改善土壤通气性促进甘薯源库间光合产物运转的原因解析
2020-02-20刘永晨司成成柳洪鹃张彬彬史春余
刘永晨 司成成 柳洪鹃,* 张彬彬 史春余,*
研究简报
改善土壤通气性促进甘薯源库间光合产物运转的原因解析
刘永晨1司成成2柳洪鹃1,*张彬彬1史春余1,*
1山东农业大学农学院/ 作物生物学国家重点实验室, 山东泰安 271018;2海南大学园艺学院, 海南海口 570228
为了明确土壤通气性对甘薯源库间光合产物运转的调控机制, 本研究以淀粉型品种商薯19和济徐23为试验材料, 设置疏松、对照和紧实3个处理进行大田试验, 研究结果表明, 与对照处理相比, 疏松处理显著提高2个品种的块根产量和经济系数, 2年平均增幅分别为27.03%~38.74%和6.30%~13.05%, 紧实处理则显著降低2个品种的块根产量和经济系数, 2年平均降幅分别为17.87%~15.92%和10.83%~15.63%。功能叶13C标记结果显示, 疏松处理显著提高块根中光合产物的输入效率。疏松处理显著提高块根中蔗糖和淀粉含量, 显著降低地上部器官中淀粉含量和茎中尤其是茎的中下部中蔗糖含量; 紧实处理则显著降低块根中蔗糖和淀粉含量, 而显著提高地上部器官蔗糖和淀粉含量, 且茎中下部蔗糖含量增幅较大。疏松处理显著降低50~150 d茎基部与茎顶部间和茎基部与块根间的蔗糖含量差; 紧实处理则显著提高茎基部与茎顶部间和茎基部与块根间的蔗糖含量差, 且茎基部与块根间蔗糖含量差的变幅大于茎基部与茎顶部间的蔗糖含量差。相关分析表明, 茎基部与块根间、茎基部与茎顶部间蔗糖含量差与块根蔗糖和淀粉含量呈极显著负相关。说明改善土壤通气性可促进茎基部光合产物向块根的运转, 提高块根中碳水化合物含量, 增加块根产量。
甘薯; 土壤通气性; 块根产量; 光合产物; 运转
甘薯用途广, 适应性强[1-4], 丘陵山地多有种植[5-6]。近年来, 随着甘薯生产收益的增加, 甘薯种植也不断向平原地区扩张[7]。甘薯是以地下块根为收获物, 土壤疏松、通气良好是其获得高产的主要土壤条件之一[8-9]。土壤黏度大、板结、通气性差时, 块根产量降低、品质变差。丘陵山地因水浇条件差, 土壤极易因干旱而板结; 平原地区随着机械化的普及和肥料的不合理施用, 耕作层土壤的紧实度逐年增加[10-12], 土壤通气状况日益恶化, 严重影响甘薯块根产量。土壤通气状况已经成为制约甘薯高产稳产的主要因素之一。
改善土壤通气性可以促进块根膨大过程中光合产物由地上部向块根的运输, 提高干物质在块根中的分配率, 显著提高甘薯块根产量[13-15]。关于土壤通气性调控光合产物运转分配的生理原因, 前人进行了有益的探索, 一般认为改善通气性可以提高块根形成层的活动能力[16], 增加块根中ATP含量和脱落酸(ABA)含量[17], 降低淀粉酶活性[14-15]等促进源库间光合产物运转。但前人的研究多集中于块根的膨大特性, 而对光合产物由叶片装载、经茎运输后卸载到块根的整个运转过程的关注较少, 缺乏对土壤通气性调控光合产物运转关键环节的认识。因此, 本研究设置不同通气性的土壤条件, 从源库间光合产物运转效率、不同器官和功能叶以下茎不同部位蔗糖和淀粉含量、不同器官间蔗糖含量差等方面系统分析土壤通气性引起光合产物由源到库运转差异的关键环节, 研究结果可以为促进甘薯源库间光合产物运转及栽培措施的改进提供理论依据。
1 材料与方法
1.1 试验材料与设计
2017年和2018年在山东农业大学农学试验站进行大田试验。供试品种为商薯19和济徐23, 供试土壤为沙壤土。2017年0~20 cm土层含碱解氮106.29 mg kg-1、速效磷36.93 mg kg-1、速效钾109.67 mg kg-1、有机质0.97%。2018年0~20 cm土层含碱解氮88.82 mg kg-1、速效磷33.27 mg kg-1、速效钾90.35 mg kg-1、有机质1.05%。依据土壤容重[18]和紧实度设置3个处理: (1)紧实区(JS), 压实试验地而成, 0~20 cm土层的容重为1.40~1.50 g cm-3, 紧实度大于600 kPa, 一般不高于1200 kPa。(2)标准区(CK), 0~20 cm土层的容重为1.30~1.40 g cm-3, 紧实度为300~400 kPa。(3)疏松区(SS), 试验地土壤、有机肥和沙混合而成, 其土壤有机质含量和标准区一致并将3个处理的速效氮、磷、钾含量调到基本一致, 0~20 cm土层的容重为1.20~1.30 g cm-3, 紧实度为100~200 kPa。3个处理土壤物理性状如表1所示。采取裂区试验设计, 品种为主区, 紧实度为副区, 重复3次, 小区面积为10 m2, 行距80 cm, 株距25 cm。在栽秧期、块根膨大前期、块根膨大高峰期、块根膨大后期用土壤紧实度仪测量土壤紧实度。栽秧后间隔5 d测量土壤0~20 cm和20~40 cm土层土壤体积含水量。小区每平方米含水量(m3)为H×1 m2×土壤体积含水量(%), 其中H为土层深度。以含水量最高的小区为标准将其余小区含水量补齐。
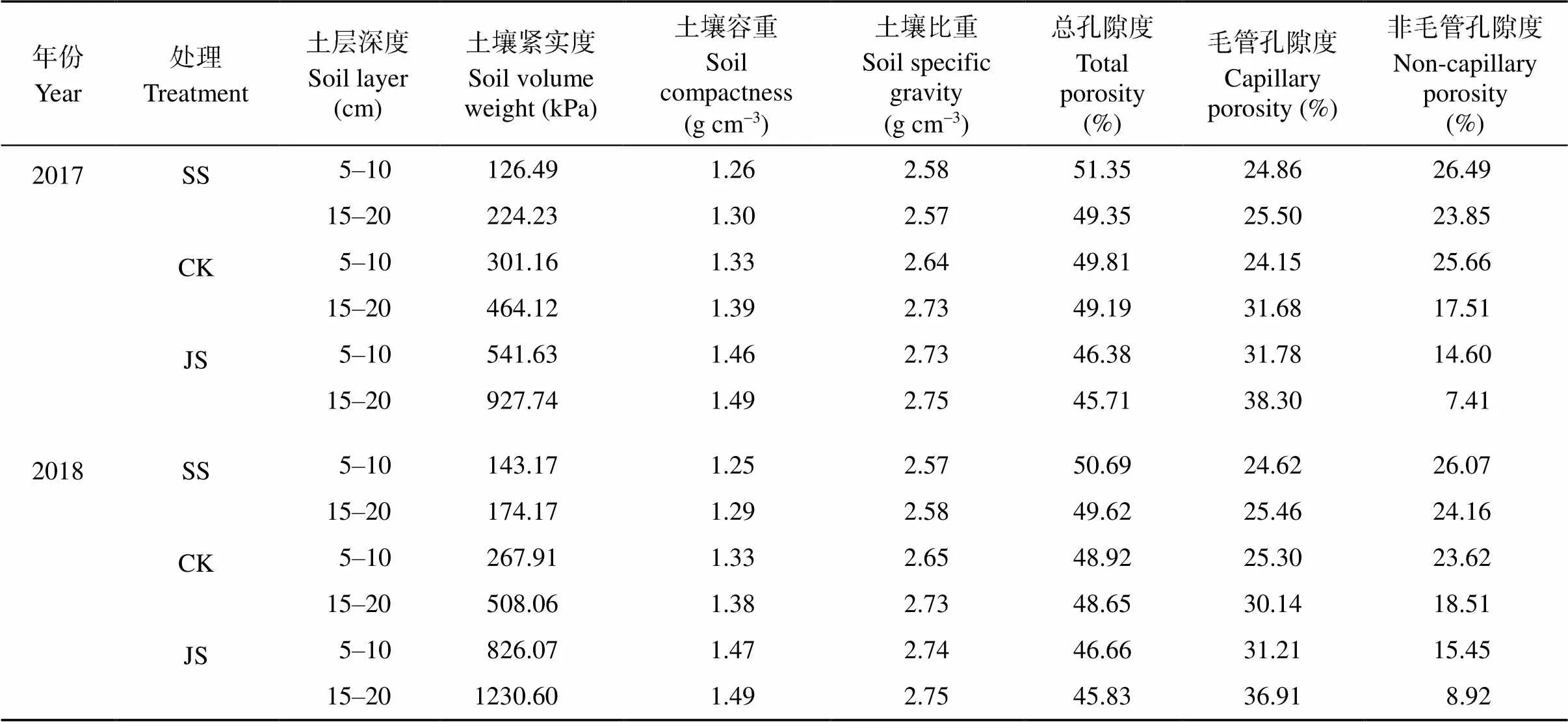
表1 栽秧期土壤物理性状
SS: 疏松土壤; CK: 对照土壤; JS: 紧实土壤。
SS: loose soil; CK: control soil; JS: compact soil.
1.2 测定项目与方法
1.2.1 收获期测产 在收获期测定每个小区每行的薯块数和块根鲜重。
1.2.2 甘薯各器官中碳水化合物含量 自栽后50 d开始取样, 每隔20 d取样一次, 共计取样6次。留样时选取各品种每个处理长势基本一致的植株5株, 分为叶片、叶柄、茎蔓及块根四部分。叶片和叶柄各200 g; 块根切片混匀后留取200 g; 茎蔓中的主茎留取茎顶部、茎中部和茎基部三部分样品, 茎顶部为主茎倒5叶叶柄所在位置向下延伸10~15 cm, 茎中部为主茎(去掉自生长点向下第5片展开叶以上部分)对折后, 自中点向两侧延伸5.0~7.5 cm, 茎基部为主茎最下面10~15 cm (图1), 将主茎剩余部分和侧茎剪碎混匀后留取200 g。以上样品105℃杀青, 60℃烘干, 磨成粉末过筛, 用于蔗糖和淀粉含量的测定。采用蒽酮比色法测定蔗糖和淀粉含量[19]。
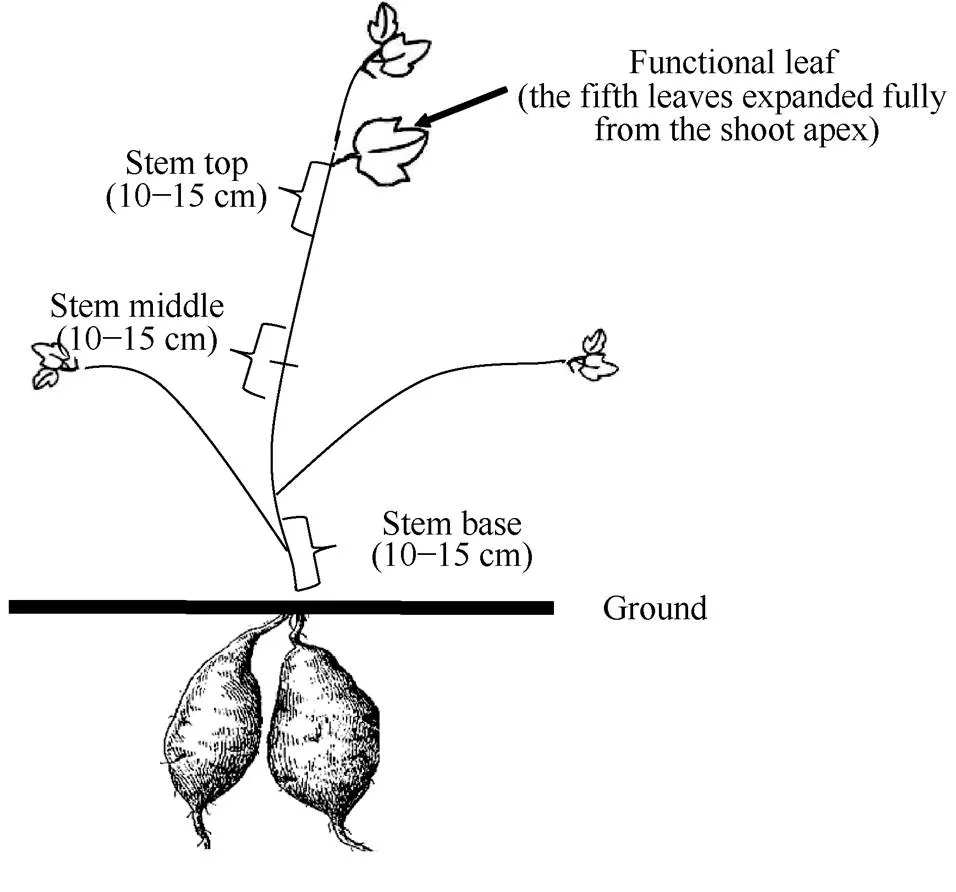
图1 主茎各部位划分模式图
1.2.3 经济系数 块根鲜重与整株鲜重的比值。
1.2.4 器官间蔗糖含量差 均以前面器官的蔗糖含量作为被减数, 计算公式如下:
柄与叶间(%)=(柄-叶)/[(柄+叶)/2]×100
柄与茎顶间(%)=(柄-茎顶)/[(柄+茎顶)/2]×100
茎基部与茎顶部间(%)=(基部-顶部)/[(基部+顶部)/2]×100
茎基部与块根间(%)=(基部-块根)/[(基部+块根)/2]×100。
1.2.513C标记 于块根膨大中期(栽秧后100 d左右), 选择晴朗无风天气9:00-11:00, 从每个小区选择生长基本
一致、具有代表性的植株3株, 在其主茎顶部第4片和第5片展开叶上标记13CO2。由Ba13CO3(99%13C)和磷酸在反应器中生成13CO2, 并用气球收集; 标记前将欲标记叶用体积约为400 mL的聚氯乙烯透明塑料薄膜袋密封, 用医用注射器注入50 mL13CO2(1%); 光合同化40 min, 之后撤掉塑料薄膜袋。标记完成24 h后, 剪取植株地上部, 挖出地下部块根。主要留取: 块根、标记叶及其所在的标记茎和标记柄、主茎、侧茎、侧叶。分样后将块根切片, 茎切段, 装袋, 经105ºC杀青10~30 min, 在60ºC烘箱中烘干至恒重; 然后称重、粉碎, 用质谱仪(Isoprime 100)测定δ13C。
1.3 数据处理与分析
采用Microsoft Excel 2007分析数据和作图, 统计分析中的方差分析检验采用DPS (Data Processing System) v7.05数据处理系统。采用SPSS Statistics 20进行相关性分析。
2 结果与分析
2.1 块根产量及经济系数
由表2可知, 与对照处理相比, 疏松处理显著提高两品种的块根产量和经济系数, 2年平均增幅分别为27.03%~38.74%和6.30%~13.05%, 紧实处理则显著降低块根产量和经济系数, 2年平均降幅分别为17.87%~ 15.92%和10.83%~15.63%。疏松处理的单薯重显著高于对照, 而紧实处理则显著低于对照。即疏松处理主要通过提高单薯重, 提高经济系数而增产, 紧实处理则因降低单薯重, 降低了经济系数而减产。
2.2 功能叶13C的分配率和运转效率
由表3可知, 块根快速膨大期, 与对照处理相比, 疏松处理显著提高块根中13C同化物的分配率, 显著降低其他器官13C同化物分配率; 紧实处理与之相反。13C标记后24 h后, 与对照处理相比, 疏松处理显著提高块根中13C丰度单位时间增加量, 显著降低标记叶中13C丰度单位时间降低量和主茎13C丰度单位时间增加量; 紧实处理变化规律与之相反。
2.3 不同器官蔗糖和淀粉含量
由表4可知, 与对照处理相比, 疏松处理2个品种叶片、叶柄和块根中蔗糖含量显著提高, 而茎中蔗糖含量显著降低; 紧实处理则与之相反, 两年数据规律一致。由表5可知, 疏松处理块根中淀粉含量显著高于对照处理, 紧实处理则显著低于对照处理; 疏松处理叶、柄和茎中淀粉含量分别自栽秧后90 d、70 d和50 d至收获期均显著低于对照处理, 而紧实处理则显著高于对照处理。即改善土壤通气性块根中蔗糖和淀粉快速积累开始的早、积累时间长; 降低土壤通气性茎部蔗糖和淀粉的积累效率高。

表2 块根产量及经济系数
标以不同字母的值在处理间差异显著(< 0.05)。缩写同表1。
Values followed by different letters within the same column are significantly different among different treatments at the 0.05 probability level. Abbreviations are the same as those given in Table 1.

表3 块根快速膨大期各器官内13C同化物的分配率(%, 2017年, 品种为商薯19)
标以不同字母的值在处理间差异显著(<0.05)。缩写同表1。
Values followed by different letters in the same column are significantly different among different treatments at the 0.05 probability level. Abbreviations are the same as those given in Table 1.

图2 标记叶、主茎和块根中13C丰度变化特点(2017年)
缩写同表1。Abbreviations are the same as those given in Table 1.
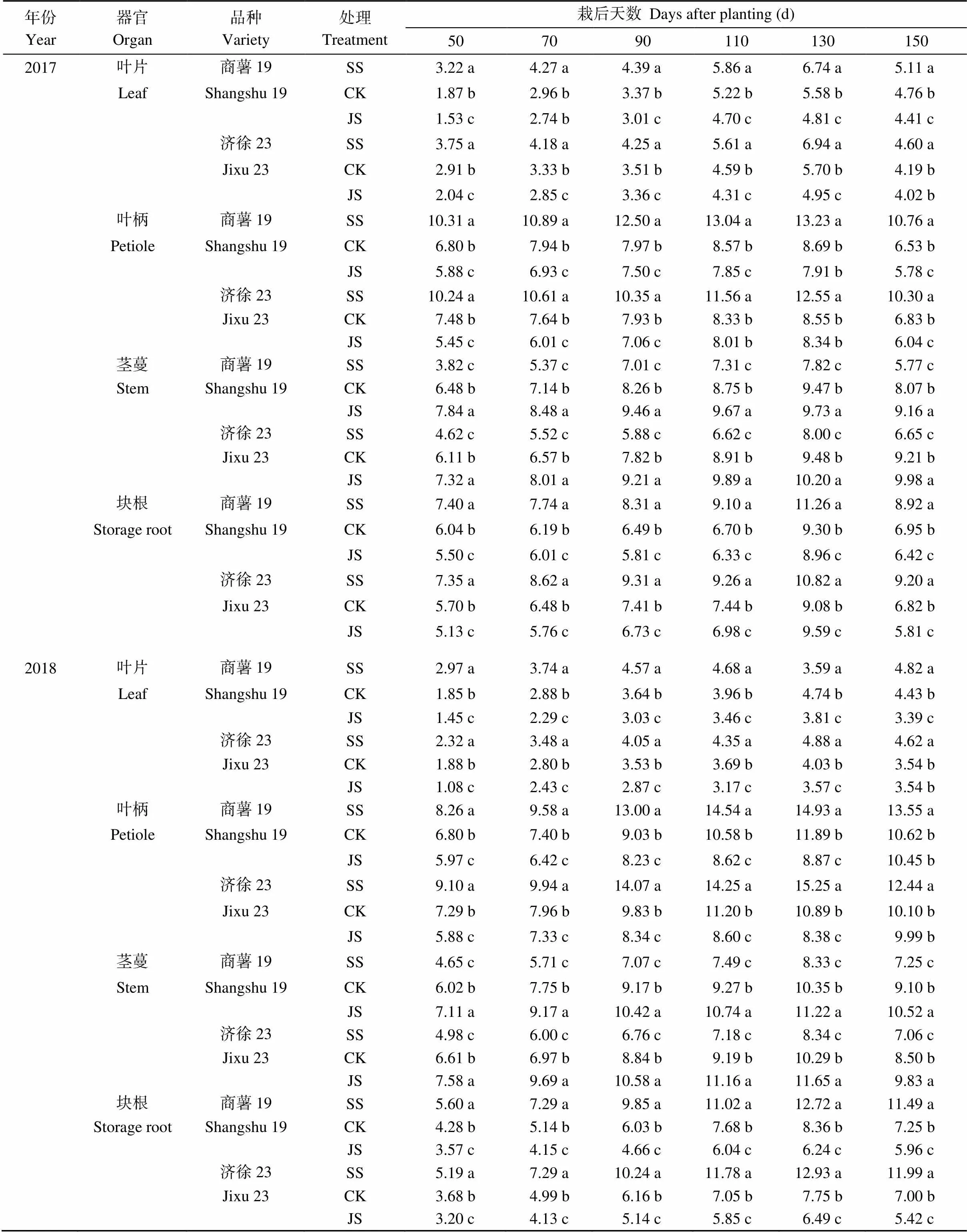
表4 不同器官蔗糖含量
标以不同字母的值在处理间差异显著(< 0.05)。缩写同表1。
Values followed by different letters within the same column are significantly different among different treatments at the 0.05 probability level. Abbreviations are the same as those given in Table 1.

表5 不同器官淀粉含量
标以不同字母的值在处理间差异显著(< 0.05)。缩写同表1。
Values followed by different letters within the same column are significantly different among different treatments at the 0.05 probability level. Abbreviations are the same as those given in Table 1.
2.4 功能叶以下茎不同部位蔗糖和淀粉含量
由表6可知, 茎基部蔗糖含量显著高于茎顶部和茎中部。与对照处理相比, 疏松处理显著提高茎顶部蔗糖含量而显著降低茎中部和基部蔗糖含量; 紧实处理变化规律与之相反。疏松处理主茎各部位淀粉含量均显著下降, 而紧实处理主茎各部位淀粉含量均显著提高, 2个处理均以茎中部和基部的变化幅度较大。即改善土壤通气性减少了蔗糖和淀粉在茎中部和基部的积累。

表6 茎不同部位蔗糖和淀粉含量(%, 2018年)
标以不同字母的值在处理间差异显著(< 0.05)。缩写同表1。
Values followed by different letters within the same column are significantly different among different treatments at the 0.05 probability level. Abbreviations are the same as those given in Table 1.
2.5 器官间蔗糖含量差
器官间蔗糖含量差可以反映器官间光合产物运转的情况。由表7可知, 疏松处理显著降低茎基部与茎顶部和茎基部与块根间的蔗糖含量差, 而显著提高柄与叶片和柄与茎顶部间的蔗糖含量差; 紧实处理显著提高茎基部与茎顶部和茎基部与块根间的蔗糖含量差, 对柄与叶片和柄与上茎的蔗糖含量差的影响存在年份差异。两处理引起器官间蔗糖含量差的变化幅度表现为茎基部与块根间>茎基部与茎顶部间>柄与叶片间和柄与茎顶间。即改善土壤通气性主要提高了茎基部到块根间光合产物的运转效率。
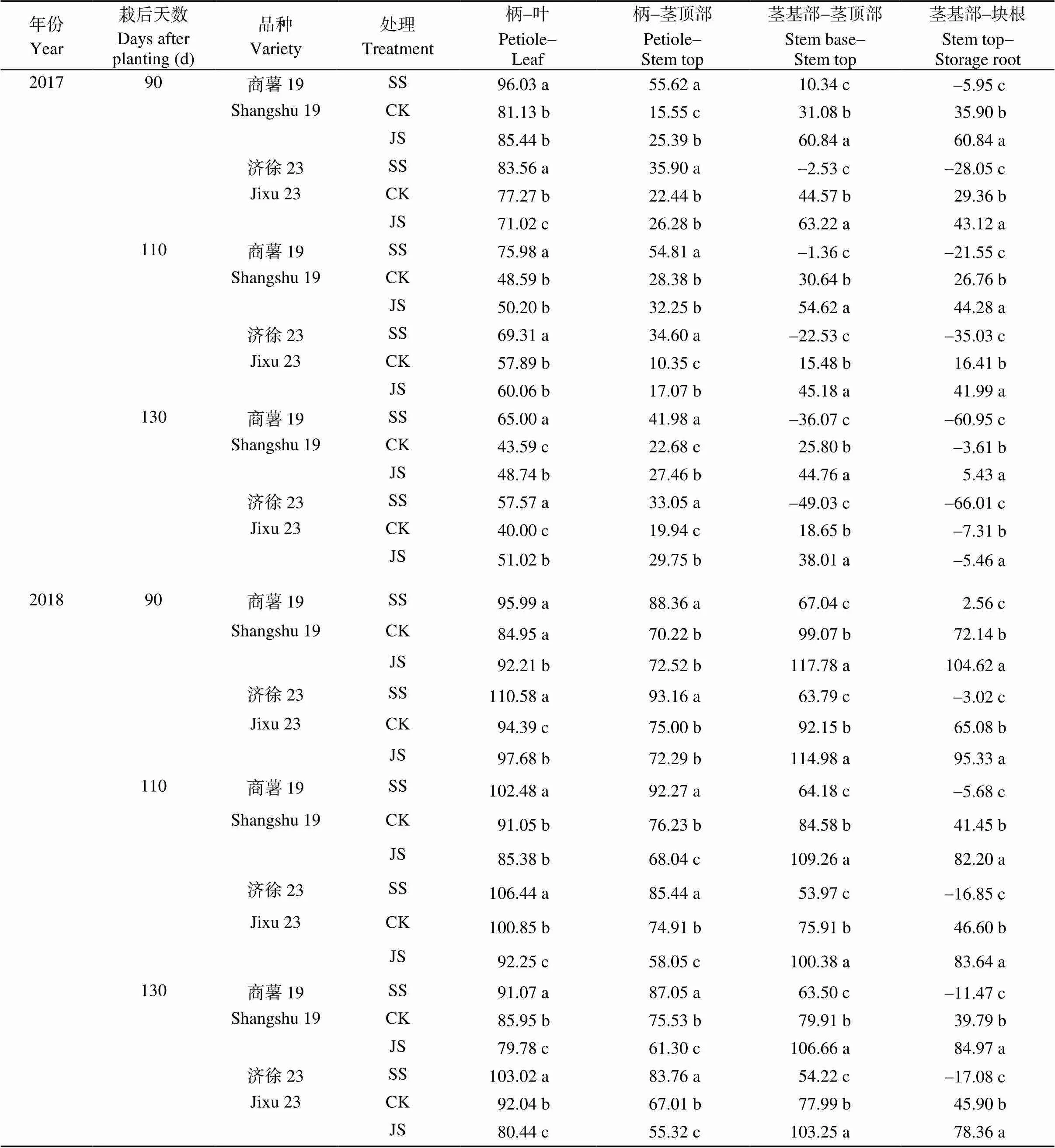
表7 不同器官间蔗糖含量差
标以不同字母的值在处理间差异显著(< 0.05)。缩写同表1。
Values followed by different letters within the same column are significantly different among different treatments at the 0.05 probability level. Abbreviations are the same as those given in Table 1.
2.6 甘薯器官间蔗糖含量差与块根碳水化合物含量的相关分析
由表8可知, 茎基部与茎顶部和茎基部与块根间的蔗糖含量差与块根中蔗糖和淀粉含量均呈极显著负相关, 且茎基部与块根间蔗糖含量差与块根蔗糖含量相关系数较大、茎基部与茎顶部间蔗糖含量差与块根淀粉含量相关系数较大。
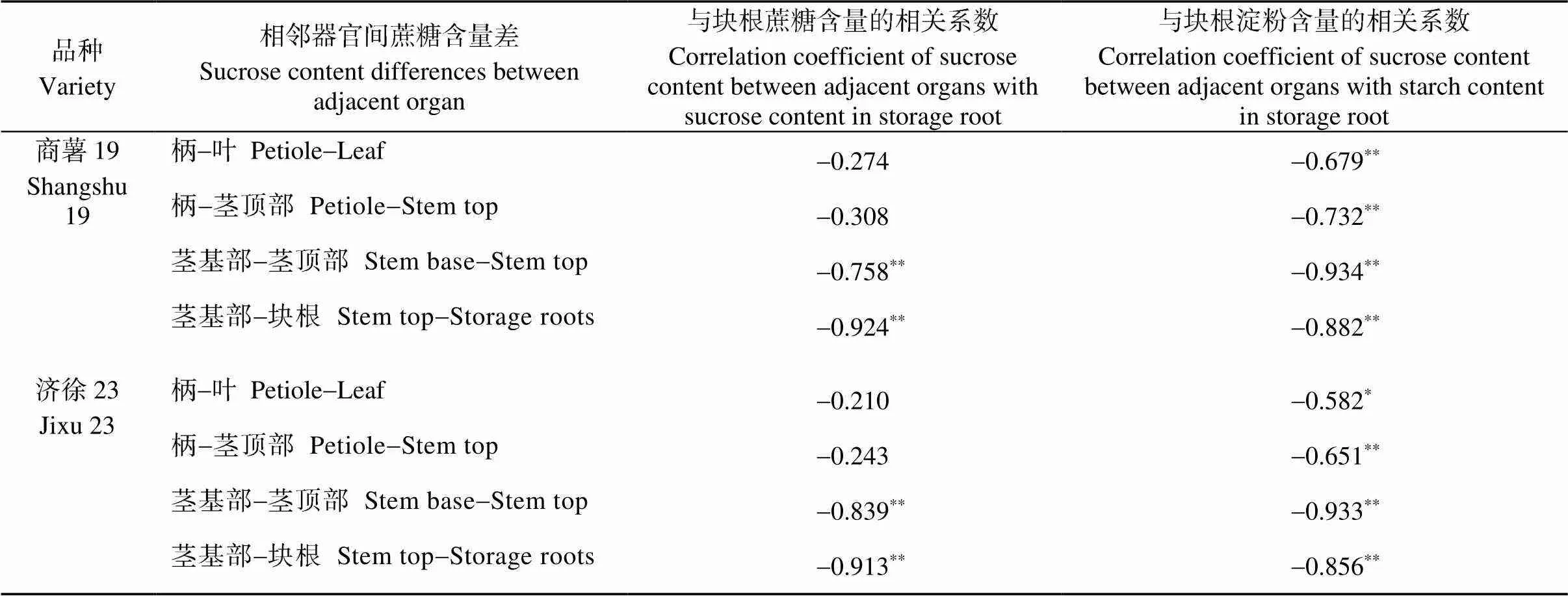
表8 甘薯器官间蔗糖含量差与块根碳水化合物含量的相关性分析
*表示在0.05水平(双侧)上显著相关,**表示在0.01水平(双侧)上显著相关
*and**mean significant at the 0.05 and 0.01 probability levels, respectively.
3 讨论
3.1 土壤通气性影响甘薯块根产量的原因分析
改善土壤通气性能显著提高甘薯块根产量[13,17,20-22], 增加土壤容重或向土壤中冲入氮气, 降低氧气浓度, 则显著降低块根产量[14-15]。土壤通气性好, 块根形成早、数量多, 块根产量高, 而土壤通气性差, 块根膨大慢[14-15], 块根产量低。也有研究认为, 改善土壤通气性通过提高甘薯单薯重和收获指数增加块根产量[22]。本研究结果表明, 与对照处理相比, 疏松处理能显著提高块根产量, 商薯19和济徐23两年的平均增幅分别为27.03%和38.74%; 紧实处理则显著降低两品种块根产量, 降幅分别为17.87%和15.92% (表1)。与对照处理相比, 疏松处理显著提高块根中13C同化物的分配率(表3), 块根单薯重和经济系数; 紧实处理则提高地上部器官中13C同化物的分配率, 降低块根单薯重和经济系数。即改善土壤通气性能通过促进光合产物向块根中运转, 促进块根膨大而增加块根产量。
3.2 土壤通气性引起甘薯源库间光合产物运转差异的原因解析
目前, 改善土壤通气性提高甘薯块根产量的原因分析多集中于块根的形成及膨大特性, 认为改善土壤通气性能提高块根形成过程中土壤温度的日较差[23]; 减少初生木质部的数量[15], 增强根中初生形成层的活动能力, 促进块根的形成[14,16]。改善土壤通气性能提高块根中焦磷酸化酶活性而降低淀粉酶活性[15]; 提高块根中ATP含量和ATP酶活性、提高脱落酸含量[17]; 提高块根中蔗糖合酶和ADPG焦磷酸化酶的活性[23], 促进块根中淀粉的积累, 促进块根的膨大。而对甘薯源库间光合产物运转差异的分析较少, 仅有部分研究认为改善土壤通气性能增加叶片中钾、钙、锰、硼和锌的含量, 提高ATP含量和ATP酶活性, 促进叶片中光合产物的输出[13,17]。而甘薯源库间光合产物的运转需要叶、柄、茎蔓和块根共同完成, 只关注某一个器官的生理变化, 无法确定土壤通气性引起光合产物运转差异的关键环节。本研究结果表明, 与对照处理相比, 疏松处理显著提高块根中13C同化物的输入效率, 紧实处理与之相反(图2)。疏松处理显著提高块根中蔗糖和淀粉含量; 显著降低地上部各器官淀粉含量, 且对茎的作用时间早, 并显著降低茎基部蔗糖含量(表4和表5)。紧实处理显著降低块根中蔗糖和淀粉含量; 显著提高茎、柄和叶中淀粉含量, 并显著提高茎基部蔗糖含量(表6)。改善土壤通气性显著降低茎基部与块根间蔗糖含量差(表7)。相关分析的结果也表明, 茎基部与块根间蔗糖含量差与块根蔗糖和淀粉含量呈极显著负相关关系(表8)。说明改善土壤通气性促进甘薯源库间光合产物运转的关键过程是光合产物由茎基部向块根的运转, 该过程运转顺畅, 源库间光合产物的运转效率高, 块根中光合产物积累多, 块根产量就高。
[1] Rankine D R, Cohen J E, Taylor M A, Coy A D, Simpson L A, Stephenson T. Parameterizing the FAO Aquacrop model for rainfed and irrigated field-grown sweet potato., 2015, 107: 1.
[2] Hazra P, Chattopadhyay A, Karmakar K, Dutta S. Sweet potato. In: Modern Technology in Vegetable Production. New Delhi: New India Publishing Agency, 2011. pp 358–370.
[3] Abdissa T A, Chali K, Tolessa F, Tadese A G. Yield and yield components of sweet potato as influenced by plant density in Adami Tulu Jido Kombolcha District, Central Rift Valley of Ethiopia., 2001, 1: 40–48.
[4] Mu T H, Tan S S, Xue Y L. The amino acid composition, solubility and emulsifying properties of sweet potato protein., 2009, 112: 1002–1005.
[5] Bourke R M. Sweet potato () production and research in Papua New Guinea., 1985, 33: 89–108.
[6] Bourke R M. Influence of soil moisture on sweet potato yield in the Papua New Guinea highlands., 1989, 9: 322–328.
[7] Duan W, Wang Q, Zhang H. Comparative study on carbon- nitrogen metabolism and endogenous hormone contents in normal and overgrown sweetpotato., 2018, 115: 199–207.
[8] Kazuyki W, Toshio K. Effects of the capacity and composition of soil air on the growth and yield of sweet potato plants., 1964, 33: 418–422.
[9] Anikwe M A N, Ubochi J N. Short-term changes in soil properties under tillage systems and their effect on sweet potato (L.) growth and yield in an Ultisol in south-eastern Nigeria., 2007, 45: 351–358.
[10] Bogunovic I, Pereira P, Kisic I, Sajko K, Sraka M. Tillage management impacts on soil compaction, erosion and crop yield in Stagnosols (Croatia)., 2018, 160: 376–384.
[11] Ungureanu N, Croitoru S T, Biriş S, Voicu G, Vlǎ Duţ V, Selvi K C. Agricultural soil compaction under the action of agricultural machinery., 2015, 43: 31–42.
[12] Botta G F, Tolon-Becerra A, Lastra-Bravo X, Tourn M. Tillage and traffic effects (planters and tractors) on soil compaction and soybean (L.) yields in., 2010, 110: 167–174.
[13] 史春余, 王振林, 郭风法, 余松烈. 土壤通气性对甘薯养分吸收、14C-同化物分配及产量的影响. 核农学报, 2002, 16: 232–236.Shi C Y, Wang Z L, Guo F F, Yu S L. Effects of the soil aeration on nutrient absorption,14C-assimilates distribution and storage root yield in sweet potato., 2002, 16: 232–236 (in Chinese with English abstract).
[14] Watanabe K, Ozaki K. Studies on the effects of soil physical conditions on the growth and yield of crop plants: III. Effects of the capacity and composition of soil air on the growth and yield of sweet potato plants., 1964, 33: 418–422.
[15] Watanabe K, Kodama T, Nomoto T. Studies on the effects of soil physical conditions on the growth and yield of crop plants: IV. Effects of the different soil structures on a few physiological characters of sweet potato plants., 1966, 34: 409–412.
[16] 王树钿, 于作庆. 甘薯在不同土壤条件下高产规律的初步研究. 中国农业科学, 1981, 14(1): 49–55.Wang S D, Yu Z Q. A preliminary study on the high-yielding law of sweet potato in different kind of soil., 1981, 14 (1): 49–55 (in Chinese with English abstract).
[17] 史春余, 王振林, 余松烈. 土壤通气性对甘薯产量的影响及其生理机制. 中国农业科学, 2001, 34: 173–178.Shi C Y, Wang Z L, Yu S L. Effects of soil aeration on sweet potato yield and its physiological mechanism., 2001, 34: 173–178 (in Chinese with English abstract).
[18] 孙向阳. 土壤学. 北京: 中国林业出版社, 2005. pp 131–136.Sun X Y. Soil Science. Beijing: China Forestry Publishing House Publishers, 2005. pp 131–136 (in Chinese).
[19] 朱伟. 蒽酮-硫酸比色法测定香菇多糖含量. 北方药学, 2011, 8(8): 8–9.Zhu W. Determination of the lentinan content by anthrone-sulfuric acid colorimetry., 2011, 8(8): 8–9 (in Chinese with English abstract).
[20] Kodama T, Nomoto T, Watanabe K. The effect of soil density and amount of fertilizer on the growth and yield., 1959, 27: 372–374.
[21] Kaoru E, Hakabu S. Effect of atmospheric humidity and soil moisture on the translocation of sucroce-14C in the sweet potato plant., 1962, 32: 41–44.
[22] Kazuyuki W, Toshio K. Effects of the different soil structures on a few physiological characters of sweet potato plants., 1965, 34: 409–412.
[23] 史文卿, 张彬彬, 柳洪鹃, 赵庆鑫, 史春余, 王新建, 司成成. 甘薯块根形成和膨大对土壤紧实度的响应机制及与产量的关系. 作物学报, 2019, 45: 755–763.Shi W Q, Zhang B B, Liu H J, Zhao Q X, Shi C Y, Wang X J, Si C C. Response mechanism of sweet potato storage root formation and bulking to soil compaction and its relationship with yield., 2019, 45: 755–763 (in Chinese with English abstract).
Reason exploration for soil aeration promoting photosynthate transportation between sink and source in sweet potato
LIU Yong-Chen1, SI Cheng-Cheng2, LIU Hong-Juan1,*, ZHANG Bin-Bin1, and SHI Chun-Yu1,*
1College of Agronomy, Shandong Agricultural University / State Key Laboratory of Crop Biology, Tai’an 271018, Shandong, China;2College of Horticulture and Landscape Architecture, Hainan University, Haikou 570228, Hainan, China
Fieldexperiments were performed using the varieties of starchy sweet potato Shangshu 19 and Jixu 23 with three treatments including loose soil, control soil and compact soil, to clarify the regulatory mechanism of soil compaction on transportation of photosynthates between sink and source of sweet potato. Compared with control treatment, storage root yield and economic coefficient of loose soil treatment were significantly increased by 27.03%–38.74% and 6.30%–13.05% in two years, respectively, while those of compact soil treatment significantly decreased by 17.87%–15.92% and 10.83%–15.63%, respectively. The13C labeling results of functional leaves showed that loose treatment significantly improved the import efficiency of photosynthate in storage roots. Loose soil treatment significantly increased sucrose and starch contents in storage roots, but significantly reduced starch content in aboveground organs, especially in lower-middle position of stem. Compact soil treatment significantly decreased sucrose and starch contents in storage roots, but significantly increased starch and sucrose contents in aboveground organs especially in lower-middle position of stem. Both difference of sucrose content between stem base and stem top and between stem base and storage root at 50–150 days after planting in loose treatment were significantly decreased. While, significantly increased in compact treatment. The variation range of sucrose content difference between stem base and storage root was larger than between stem base and stem top. There was a very significantly negative correlation between the sucrose content difference of stem base and storage root, and the sucrose and starch content in storage root. Improvement of soil aeration, can promote the transportation of photosynthates from stem base to storage root, increase carbohydrate content in storage root and enhance storage root yield.
sweet potato; soil aeration; storage root yield; photosynthate; transportation
2019-03-08;
2019-09-26;
2019-10-12.
10.3724/SP.J.1006.2020.94038
史春余, E-mail:scyu@sdau.edu.cn, Tel: 0538-8246259; 柳洪鹃, E-mail: liumei0535@126.com
E-mail: liuyongchensdau@163.com
本研究由国家自然科学基金项目(31371577, 31701357)和山东省薯类产业创新团队首席专家项目(SDAIT-16-01)资助。
This study was supported by the National Natural Science Foundation of China (31371577, 31701357) and the Potato Innovation Program for Chief Expert of Shandong Province (SDAIT-16-01).
URL:http://kns.cnki.net/kcms/detail/11.1809.S.20191012.1214.004.html
