value for a 95%confidence level.In order to assess whether there were matrix interferences,slopes of the calibration curves obtained from the external standard and standard additions calibration methods were compared using analysis of variance(ANOVA)test.Both for tryptophan and norvaline,the pvalues of ANOVA were above 0.05 which means that there were no matrix interferences for a 95% confidence level.Therefore,the external standard calibrations method was considered appropriateto perform the quantitation of the analytes in the dietary supplements.Accuracy was evaluated as the recovery obtained from both spiked solutions of norvaline and tryptophan at different labeled content percentages(80,100 and 120%)injected in triplicate.As shown in Tables 2 and 3,recovery values for the two analytes were satisfactory as all of them were near the 100%.Precision was evaluated as the instrumental repeatability,method repeatability and intermediate precision.As Tables 2 and 3 show,regarding the instrumental repeatability,the RSD values(%)for areas and retention times were lower than 1 and 2%,respectively.For the method repeatability,the RSD values(%)for areas and retention times were also adequate,with values lower than 2 and 6%,respectively.Moreover,in the case of intermediate precision,the RSD values(%)were lower than 10%for both the areas and retention times.

Table 3Analytical characteristics of the developed method for the enantiomeric determination of tryptophan in dietary supplements.
Regarding LOD and LOQ values,as can be seen in Tables 2 and 3,these values were 9.3 and 31μM for norvaline enantiomers,and 7.5 and 25μM for tryptophan enantiomers.Figs.2A and B,and 3A and 3B display the chromatograms corresponding to the enantiomeric separation of norvaline and tryptophan,respectively,at concentrations corresponding to their LOD and LOQ.

Fig.2.Chromatograms corresponding to(A)the LOD(9.3μM)and(B)the LOQ(31μM)of D-norvaline;(C)FMOC-derivatized racemic norvaline standard solution(0.5 M)and analyzed dietary supplements whose label indicated presence of L-norvaline(samples 1 and 2).Experimental conditions:15 cm×100μm I.D.;mobile phase:ACN/3 mM ammonium acetate(65/35,v/v)(apparent pH=4.8);UV detection wavelength:254 nm;flow rate:10μL/min;backpressure:23 bar;injection volume:20 nL.

Fig.3.Chromatograms corresponding to(A)the LOD(7.5μM)and(B)the LOQ(25μM)of D-tryptophan;(C)FMOC-derivatized racemic tryptophan standard solution and analyzed dietary supplements whose label indicates presence of L-tryptophan(samples 1-4).Experimental conditions:15 cm×100μm I.D.;mobile phase:ACN/3 mM ammonium acetate(65/35,v/v)(apparent pH=4.8);UV detection wavelength:254 nm;flow rate:10μL/min;backpressure:23 bar;injection volume:20 nL.
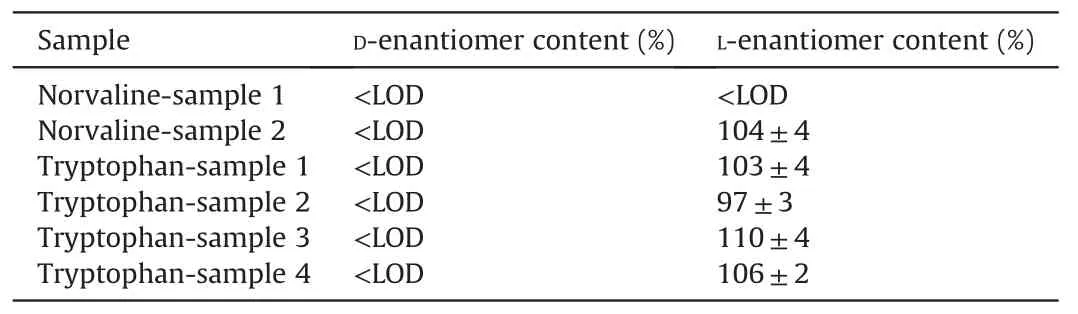
Table 4The results obtained in the analysis of norvaline and tryptophan in dietary supplements.
Once the developed method was proven to be adequate for the enantiomeric determination of tryptophan and norvaline,it was applied to the analysis of four dietary supplements containing Ltryptophan and two containing L-norvaline.As can be seen in Fig.2C,D-norvaline content was below the LOD(9.3μM)in samples 1 and 2,while the content of L-norvaline in sample 2 was above the LOQ value and the amount quantified by the standard external calibration method was in agreement with the labeled content(Table 4).Regarding sample 1,content of L-norvaline did not match the labeled content,since it was below the LOD.Preconcentrating 4 times sample 1 still led to no detection of L-norvaline.Spiking sample 1 with 125μM of L-norvaline resulted in the detection of this peak being the calculated concentration equal to the added one.A second batch of sample 1 was also injected(data not shown)and the outcome was the same as with the first batch that L-norvaline could not be detected.Consequently,it could be concluded that L-norvaline was not present in this sample in spite of the fact that the label declared its presence.
As in the case of L-norvaline dietary supplements,the content of D-tryptophan in the four supplements analyzed was below the LOD value(Fig.3C).Content of L-tryptophan was in agreement with the label in all cases(Table 4).
4.Conclusions
The enantiomeric separation of FMOC-derivatized protein and non-protein amino acids was achieved by nano-LC using an MQDsilica hybrid monolithic column.First,the FMOC was selected as the derivatization reagent which has the simplest sample preparation and satisfactory enantioresolution.Second,the effect of the apparent pH,buffer concentration and content of the ACN in the mobile phase was investigated for a group of FMOC-derivatized amino acids.Third,under the optimized conditions,the MQDsilica hybrid monolithic column provided good enantiorecognition for 19 analytes,enabling baseline enantioseparation for 11 out of 27 assayed FMOC-amino acids.Finally,the method was proven to be adequate in terms of selectivity,linearity,precision,accuracy and LODs and LOQs for the enantiomeric determination of L-norvaline and L-tryptophan in dietary supplements.From the analyzed dietary samples,none of them presented detectable amounts of the D-enantiomers while the content of the L-enantiomers was in agreement with the label content in all cases,except for one L-norvaline sample whose presence was completely missing although the label indicated its presence.This demonstrates the applicability of the developed strategy in the quality control of dietary supplements in a fast and accurate manner.It is also the first report showing the application of the MQD silica-hybrid monolithic column to analysis of real samples.
猜你喜欢
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Karacoline,identified by network pharmacology,reduces degradation of the extracellular matrix in intervertebral disc degeneration via the NF-κB signaling pathway
- Nanodiamonds with powerful ability for drug delivery and biomedical applications:Recent updates on in vivo study and patents
- Comparing different domains of analysis for the characterisation of N-glycans on monoclonal antibodies
- Rapid identification of chemical profile in Gandou decoction by UPLCQ-TOF-MSE coupled with novel informatics UNIFI platform
- GC-NICI-MS analysis of acetazolamide and other sulfonamide(R-SO2-NH2)drugs as pentafluorobenzyl derivatives[R-SO2-N(PFB)2]and quantification of pharmacological acetazolamide in human urine
- Analysis of pesticide residues in commercially available chenpi using a modified QuEChERS method and GC-MS/MS determination
