Nanodiamonds with powerful ability for drug delivery and biomedical applications:Recent updates on in vivo study and patents
2020-02-28SwatiChauhanNehaJainUpendraNagaich
Swati Chauhan,Neha Jain,Upendra Nagaich
Department of Pharmaceutics,Amity Institute of Pharmacy,Amity University,Noida,U.P.,India
Keywords:
ABSTRACT
1.Introduction
With the swift expansion in the field of nanoscience and nanotechnology,nanomaterials based on carbon have been a centre of attention ever since their innovation.Carbon-based nanomaterials include fullerene,carbon nanotubes,nanodiamonds and graphene.Their distinctive nature brands them to be used in many disciplines extending within material science,energy,environment,biology,pharmaceutical sciences and for drug delivery systems[1].Diamond nanoparticles,also known as nanodiamonds(NDs),are single crystal diamonds consisting of carbon as the basic component with high physical and chemical properties.These are nanoscopic version of sp3carbon,while other carbon nanotubes and fullerenes are of sp2configuration.The average particle size of diamond nanoparticle is 4-5 nm(thousands of times smaller than human hair).On primary particle sizes basis,Shenderova and McGuire classified NDs into nanocrystalline particles(1 to≥150 nm)and ultra-nanocrystalline particles(2-10 nm)as shown in Fig.1.
NDs are famous as ultradisperse diamonds which have exclusive properties of diamond core viz.chemical inert core,prominent hardness and superior thermal conductivity[2].There are several techniques for the synthesis of NDs viz.chemical vapor deposition,high temperature high pressure,and detonation of explosives[3].The process of synthesis has been detailed in Section 3 of the paper.NDs are rapidly emerging as promising carriers for next-generation therapeutics and drug delivery.NDs exhibit tunable surface,excellent biocompatibility and large surface area for conjugation of molecules like drugs and genes for their intracellular and extracellular delivery.Additionally,fluorescence imparts a diagnostic feature to NDs,thus they can be utilized as image probes.Throughout the past couple of decades,many investigations have potentially added for the advancement of NDs,exploiting their numerous properties for biological applications[4].On the contrary,there are several crucial concerns and challenges associated with these carbon-based nanocarriers for clinical application.The foremost challenge is toxicity of carbon-based nanocarriers viz.how their nature and mechanism affect the living cells.Therefore,an in-depth study is necessary to ascertain the mechanism of nanoparticle induced toxicity via in vivo and in vitro cytotoxicity studies.Moreover,short circulation half-life of nanoparticles causes their faster elimination via opsonization by phagocytes in human body,which makes them unsuitable for sustained drug delivery[5].Thus,as a solution to this problem,surface functionalization,i.e.,modification of surface by attaching ligands has emerged as a boon technique for making NDs best for delayed drug delivery.

Fig.1.Schematic representation of nanodiamond classification.
Therefore,it is envisaged that NDs can serve as good drug carriers,image probes,or implant coatings in biological systems.However,developing future nanoscale devices and arrays that harness these nanoparticles will require unprecedented spatial control[6].
2.Unique structure and properties of NDs
The unique properties of NDs are attributed to the exclusive properties of diamonds and nanoparticles as shown in Fig.2.Diamonds are the hardest known material and transparent electrical insulator.These consist of tetrahedral sp3carbon atoms which form unique large crystals[7].
NDs are supposed to have core-shell structural design as they have diamond inner core(sp3carbon atoms)and graphitic outer shell(sp2carbon atoms)with hanging bonds ended with functional groups[8].The accurate nature of the outer shell remains unclear,but two general models have emerged.One is an amorphous shell with significant sp2carbon content and the other is a sp2graphenetype sheet of a fullerene structure,giving rise to a structure described as‘bucky-diamond’[9].The two types of bonds can be interchangeable viz.the stretched face of diamond is a graphene plane while the puckered graphene may become a diamond surface.This interchangeability allows NDs particles to act as flexible templates,particularly around the curved surface where electrons are unstable.These include a uniquely faceted truncated octahedral architecture that enables potent drug binding and dispersibility in water[10].The surface mainly consists of carbon,phenols,pyrones and sulfonic acid as shown in Fig.3.NDs also contain carboxylic acid groups,anhydride,hydroxyl groups and epoxide groups but in smaller amount.Due to the presence of carboxylic groups,NDs suspensions are stable in water and have the capability of complexing with water soluble drugs like purvalanol A(drug for liver cancer),4-hydroxytamoxifen(drug for breast cancer),and dexamethasone(an anti-inflammatory agent)[11].
Properly purified nanodiamond can have almost perfect crystalline structure with insignificant parts of non-diamond carbon.Furthermore,NDs have naturally occurring nitrogen-vacancy(N-V)centers or nitrogen impurities(as shown in Fig.4),which can form complexes in the core of nanodiamond particles like peptides,and amines.NDs holding N-V centers provide fluorescence characteristic,and impart optical properties to NDs[12].It is also known as fluorescent nanodiamond(FND),and has been utilized for biolabeling agent.
Exploiting this fluorescence trait,it has been investigated that NDs complexes with doxorubicin significantly diminish the brain tumor by convection enhanced intracranial delivery.This indicates the possibility of using NDs to treat nervous system related diseases and injuries[13].They absorb at 550-800 nm and emit bright fluorescence efficiently in the far-red region,without photoblinking and photobleaching,which is the key reason for application of NDs as cellular markers as a fluorescent probe for single particle tracking with the advantage of surface functionalization and noncytotoxic nature of NDs[14].N-V centers in NDs can also be formed in two steps,i.e.,generation of vacancy by irradiation and then migration of atom through crystal lattice which is then trapped by foreign atom.There can be two types of charges on N-V center,i.e.,neutral or negatively charged ones,which can easily be differentiated on the basis of their emission spectra.Negatively charged N-V centers are paramagnetic which upon optical pumping cause spin polarization.Spin polarization exhibits a long coherence time which has significant applications in diagnostics,particularly optical coherence tomography[6].Optically,NDs reveal the largest optical band gap(5.4-5.6 eV at room temperature),and thus become a wide-band semiconductor.NDs robustly possess excellent electrical properties.An investigation on NDs showed that the aggregated NDs powder demonstrated a low dielectric loss tangent,indicating its good dielectric properties.Mechanically,NDs have high Young's modulus,thus making them strong and hard with elevated refractive index,electrical resistivity,and thermal conductivity.Maitra et al.synthesized poly(vinyl alcohol)-matrix(PVA)reinforced with nanodiamond particles(0.6 wt %)and characterized by nano-indentation technique for mechanical properties.The results displayed a noteworthy improvement in hardness and elastic modulus of PVA by small addition of NDs.The reason attributed to the increased covalent linkages between ND and PVA matrix[15].Likewise,Gogotsi group produced multifunctional bone scaffold materials with the help of poly(L-lactic acid)(PLLA)and 1%-10% octadecylamine-functionalized nanodiamond(NDODA).The outcomes of mechanical testing displayed a 280% increase in the strain at failure and a 310%increase in fracture energy in tensile tests.Thus,an overall enhancement in mechanical properties of matrix was observed.The NDs have assured characteristics which concoct them a functional matter in several disciplines[16].

Fig.2.Pictorial representation of properties of nanoparticles and diamonds.

Fig.3.Basic structure of nanodiamonds with surface functional groups.
3.Synthesis of NDs
NDs were first formed in USSR in 1963 when detonation of carbon-based explosives resulted in formation of diamonds nanoparticles.NDs can also be formed by applying special techniques,principle and procedures like detonation technique,high-pressure high-temperature(HPHT),chemical vapor deposition(CVD)technique,ultrasonic synthesis,hydrothermal synthesis,ion and laser bombardment and electro-chemical synthesis[17].The size,shape,surface structure(groups attached on surface like carboxyl and lactones)and quality of NDs are determined by the methods used in their production.There are several techniques for the synthesis of NDs viz CVD,HPHT and detonation technique.The properties of yielded NDs may vary from technique to technique.Detonation technique includes controlled explosion of carbon containing compounds which results in NDs of narrow size distribution having majorly round or oval shape.Detonated NDs have sp2carbon on surface with functional groups and large surface area,which facilitates the attachment of drug molecules,thus proving an excellent drug carrier system.These types of NDs attain various drawbacks like presence of high number ofimpurities and lattice defects.Due to their small size and high impurities,these can keep stable fluorescent defects to a very less extent.Therefore they tend to be a poor candidate for sensing and labeling techniques.The smallest member of diamond family is diamondoid,which is obtained by applying the process of grinding.Diamondoids are extracted from crude oil.The lower diamondoids are known as adamantine.The resultant NDs are in more pure form and possess very few lattice defects than the detonated NDs.These have flattened surface like flakes having uniform structure with broad size distribution.Different sizes of NDs can be selected by centrifugation.Higher particles will settle down and the lower particles will remain in supernatant.Unlike the detonated NDs,these NDs possess stable defects with numerous colored centers.These stable defects result in fluorescence effect which is non-bleachable as compared to the fluorescence imparted by organic dyes.Negatively charged N-V centers are responsible for this stable fluorescence which can be exploited for biolabeling applications.These N-V centers are also responsible for imparting excellent spin properties to NDs,due to which they can react even in the presence of limited magnetic fields.This property can be utilized for sensor applications.NDs also have an excellent electrical property and thus can act as transducers in chemical sensing viz.hydrogen terminated diamond is surface conductive while boron doped diamond is a semiconductor.

Fig.4.Diagrammatic illustration of nitrogen-vacancy(N-V)centers in nanodiamonds.
Detonated NDs are formed by mixing two explosives,i.e.,trinitrotoluene and hexogen(octogen),as shown in Fig.5.By this procedure,average particles size of NDs can be obtained in the range of 4-5 nm.It is notable to mention the parameters to increase the yield of NDs,i.e.,rapid cooling by increasing the quantity of coolant present in the system like gases(argon)and water,water base foams and ice[18].The process of synthesis is depicted in Fig.1.The resultant product obtained from detonation soot is a mixture of diamond particles(up to 75 wt %)with other carbon allotropes(25 wt%-85 wt%)and incombustible impurities(metals and oxides,1 wt %-8 wt %),which has to be purified for the most applications.Another technique for nanodiamond synthesis is chemical vapor deposition(CVD).CVD is the common process used and includes techniques like hot filament chemical vapor deposition(HFCVD),microwave-plasma-assisted chemical vapor deposition(MWPCVD),and combustion flame chemical vapor deposition(CFCVD)for increased yield[19].The process of CVD is clearly depicted in Fig.5.
Neu et al.have introduced a process for the fabrication of highquality,spatially isolated nano-diamonds on iridium via MWPCVD growth[20].Tsugawa et al.developed a low-temperature and large-area nanodiamond coating method by MWPCVD sustained using surface[21].Lin et al.used microwave plasma jet chemical vapor deposition system(MPJCVD)for synthesizing smooth ultrananocrystalline diamond(UNCD)films[22].Ultrasonication is another technique for NDs preparation,utilizing high pressure and high temperature.The entire process of preparation via ultrasonic synthesis is mentioned in Fig.6[23].The major concern with the nanodiamond synthesis is its yield and cost-effective production.Several researches have been conducted to obtain the cost-effective industrial production techniques.
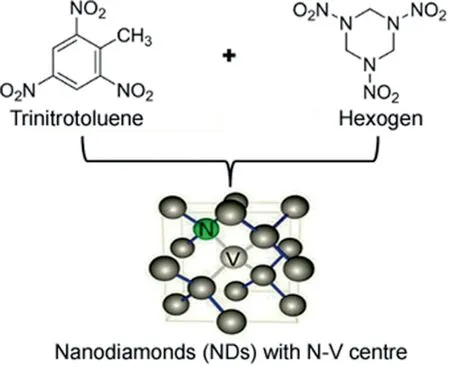
Fig.5.Diagrammatic presentation of nanodiamonds synthesis via detonation technique.

Fig.6.Flowchart representation of nanodiamonds synthesis via chemical vapor deposition technique and ultrasonic technique.
Boudou et al.explored a fabrication technique for the production of fluorescent NDs with high yields.The process includes the conversion of diamond microcrystal powder obtained from ultrasonic method into aqueous concentrated colloidal dispersion of ultra-small nanoparticles(with a mean size less than 10 nm).The fabrication yield of NDs was higher than the previously reported microdiamonds.The results demonstrated an industrial costeffective fabrication of fluorescent NDs with controlled properties[24].
4.In vitro and in vivo cytotoxicity
NDs mediated drug delivery has drawn attention of scientists for secure and reliable transport of active moieties to the target site in a living system.However,for drug delivery significant toxicity is the major concern of these nano-carriers.Hence,it becomes obligatory to comprehend the in-depth knowledge of nanodiamond's biocompatibility via in vitro and in vivo studies[25].In vitro cytotoxicity investigations are more simple,reproducible,more useful and cost-effective than in vivo studies in animal models.These tests are based on various cell functions like enzyme activity,cell proliferation,ATP(Adenosine tripolyphosphate)production,and nucleotide uptake activity.Primarily,methyl tetrazolium(MTT)assays are very common in determining cell viability and proliferation[26].Generally,it is a two-step process(as shown in Fig.7).The first step involves reduction of yellow tetrazolium MTT(3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide)by metabolically active cells(by the action of dehydrogenase enzymes)to generate nicotinamide adenine dinucleotide(NADH)and nicotinamide adenine dinucleotide phosphate(NADPH)and insoluble formazan.

Fig.7.In vitro cytotoxicity(MTT)assay for nanodiamonds.
The second step involves solubilization of intracellular purple formazan which can be quantified by means of absorbance values spectrophotometrically.If the absorbance values are less than those of absorbance of control cells,there is reduction in rate of cell proliferation.Likewise,ATP assay is also known for its quick results and small sample cells.It is based on the assumption that live cells produce luminescence as it contains ATP inside and the level of luminescence will be proportional to ATP content of cells.It comprises cell lysis,which is followed by reaction between assay and ATP content of cell which finally generates luminescence.Photometer is used for the measurement of luminescence.NDs have also been estimated for in vitro cytotoxicity studies via MTT assay and ATP production assay[27].The first reported toxicity of NDs at cellular level via MTT and ATP production assay was done by Schrand et al.The experiment turns out to be positive with no toxicity to cells with this size range and cell morphology remains unaffected after incubation with NDs.Further,they compared carbon-based nanomaterials(carbon black,carbon nanotubes,NDs)on neuroblastoma cells and macrophages.The results revealed the mildest toxic effect exhibited by NDs as compared to others[25].Moreover,biocompatibility of NDs was also investigated on kidney cell culture by Yu et al.They explored the localization of NDs within the cytoplasm of cells via fluorescent confocal microscopy and the outcomes showed less toxicity within the cells[28].Supplementary to the above assays the toxicity or biocompatibility of NDs,reactive oxygen species(ROS)assay can also be performed.This assay is based on the generation of ROS by nanoparticle induced oxidative stress.Normally,ROS are produced as consequence of cellular redox/enzymatic reactions viz.metabolism and phagocytosis.In their further studies,Schrand et al.compared the ROS generation by carbon black and NDs via fluorescence intensity of dichlorofluorescein(DCF).The outcome revealed a lower level of ROS generation by NDs as compared to carbon black[29].Additionally,Keremidarska et al.investigated the cytotoxicity of different sizes of NDs on two types of cell models,i.e.,a human osteosarcoma cell line,MG63,and primary rat mesenchymal stem cells(rMSCs).Optical microscopy and proliferation assay were employed as assessing tools for cytotoxicity after 72 h of exposure to cells.NDs of small size were more toxic as they have shown elevated cell proliferation than those of higher size.Furthermore,genetoxicity studies have also become significant to envisage the effect of NDs at a chromosome level[30].An investigation on TNFαand Bcl-x genes was done by Huang et al.by incubating acid treated NDs alongwith genes.The findings revealed no change in genes expression as compared to control.A significant biocompatibility was observed with HT-29 human colorectal adenocarcinoma cells by examining DNA fragmentation.A research on A549 lung cancer cells and 3T3-L1 embryonic fibroblasts revealed the non-cytotoxicity and significant viability of cells by incubating them with NDs for 10 days.Even,the cellular uptake of NDs did not lead to any interference in cell division and gene or protein expression[31].Apart from in vitro cellular toxicity studies,animal models have also been used to study the effect of NDs on surrounding environment and thus,human health.Fig.8 shows the different model organisms used for conducting in vivo study.
During manufacturing of NDs powder,some powder may spread in the surrounding air and may cause contamination due to its low density.This may also lead to pulmonary toxicity owing to respiration in that environment.To assess it,Yuan et al.performed the intratracheal instillation of NDs in mice and confirmed the low toxicity results via histopathological and ultrastructural investigations.Besides pulmonary toxicity and subcutaneous studies,a long-term toxicity study of NDs hydrosols was also performed via oral administration to mice.The aim was to study the effect of NDs on natural growth and reproducibility of mice via weight dynamics and production of offspring respectively.The experimental protocol includes water substitution by 0.002 wt%-0.05 wt%NDs hydrosols in mice regular diet.The results of weight dynamics displayed normal growth of internal organs viz.lungs,liver,kidneys and pancreas.Concurrently,healthy offsprings were observed,which indicates no loss in reproduction ability of mice[32].The more detailed studies are shown in Table 1[33-38].
5.Surface functionalization
Most of the commercially available NDs surface is found to be highly oxidized and carries numerous functional groups like hydroxyl,carboxyl,lactone and ketone.The presence of functional groups and sp2carbon directly affects the stability of NDs in a variety of media and leads to agglomeration.Moreover,NDs are thermodynamically unstable due to high surface energy.Thus,it becomes the need to alter the functional groups present on the surface of NDs.Surface functionalization involves modification in surface strategies of any compound(NDs),by attaching a variety of functional groups such as hydroxyl,carbonyl,carboxyl,anhydrides and lactones[39].Surface functionalization also enhances the solubility in a variety of polar organic solvents and stability(by preventing agglomeration).However,as per the literature,scientists have explored significant mechanical and chemical techniques to reduce agglomeration and substitute functional group on their surface.Mechanical techniques comprise ultrasonic and ball milling.Apart from this,stirred media milling with ceramic beads followed by sonication has also emerged as a novel technology for the disintegration of NDs.Chemical technique involves nanodiamond powder heating in air at 400-430℃ so as to eliminate the sp2carbon impurities which may reduce the chances of agglomeration[40].Osswald et al.highlighted the use of narrow temperature(400-430℃)for maximum oxidation of sp2carbon with minimum wastage of diamond.NDs should be stable enough when their utilization is aimed for biological applications[41].Alwani et al.formulated ND-gene complexes called diamoplexes for cervical cancer.Synthesis was done by covalent conjugation of lysine amino acid to carboxylated NDs surface generated through reoxidation in strong oxidizing acids.Results for particle size and zeta potential showed minimum sedimentation and good stability.The study concludes that functionalization of NDs with lysine maintains long term stability and also enables NDs to successfully interact with biological system[42].Fluorination of NDs surface was done by Liu et al.Gaseous fluorine and hydrogen mixture reacted with NDs at 150-470℃ to produce a fluorinated NDs.Fluorinated NDs can further be functionalized with alkyl-lithium reagents,diamines and amino acids[43].Burleson et al.aimed at the surface modification of NDs in a controlled manner so as to differentiate the influence of different functional groups on in vitro cellular response.Surface modification was done in hydro-and solvo-thermal conditions.The outcomes with in vitro cellular assays revealed no cellular toxicity with CO,OH,or NH-groups on the surface of the diamond particle[44].Another successful targeted cellular interaction was explored by Krueger et al.They reduced all surface oxygen groups to hydroxyl by reacting borane in tetrahydrofuran(THF).Additionally,peptides synthesis was done by silane linker molecules to form an amine terminated surface[45].These schemes are presented in Fig.9.There are a variety of ligands utilized for the surface functionalization of NDs viz.carboxylic acid,fluorine,chlorine,lysine,and glutaraldehyde as stated in literature.Chemical modification of diamond surface is essential for diamond to be applied as potential biosensor or biochip or a substrate to immobilize biological molecules[46].
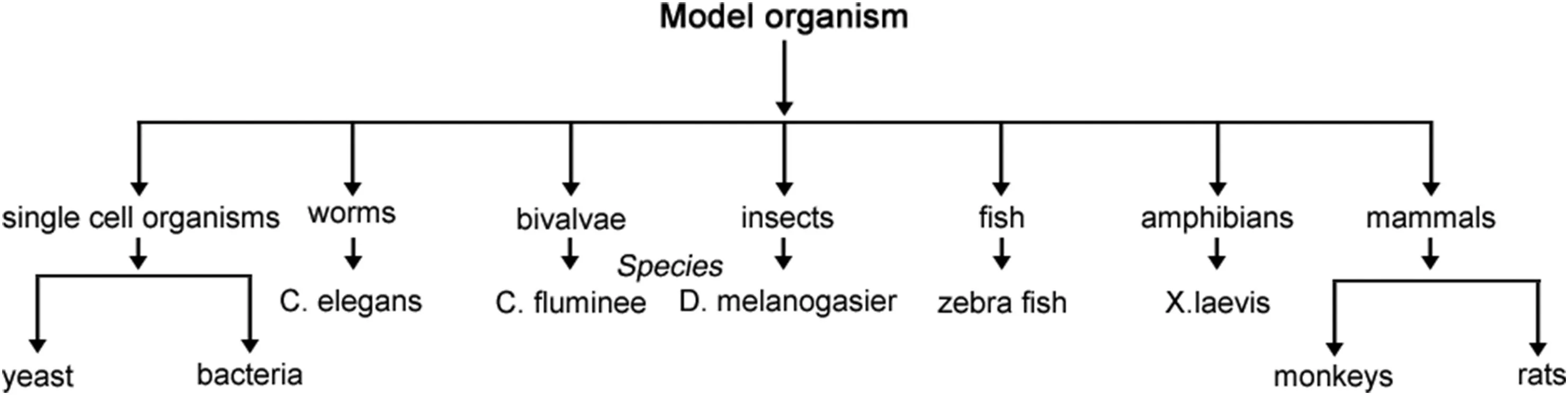
Fig.8.Model organisms used for in vivo studies with nanodiamonds.
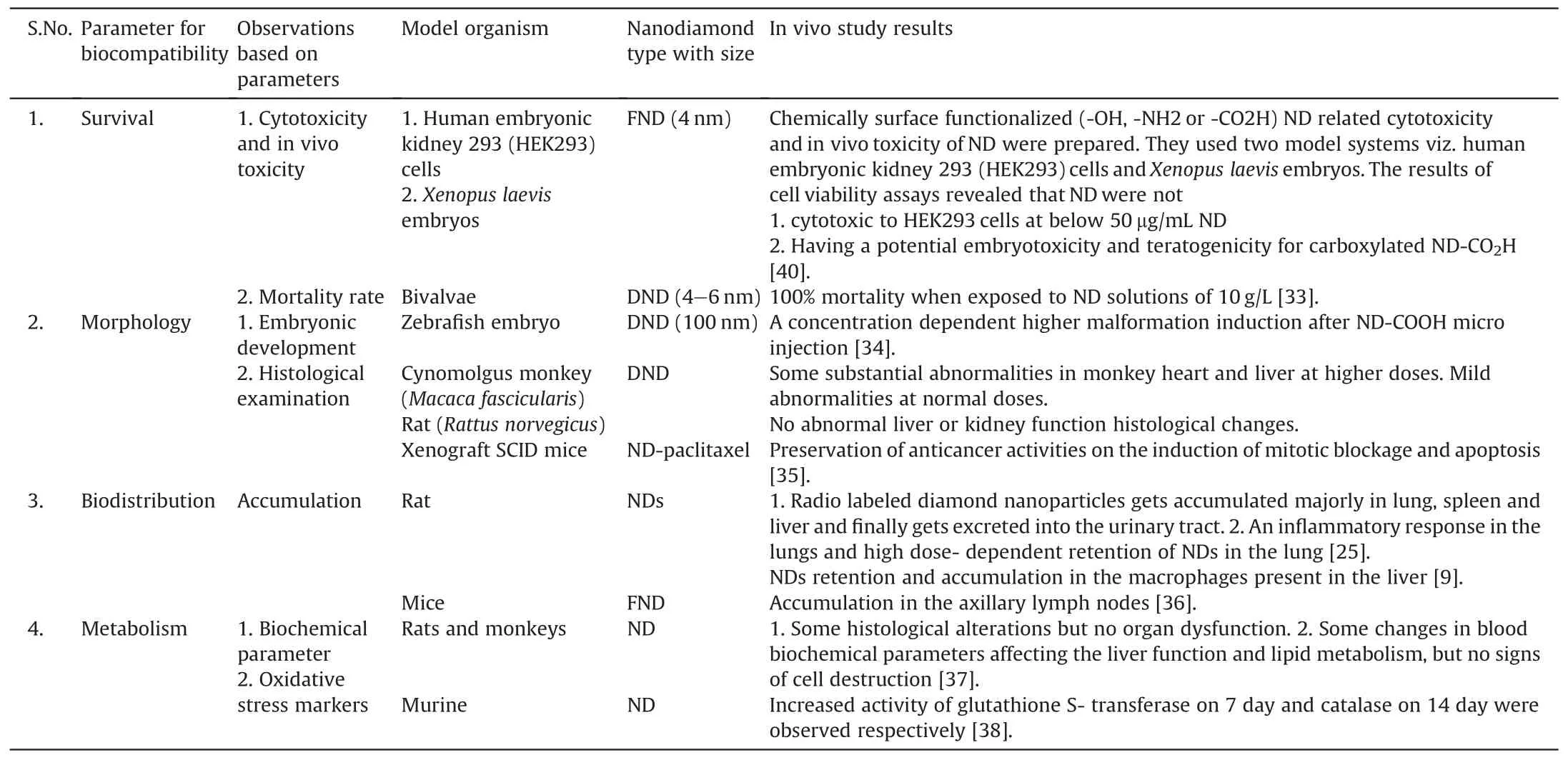
Table 1In vivo studies conducted with nanodiamonds.
NDs can be used in various sectors including medical and nonmedical.These are widely used as vehicle for drug delivery,in bio-imaging and cell tracking,skin care and hair implants,dental care,and many more.NDs tend to form agglomerates which may be useful in chromatography and drug delivery.The characteristics,such as photo stability,chemical non-reactivity,biocompatibility,and emission in the far-red bandwidth,make NDs an interesting candidate for noninvasive imaging[34].These tiny gems possess a broad range of promising applications in drug delivery,bio-imaging and tissue engineering.
6.Biomedical applications of NDs

Fig.9.Illustration of several schemes for the surface functionalization of nanodiamonds.
NDs exhibit special traits of nanoparticles and diamonds,which can widely be used in various fields viz.biomedical and imaging procedures.NDs are very prominent as targeted drug delivery system with increased drug efficacy and decreased toxicity level,and thus can also be known as safer medication.Diamond containing structures will provide significant improvements in the diagnosis and treatment of medical conditions over the coming years.Diamond coatings have been applied to a number of medical devices in recent years,including temporal mandibular joint prostheses,heart valves,and micro electro-mechanical systems,microscale devices for sensing or drug delivery.Diamond nanowire based nucleic acid sensors have been developed.NDs are also used as targeted drug delivery vehicle for bone diseases and bone regeneration[47].There are many potential biological and medical applications of NDs,including its use in biocompatible composites and implants,targeted drug delivery,components of biosensors and stable solid supports for the synthesis of peptides.But there are three main reported use of NDs,i.e.,immobilization of proteins,as fluorescent markers for cell imaging and as a drug delivery vehicle.
6.1.NDs in imaging and therapy
The unique fluorescence properties of NDs have already been discussed.These properties could be explored further to obtain advantages;high quantum efficiency of NDs may result in bright fluorescence;NDs are photostable over longer periods which might help in long-term NDs tracking in cell;NDs have long time fluorescence and emission wavelength less than 690 nm that may contribute to high contrast imaging;photobleaching related to fluorophore can be avoided.The imaging and therapy utilizing nanodiamond helps in early diagnosis,intervention,and effective prevention.Imaging techniques are useful in determining the proper stage of the disease,follow-up after treatment and,as highlighted in recent times,in predicting prognosis[48].Fu et al.examined the binding of positively charged NDs to negatively charged DNA by exploring a red fluorescent NDs(100 nm in size).Primarily,DNA molecules were labeled with TOTO-1(nucleic acid stain)and then allowed to associate ionically with poly-L-lysinecoated carboxylated fluorescent NDs in a buffer medium(diluted solution).After this,the diluted solution was passed through micro channel-combining technology and laser excitation at different wavelengths,DNA was discovered to interact with positively charged NDs via packing.This research reveals that interaction between biomolecules could be studied by single fluorescent nanodiamond[48].Furthermore,another research group utilized fluorescent properties of NDs(100 nm red fluorescent NDs)to explore the uptake mechanism of transferring in HeLa cells.NDtransferrin complexes were made by linking NDs with transferrin via amide linkage[49].In addition,Mohan et al.outlined the motion of free and bioconjugated red fluorescent NDs in Caenorhabditis elegan(worms).Worms were divested from E.coli and then given free NDs and dextran/albumin conjugated carboxylated NDs.After 12 h of administration,free NDs were localized in worm's intestinal tract with no absorption while bioconjugated NDs were captured up by intestinal cells of worms and remained there for 24 h[50].Hsu et al.used fluorescent nanodiamond(FND)for the labeling and tracking of neuronal differentiation and neuron cells derived from embryonal carcinoma stem(ECS)cells[51].Zurbuchen et al.had demonstrated a subcellular multimodal imaging technique which facilitates the localization of NDs having fluorescent N-V centers.Surface was functionalized to target specific locations which were observable by both optical and electron microscopies.The images of optical microscopy displayed NDs in vitro tracking and confirmed its uptake.Due to exclusive fluorescent property of NDs,these are utilized as biolabeling agent even for diagnosing diseases of the nervous system[52].Huang et al.investigated the compatibility of NDs on neurons.The results revealed that NDs possess very low neuronal toxicity,except that it hinders the morphogenesis of neural cells[53].
6.1.1.Disadvantages
Complications exist while the application of NDs from physics field to biomedical field.Methods used for characterizing NDs in dry state are hardly applicable for detecting NDs in living organisms such as cells[54].
Reineck et al.observed weak correlation between FND size and fluorescence brightness which may be due to surface interactions[55].
6.2.Carrier for drug and peptide delivery
For the conjugation of active pharmaceutical ingredients,NDs are ideal candidate owing to their huge surface area and surface functionalities.A drug carrier is found to be suitable only in terms of its loading capacity,capability of protection from surrounding environment and inert nature.A prominent drug loading efficiency with less concentration of carrier is highly appreciated.Simultaneously,timely release of drug from the carrier is also of great significance for desired therapeutic effect.Huang et al.investigated the loading and release of a chemotherapeutic agent viz.doxorubicin hydrochloride(DOX)from NDs.The research was based on the concept of ionic interaction between carboxylic and hydroxylic groups present on the surface of NDs and amine group of DOX to form NDs-DOX loose cluster.In further studies,it was found that DOX was adsorbed on surface of NDs and also in the fissures of cluster.Additionally,cytotoxic studies of DOX-NDs on mouse macrophages and human colorectal cancer cells revealed a lower toxic effect with sustained release than free-DOX[56].Apart from the large surface area for conjugation,NDs have also emerged as dispersibility enhancing agents of hydrophobic drugs[57].There are certain chemotherapeutic moieties which have their solubilities in organic solvents that limit their parenteral administration viz.a liver cancer drug‘purvalanol A’[58]and a breast cancer moiety‘4-hydroxytamoxifen’[59].The characteristic of enhancing dispersibility in water is attributed to NDs'nature of adsorbing drug on surfaces and retaining therapeutic effectiveness of the drug.The therapeutic activity of NDs formulations was confirmed by MTT assay.These outcomes revealed that NDs could play a significant role in formulation development of poor water-soluble drugs[60].
Enzyme immobilization is an attempt of technique which includes matrixing or engraving enzymes onto a polymer matrix and increasing the availability of enzymes to substrate.Immobilization of proteins on nanodiamond is much in use for drug delivery.Properties of having high colloidal stability and enterosorbent potential in aqueous and non-aqueous media allow NDs to be efficiently used for the targeted drug delivery[61].But stability of the complexes is based on bonding of NDs with respective proteins.As concluded by Purtov et al.complexes obtained by covalent immobilization are more stable than non-specific absorption of proteins[62].Researchers have proved the uptake of NDs by living cells.NDs can easily be complexed with any compound which can either be an antigen,an antibody or an immunoglobulin.Various researchers have shown their work on immobilization with NDs.Kossovsky et al used NDs coated with a disaccharide to immobilize an antigen,which was then directly injected into rabbits to elicit an immune response.The antigen carrier plays an important role in controlling the conformation of the antigen and exposing or shielding important functionalities;it is believed that NDs immobilization results in less distortion of the protein conformation allowing better binding by antibodies and hence,a stronger immune response[63].Bondar et al.used detonation NDs for the separation of recombinant protein from E.coli.by physical adsorption of protein onto NDs particles[64].Huang et al.described the immobilization of antibodies and bacterial binding on NDs[65].Kong et al.used this principle to capture proteins for analysis by MS spectra[66,67].Researcher have also demonstrated that NDs seeded electrodes can be bio-functionalized using previously published UV alkene surface chemistry of diamond films and used for pathogen detection.This is basically nano-structuring of biosensing electrodes with NDs for antibody immobilization[68].
Various researches have been carried out,which allow to consider the possibility of applying NDs as carriers to address deliveries of bioactive substances(i.e.,drugs)to various biological targets(in vitro as well as in vivo).NDs have the potential to deliver the biological moieties into a single cell,or targeted cells;hence this technology is widely used in cancer treatment[69].In cancer treatment,it is required to target the cancer cells,not the normal cell or tissue.Clusters of the NDs surround the drugs to ensure that they remain separated from healthy cells until they reach the cancer cells,where they are released.Therefore,it enables the whole amount of drug to reach the target site and the size of NDs enables kidney for their easy removal from the body without blocking other blood vessels[70].
Man et al.synthesized the daunorubicin-NDs conjugates for the treatment of chemoresistant leukemia which have the potential to improve treatment efficacy,especially towards resistant strains.Cell line studies on a K562 human myelogenous leukemia cell line with multidrug resistance and augmented daunorubicin exposure was carried out for efficacy enhancement demonstration.NDs enhanced the daunorubicin delivery to resistant cells[71].NDs are used as a drug carrier mainly in any of the forms viz.NDs assemble on a chemical substrate to form a thin film,having interactions with a drug in two dimensions(forming spontaneous clusters also named as NDs hydrogel with low free energy in an aqueous solution),having interactions with a drug in three dimensions[16].Nanostructured diamond(NSD)coatings or diamond like carbon coatings enhance the wear resistance and prevent leaching of metallic ions from orthopedic and dental implants into the body.NDs-based drug delivery against cancer is one of the most developed biomedical applications.Moreover,NDs also play a vital and therapeutic role in tissue engineering and anti-microbial applications[19].Toh and his research team describe a ND-mitoxantrone(MTX)complex that can be rapidly synthesized and mediate marked improvements in drug efficacy.This study concluded that ND-MTX complex markedly enhanced MTX retention and improved therapeutic efficacy in chemoresistant breast cancer cells[72].Another research for improving therapeutic efficacy in chemoresistant cancer cell was done by reversibly bounding NDs to epirubicin by physical adsorption.This results in formation of nanodiamond-epirubicin drug complex.The results prove that NDs mediated drug delivery may serve as a powerful method for overcoming chemoresistance in cancer stem cells and markedly improving overall treatment against hepatic cancers[73].NDs prove to be very efficient vehicle for drug delivery.A potential anticancer investigation has been conducted by researchers regarding the biomedical use of plant drug-functionalized NDs.Coupling of reduced NDs was done with 5,7-dimethoxycoumarin(citropten,a plant secondary metabolite)to form a complex which shows its ability to reduce B16F10 tumor cell growth more effectively than treatment with the pure molecule.The ND-citropten complex determines cell cycle arrest,morphological changes and alteration of mRNA levels of cytoskeletal related genes[74].In the field of ocular drug delivery,NDs have been found significant application for the treatment of diseases like glaucoma.In this piece of work,researchers presented lysozyme-trigerred release of timolol maleate loaded NDs and then embedding NDs onto contact lenses.They coated individual NDs with polyethyleneimine(PEI)and then cross-linked with an enzyme cleavable polysaccharide,chitosan,forming an NDs nanogel loaded with timolol maleate(TM).These NDs nanogels are then embedded within a poly-HEMA matrix and cast into contact lenses.The results revealed effective drug complexation and enzyme activation[75].NDs are also excellent candidates for topical protection of skin form sun burn.They do not exhibit photo-catalytic activity which is the main factor of producing reactive oxygen species(ROS),resulting in the damaged skin.They exhibit the property of scattering by which they prevent UV B induced skin damage.Wu et al.evaluated the efficacy and safety of NDs in UV B protection using cell cultures and mouse models.Results revealed that NDs shielding efficiently decline 95%UV B radiation while direct exposure of UV B radiation to cultured keratinocyte and fibroblasts causes their death[76].Lists of recent investigations are presented in Table 2[77-79].
6.2.1.Advantages
NDs in their original form can be used for drug delivery,i.e.,there is no need to apply the process of oxidative modification.This can be due to attaining good water solubility even without any acidic treatment.This may result in minimum side effects.
NDs have strong affinity towards proteins and antibodies,thus forming more stable conjugates.
6.2.2.Disadvantages
Xing et al.demonstrated that genotoxicity can occur,while introducing the chemical groups onto NDs[80].
Due to smaller size of NDs,it is very difficult to evaluate their distribution through conventional microscopy method;hence,radionuclide tracer technique is used for detection.This technique involves use of radioactive materials,which can lead to toxicity,and is an expensive process[81].
The process of complexing nanodiamond with active moiety through covalent bonding is a complicated process and it is difficult to eliminate the toxic solvents used during synthesis.Moreover,the complex formed can not show slow-release function.
6.3.NDs in gene therapy
Gene therapy can be used in treatment of various lifethreatening diseases,like cancer,heart disease and diabetes.NDs act as an emerging attractive tool for gene delivery,by which efficiency of gene therapy is much more increased.The technology requires both effective cellular uptake and cytosolic release of the gene.Taking green fluorescent protein gene as an example,Chu et al.demonstrated the successful cytosolic delivery and expression of such a gene using the prickly NDs as carrier[82].Perevedentseva et al.provided evidence that lysine functionalization enables NDs to interact effectively with the biological system to be used for RNAi therapeutics[83].Zhang et al.demonstrated NDs as viral vectors for in vitro gene delivery via surface immobilization with 800 Da polyethyleneimine and covalent conjugation in presence of amine groups.This approach represented an efficient avenue towards gene delivery via DNA functionalized NDs[84].NDs have also been explored their potential in the delivery of small interfering RNAs.Liu et al.investigated the potential of small interfering RNAs(siRNA)loaded functionalized NDs with polymer polyethylenimine(PEI)for its in vitro efficiency and cytotoxicity via simulation technique.The results showed to be highly effective for in vitro delivery with low cytotoxicity[85].In addition,Alhaddad et al.elucidated the delivery of siRNA via cationic polymers viz.polyallylamine and polyethylenimine coated diamond nanocrystals.They targeted Ewing sarcoma cells which were traceable for long time owing to their intrinsic fluorescence[86].

Table 2Recent investigations regarding Nanodiamonds for cancer therapy.
6.3.1.Advantages
NDs can act as versatile nanocarriers to deliver genes in biological systems with enhanced delivery efficiency and biocompatibility.
Efficiency of drug delivery can be increased to 70 times as compared to conventional gene delivery[84,87].
6.3.2.Disadvantages
More research is required so as to improve interaction with stem cells,bio-distribution and toxicity of NDs,thereby increasing their potential[88].
6.4.NDs as an antibacterial agent
Antimicrobial or antibacterial agents hinder/terminate the growth and reproduction of bacteria.NDs have been found to kill gram-positive and gram-negative bacteria.Wehling et al.showed that NDs can be an efficient antibacterial agent based on their surface composition.Their experiment proposed that the NDs possessing partially oxidized and negatively charged surfaces would have antibacterial property viz.acid anhydride group on surface[89].Moreover,surface functionalization of NDs with protein molecules enhances the bactericidal property of NDs.In addition to the above-mentioned research,another group investigated the antibacterial activity of ultrafine nanodiamond against gram negative bacteria,i.e.,E.coli.Functionalization of NDs surface was done with carboxyl group to form carboxylated nanodiamond(cND)and was kept in highly nutritious media.Upon scanning electron microscopy(SEM),the photomicrograph revealed that cND was attached to the bacterial cell wall surface leading to its destruction[90].Surface functionalization of NDs with glycan(sugar coating)had also uncovered the bactericidal effect of NDs specifically for type 1 fimbriae-mediated E.coli.adhesion.These have the potential in countering E.coli.biofilm formation.NDs form covalent bond with molecules on cell walls or bind to intracellular components which inhibit vital enzymes and proteins,leading to a rapid collapse of the bacterial metabolism and finally cell death[91].
6.4.1.Advantages
NDs act as a potent antibacterial agent by destroying bacterial barrier.
NDs have the potency to bind with several viruses like hepatitis B or C[92].
6.4.2.Disadvantages
Continuous interaction with the cellulose dialysis membrane can result in the loss of reactive oxygen-groups and charges[93].
6.5.NDs in bone tissue engineering
Bone tissue engineering is a technique of creating biological substitutes to repair and replace the incomplete or absolute infected tissues.Therefore,the major challenge is to fabricate a scaffold which can provide strength comparable to natural bone[94].Properties like high porosity,appropriate pore size,biocompatibility with adjacent tissue,biodegradability,ability to support adhesion,growth,and differentiation of osteogenic cells make NDs to act as a promising material for bone tissue engineering[95-97].Zhang et al.fabricated a multifunctional fluorescent composite bone scaffold exploiting poly(L-lactic acid)(PLLA)and octadecylamine-functionalized nanodiamond(ND-ODA)and observed that cell proliferation was not hampered by this scaffold[98].Moreover,Yang et al.have used PND/polymer scaffold which supports osteoblast(bone-forming cell)growth and differentiation,and also,enhances bio-mineralization and formation of bone like apatite on the scaffold in simulated body fluid(SBF).This concluded that these scaffolds are useful for a wide range of orthopedic regenerative engineering applications[99].
6.5.1.Disadvantages
It is not easy to design a biodegradable and bio-compatible scaffold attaining mechanical strength that of natural bone[95].
7.Limitations of NDs
Despite having diverse advantages and application,NDs also have some limitations which have to be overcome during their biomedical application;some of these are mentioned below:
➢Compared with standard organic dyes,the emission of an N-V center is low[83].
➢It is problematic to differentiate the particle from the background fluorescence,to overcome this problem diamonds containing many N-V centers are used.However,this requires larger diamonds;this larger size can lead to problems for some bioapplications.
➢Bound NDs might alter the structure and function of attached proteins[83,100].
➢The N-V centre in diamond is a promising candidate for a solidstate qubit.However,its charge state is known to be unstable,discharging from the qubit state NV-into the neutral state NV0 under various circumstances[101].
➢The N-V center in nanodiamond has already been used as a fluorescence marker in biological systems.However,up to now there has been no analysis of the effect of the biological environment on the quantum dynamics of the N-V center;such considerations are critical to nano-biomagnetometry applications[23].
➢The conventional method use for synthesis of NDs requires high pressure and high temperature[101].
8.Patents on NDs
NDs have emerged as innovative formulation for drug delivery.Many researches are being carried out by using NDs for biological applications.Patents granted for drug delivery have been discussed in detail in Table 3[102-111].
9.Conclusion
The extraordinary properties of NDs have enhanced the researchers'interest in diverse fields of applications.The sparkling and a style statement use of diamonds have been surmounted by their significant applications in diagnostics and drug delivery system.NDs'intrinsic structural integrity and involvement of synthetic techniques like disaggregation and functionalization have explored new horizons for biomedical applications.Still,many challenges like cellular fate,re-aggregation prevention,enhanced control on surface chemistry,and large volume manufacture of NDs are the issues of high concern.On the bright side,plethora of promising applications will drive the researchers in the biomedical field ahead.An advanced and better knowledge regarding the structure and surface chemistry will direct the investigators to achieve a significant control over properties and thus,their relevant applications.Thus,continual efforts in the development of NDs as diagnostic tool and as drug delivery system will be made to surpass the previously established carbon nanomaterials viz.nanotubes and fullerenes for biomedical applications.

Table 3Patents granted for Nanodiamonds as drug delivery system.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Karacoline,identified by network pharmacology,reduces degradation of the extracellular matrix in intervertebral disc degeneration via the NF-κB signaling pathway
- Comparing different domains of analysis for the characterisation of N-glycans on monoclonal antibodies
- Rapid identification of chemical profile in Gandou decoction by UPLCQ-TOF-MSE coupled with novel informatics UNIFI platform
- GC-NICI-MS analysis of acetazolamide and other sulfonamide(R-SO2-NH2)drugs as pentafluorobenzyl derivatives[R-SO2-N(PFB)2]and quantification of pharmacological acetazolamide in human urine
- Analysis of pesticide residues in commercially available chenpi using a modified QuEChERS method and GC-MS/MS determination
- Determination of L-norvaline and L-tryptophan in dietary supplements by nano-LC using an O-[2-(methacryloyloxy)-ethylcarbamoyl]-10,11-dihydroquinidine-silica hybrid monolithic column
